Published online Apr 21, 2019. doi: 10.3748/wjg.v25.i15.1865
Peer-review started: January 4, 2019
First decision: January 30, 2019
Revised: March 5, 2019
Accepted: March 15, 2019
Article in press: March 16, 2019
Published online: April 21, 2019
Unconjugated bilirubin (UCB) is generally considered toxic but has gained recent prominence for its anti-inflammatory properties. However, the effects of it on the interaction between intestinal flora and organisms and how it influences immune responses remain unresolved.
To investigate the role of UCB in intestinal barrier function and immune inflammation in mice with dextran-sulfate-sodium-induced colitis.
Acute colitis was induced by 3% (w/v) dextran sulfate sodium salt in drinking water for 6 d followed by untreated water for 2 d. Concurrently, mice with colitis were administered 0.2 mL UCB (400 μmol/L) by intra-gastric gavage for 7 d. Disease activity index (DAI) was monitored daily. Mice were sacrificed at the end of the experiment. The length of the colon and weight of the spleen were recorded. Serum level of D-lactate, intestinal digestive proteases activity, and changes to the gut flora were analyzed. In addition, colonic specimens were analyzed by histology and for expression of inflammatory markers and proteins.
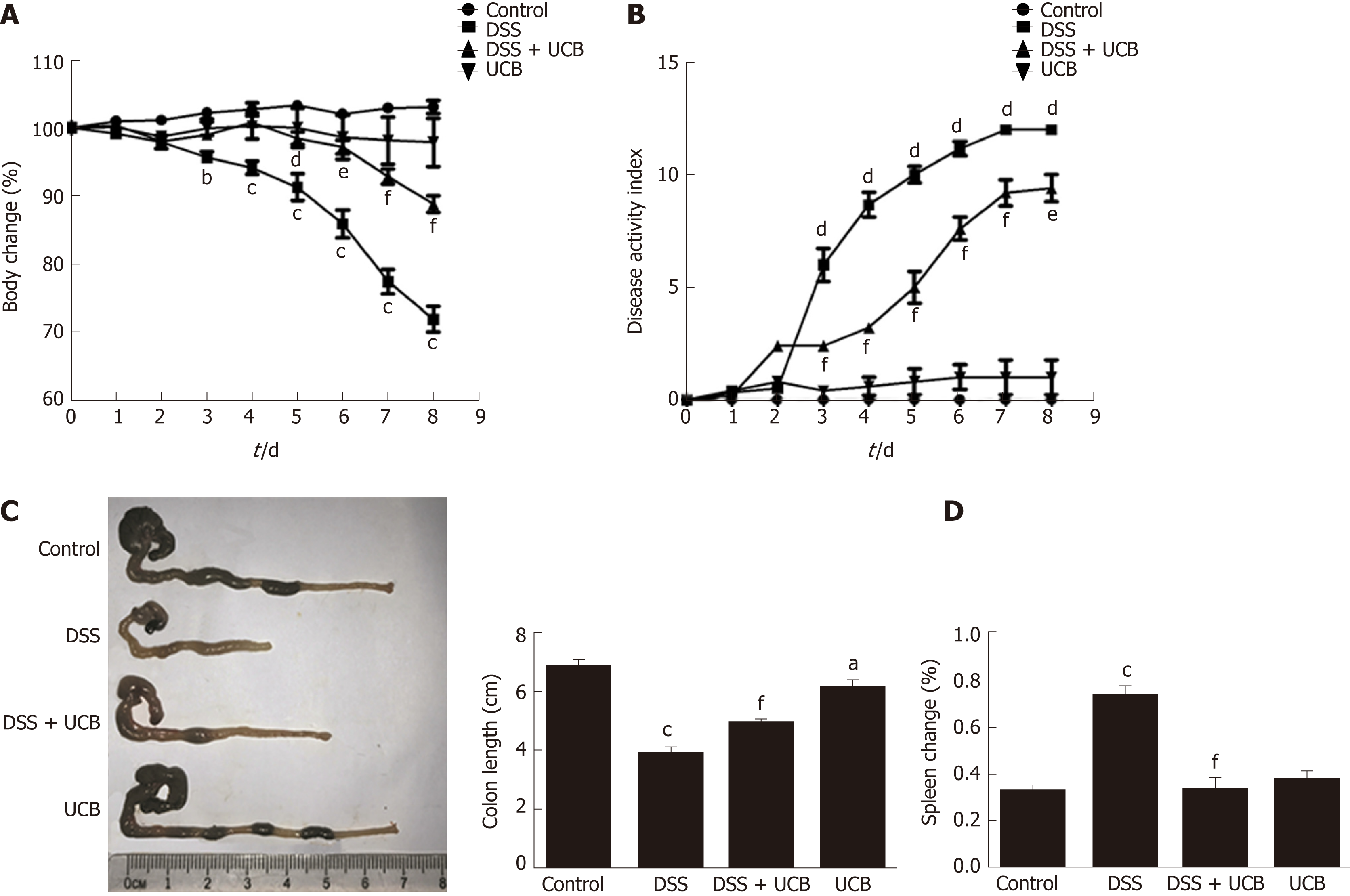
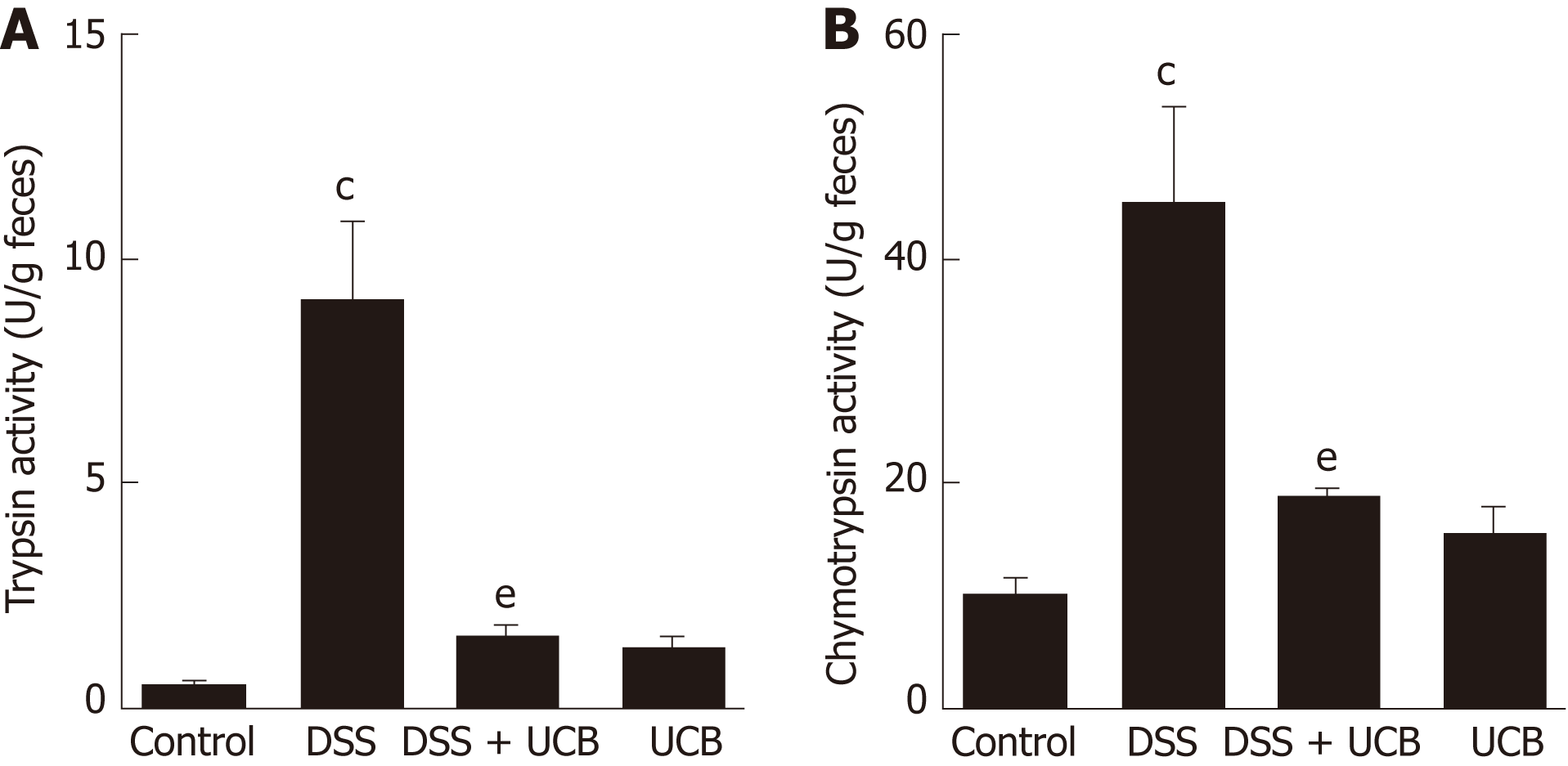
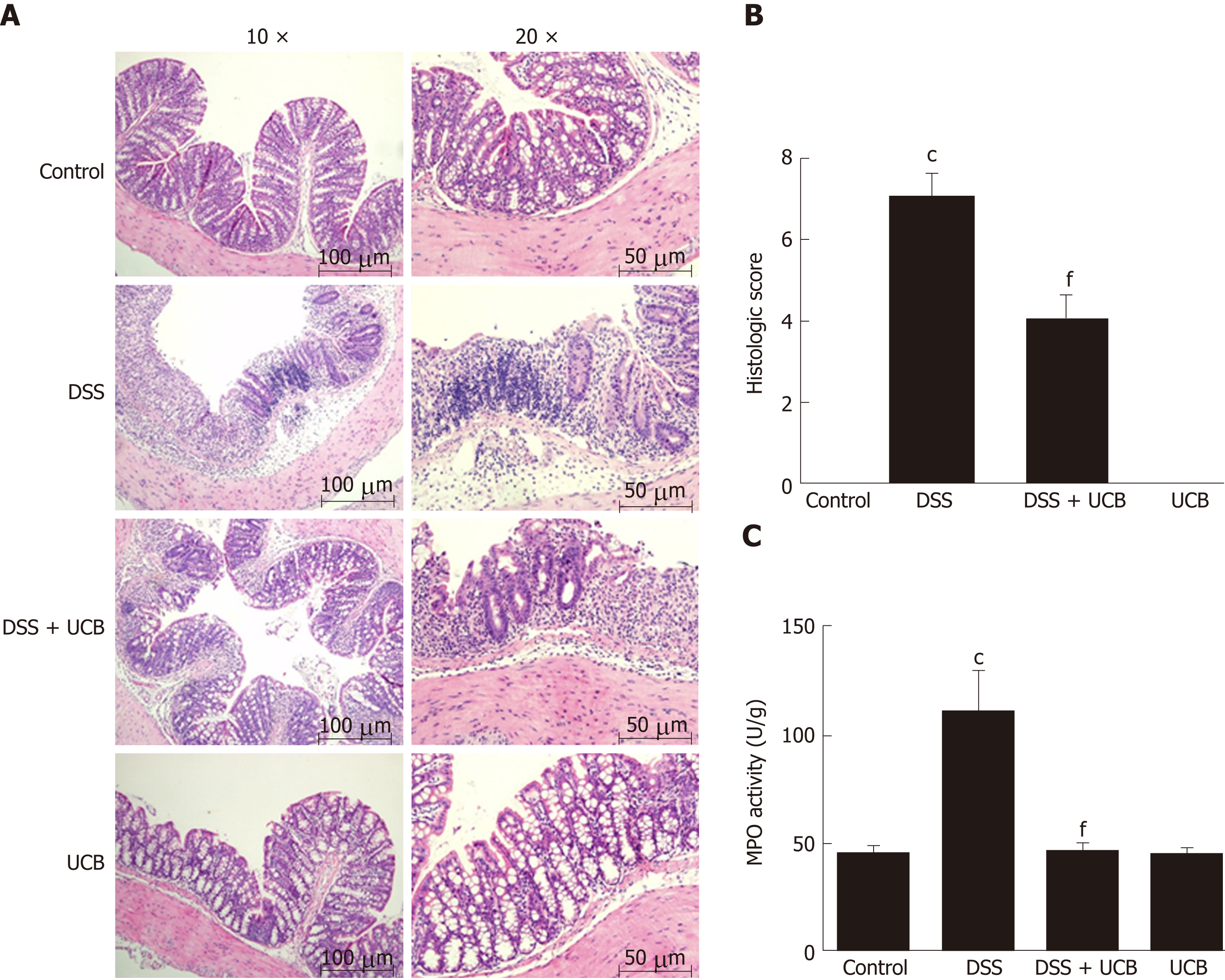
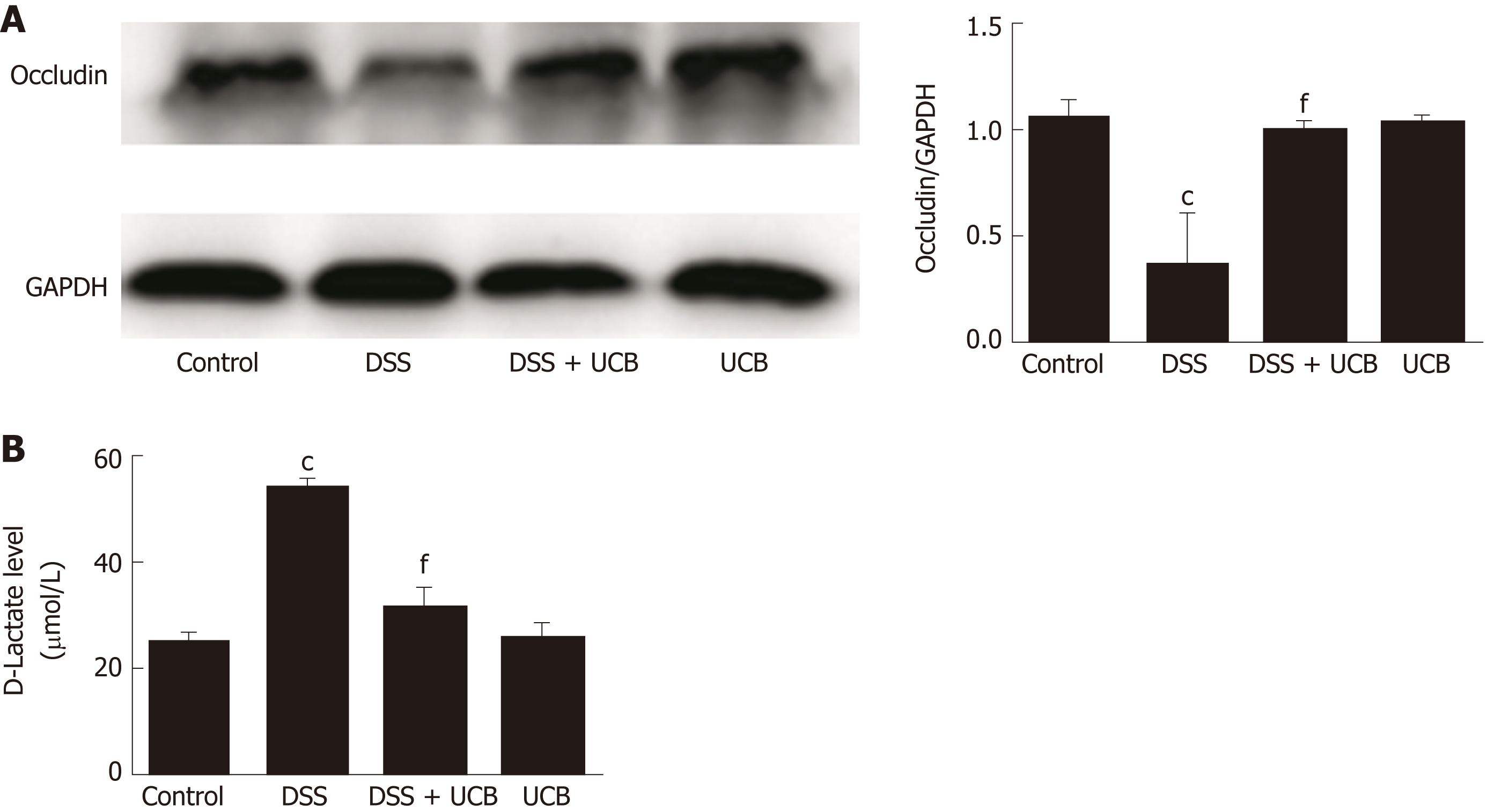
Mice treated with UCB had significantly relieved severity of colitis, including lower DAI, longer colon length, and lower spleen weight (colon length: 4.92 ± 0.09 cm vs 3.9 ± 0.15 cm; spleen weight: 0.33 ± 0.04 vs 0.74 ± 0.04, P < 0.001). UCB administration inactivated digestive proteases (chymotrypsin: 18.70 ± 0.69 U/g vs 44.81 ± 8.60 U/g; trypsin: 1.52 ± 0.23 U/g vs 9.05 ± 1.77 U/g, P < 0.01), increased expression of tight junction (0.99 ± 0.05 vs 0.57 ± 0.03, P < 0.001), decreased serum level of D-lactate (31.76 ± 3.37 μmol/L vs 54.25 ± 1.45 μmol/L, P < 0.001), and lowered histopathological score (4 ± 0.57 vs 7 ± 0.57, P < 0.001) and activity of myeloperoxidase (46.79 ± 2.57 U/g vs 110.32 ± 19.19 U/g, P < 0.001). UCB also regulated the intestinal microbiota, inhibited expression of tumor necrosis factor (TNF) α and interleukin 1β (TNF-α: 52.61 ± 7.81 pg/mg vs 105.04 ± 11.92 pg/mg, interleukin 1β: 13.43 ± 1.68 vs 32.41 ± 4.62 pg/mg, P < 0.001), decreased expression of Toll-like receptor 4 (0.61 ± 0.09 vs 1.07 ± 0.03, P < 0.001) and myeloid differentiation primary response gene 88 (0.73 ± 0.08 vs 1.01 ± 0.07, P < 0.05), and increased expression of TNF-receptor-associated factor 6 (0.79 ± 0.02 vs 0.43 ± 0.09 P < 0.05) and inhibitor of kappa B α (0.93 ± 0.07 vs 0.72 ± 0.07, P < 0.05) in the colon.
UCB can protect intestinal barrier function, regulate normal intestinal homeostasis, and suppress inflammation via the Toll-like receptor 4/ nuclear factor-κB signaling pathway.
Core tip: Intestinal function and microbiota are desired therapeutic endpoints for treatment of inflammatory bowel disease. However, a major problem is the lack of powerful and safe drugs. Unconjugated bilirubin (UCB) has anti-inflammatory and antitoxin effects. We found that UCB significantly decreased intestinal permeability and improved intestinal barrier function; regulated composition of gut microbiota; and relieved intestinal inflammation through suppressing the Toll-like receptor 4/nuclear factor-κB pathway. This study demonstrated that UCB ameliorates ulcerative colitis via intestinal barrier function through inactivating the digestive proteases and inhibiting immune inflammation through the Toll-like receptor 4/nuclear factor-κB pathway.
- Citation: Zheng JD, He Y, Yu HY, Liu YL, Ge YX, Li XT, Li X, Wang Y, Guo MR, Qu YL, Qin XF, Jiang MS, Wang XH. Unconjugated bilirubin alleviates experimental ulcerative colitis by regulating intestinal barrier function and immune inflammation. World J Gastroenterol 2019; 25(15): 1865-1878
- URL: https://www.wjgnet.com/1007-9327/full/v25/i15/1865.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i15.1865
Inflammatory bowels diseases (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), are associated with chronic, relapsing inflammation of the intestinal tract. In the early 1900s, regions such as North America, Europe, and Oceania had millions of individuals with IBD. The prevalence of IBD is highest in the western world, which was estimated to affect up to 0.5% of the general population in 2015 and equates to approximately 2.2 million Americans living with IBD in 2025[1,2]. However, newly industrialized countries have a low prevalence of IBD, but the incidence has been steadily rising. A representative study from the Asia–Pacific Crohn’s and Colitis Epidemiology Study showed that IBD was established in Asia, with an average incidence of 1.4 per 100000 people in 2011, and the incidence of UC was two-fold higher than that of CD in Asia and the incidence of IBD in China was 3.3 per 100000[3]. IBD affects millions of people around the world, producing a substantial burden on healthcare systems.
Evidence from studies on etiology and pathogenesis suggests that IBD results from a dysregulated intestinal immune response driven by complex interplay between the host and intraluminal microbiota[4]. Intestinal epithelial cells recognize conserved signature molecules in the gut commensal bacteria, called pathogen-associated molecular patterns, by pattern recognition receptors. Toll-like receptors (TLRs) are one of the major types of pattern recognition receptor families and have a pivotal effect on maintaining homeostasis of the gut microbiota[5]. During inflammation, TLR4 as an innate immune receptor is stimulated by recognition of gut pathogen-associated molecular patterns. TLR4 undergoes a conformational change and then recruits the signaling adaptors myeloid differentiation primary response gene 88 (MyD88) and tumor necrosis factor (TNF) receptor-associated factor (TRAF) 6, thereby activating downstream transcription of nuclear factor-κB (NF-κB) target genes and expression of some pro-inflammatory cytokines such as interleukin 1β (IL-1β), TNF-α, or IL-6[6]. However, anti-inflammatory and immuno-regulatory cytokines are related to the negative regulation of nitric oxide (NO), which is positively correlated with the severity of the disease. In IBD patients, NO production was positively correlated with increased levels of pro-inflammatory cytokines[7]. The role of cytokines and NO level are fundamental to regulate inflammation in IBD. Meanwhile, TLR4 was significantly more upregulated in IBD patients than in controls, especially in UC patients[8,9]. The probiotic cocktail (Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis, and Bifidobacterium breve) ameliorated clinical symptoms and histological scores, decreased NO level, and reduced TLR4, inducible NO synthase, and NF-κB expression[10]. An important mechanism of UC might be that abnormal inflammation by intestinal dysbacteriosis causes dysregulation of TLR that mediates innate immunity. The TLR4/NF-κB signaling pathway is an essential key point in the development of UC.
Unconjugated bilirubin (UCB) is generated during the physiological breakdown of heme by heme oxygenase 1. Serum levels of bilirubin increase with the accelerated release of heme from hemoglobin or with diminished hepatic conjugating activity. UCB is a potent antioxidant even at low concentrations[11,12]. It was first discovered in 1934 that bilirubin was exerts anti-inflammatory activity in patients with rheumatoid arthritis who experienced remission of symptoms after developing jaundice secondary to liver disease[13]. Studies have shown that bile acids play a role in the regulation of gut injury, immunity, and inflammation[14,15]; however, there is a lack of studies on the physiological function of bilirubin that enters the intestine together with bile. Recent investigations have demonstrated that UCB suppresses inflammatory responses in animal models of autoimmune encephalomyelitis and lung inflammation in asthma[16-18]. Nevertheless, the mechanisms on inflammation and role of UCB in the interaction between intestinal flora and immunity are not well understood. The aim of the present study was to investigate the effects of UCB on intestinal barrier function and inflammation on dextran sodium sulfate (DSS)-induced colitis in mice.
Male C57BL/6 mice aged 8-12 wk (weight approximately 25 g) were purchased from the Experimental Animal Center of the Second Affiliated Hospital of Harbin Medical University and were acclimatized for 1 wk before experiments were performed. They were reared in the Animal Laboratory Centre of Harbin Medical University under specific pathogen-free conditions (temperature 24-25 °C, humidity 70%-75%, with a 12 h light/dark lighting regimen) and were fed a standard diet of pellets and water ad libitum. The study was approved by the Institutional Animal Care and Use Committee of Harbin Medical University.
UCB, N-benzoyl-L-tyrosine ethyl ester, and N-α-benzoyl-L-arginine 4-nitroanilide hydrochloride were purchased from Sigma–Aldrich (St. Louis, MO, United States). DSS (36-50 kDa) was obtained from MP Biomedical (Solon, OH, United States). Enzyme linked immunosorbent assay (ELISA) kits for D-lactate, TNF-α, IL-1β, and myeloperoxidase (MPO) were from Beijing Propbs Biotechnology (Beijing, China). The antibodies used in this study were anti-TLR4 (19811-1-AP; Proteintech, Rosemont, United States), anti-MyD88 (4283; Cell Signaling Technology, Danvers, MA, United States), anti-TRAF6 (ab33915; Abcam, Cambridge, MA, United States), anti-inhibitor of NF-κB alpha (IκBα) (4814; Cell Signaling Technology), and anti-occludin (ab167161; Abcam). Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH), goat anti-rabbit immunoglobulin G and goat anti-mouse immunoglobulin G were purchased from ZSGB-BIO Co. Ltd. (Beijing, China). All other reagents used were of analytical grade.
Colitis was induced by oral administration of DSS as described previously, with some modifications[19]. The animals were randomly divided into five groups with five mice in each group: Control group (Control), DSS group (DSS), DSS plus UCB group (DSS + UCB), and UCB group (UCB). Colitis was induced by administering 3% DSS in drinking water for 6 d, and then drinking water without DSS for another 2 d for recovery. From the first day, UCB was administered daily via gavage at 0.2 mL for 7 d (UCB was dissolved in 0.4% dimethyl sulfoxide at concentrations up to 400 μM). During the study, weight, physical condition, stool consistency, and the presence of occult blood in feces were examined and documented daily. All animals were sacrificed after 8 d by intraperitoneal injection of an overdose of chloral hydrate. Blood specimens were collected and serum samples were prepared by centrifugation at 4000 g for 15 min at 4 °C and stored at -80 °C. The entire colon, spleen and total feces of mice were carefully removed, measured and weighted, then stored at -80 °C for further analysis.
Disease activity index (DAI) was calculated for each animal by measuring the body weight, stool consistency, and fecal blood. Each score was calculated based on the criteria listed in Table 1[20].
| Score | Weight loss, % | Stool consistency | Occult/gross bleeding |
| 0 | None | Normal | Normal |
| 1 | 1-5 | ||
| 2 | 5-10 | Loose stools | Hemoccult positive |
| 3 | 10-15 | ||
| 4 | > 15 | Diarrhea | Gross bleeding |
Colonic tissues fixed in 4% (w/v) paraformaldehyde were paraffin-embedded and sliced into 5 μm sections, followed by staining with hematoxylin and eosin for light microscopic examination to assess colon injury and inflammation. Colonic damage was graded in a blinded manner as described as Table 2[21].
| Colon damage score | 0 | 1 | 2 | 3 |
| Crypt architecture damage | None | Regeneration | Destruction | |
| Edema in sub-mucosa | None | Mild | Moderate | Severe |
| Inflammatory cells infiltration | None/rare | Lamina propria | Sub-mucosa | Muscle layer |
D-Lactate concentration in serum was measured by ELISA. Results are expressed as μmol D-lactate/mL serum.
Trypsin and chymotrypsin (amidase) activities were measured using N-α-benzoyl-L-arginine 4-nitroanilide hydrochloride or N-benzoyl-L-tyrosine ethyl ester as the substrate, respectively, as described previously[22,23].
Total RNA was isolated from colon tissues using the UNlQ-10 Column Trizol Total RNA Isolation Kit (Sangon Biotech, Shanghai, China). After treating with DNase I (TaKaRa, Tokyo, Japan), RNA was transcribed into cDNA using Prime Script TM RT regent Kit (TaKaRa) using an Eppendorf Mastercycler personal Thermo cycler. All primers were obtained from Sangon. The primers were used as followed: forward: 5’-GAGCACCTTCTTTTCCTTCATCTT-3’ and revers: 5’-TCACACACCAGCAGGTT
ATCATC-3’ for IL-1β, forward: 5’-CATCTTCTCAAAATTCGAGTGACAA -3’ and reverse: 5’-TGGGAGTAGACAAGGTACAACCC-3’ for TNF-α and forward: 5’-CATGGCCTTCCGTGTTCCTA -3’ and revers: 5’-GCGGCACGTCAGATCCA-3’ for GAPDH. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in a volume of 20 μL with FastStart Universal SYBR Green Master (Roche, Basel, Switzerland). All samples were analyzed in triplicate, and the results were normalized to the expression of GAPDH. Results were expressed as 2−ΔΔCt.
Genomic DNA from feces was extracted by Stool Genomic DNA Extraction Kit (Tiangen Biotech, Beijing, China) and used for quantitative analysis of fecal bacteria Firmicutes, Bacteroidetes, Actinomycetes, and Proteobacteria. All primers were obtained from Sangon. The primers were used as followed: forward: 5’-GCTGCTAATACCGCATGATATGTC-3’ and reverse: 5’-CAGACGCGAGTCCAT
CTCAGA-3’ for Firmicutes, forward: 5’-GAGAGGAAGGTCCCCCAC-3’ and reverse: 5’-CGCTACTTGGCTGGTTCAG-3’ for Bacteriodetes, forward: 5’-TGTAGCGGTGGA
ATGCGC-3’ and reverse: 5’-AATTAAGCCACATGCTCCGCT-3’ for Actinomycetes, forward: 5’-TAGGCTTGACATTGATAGAATC-3’ and reverse: 5’-CTTACGAAGGCA
GTCTCCTTA-3’ for Proteobacteria and forward: 5’-GCAACGAGCGCAACCC-3’ and reverse: 5’-ACGGGCGGTGTGTAC-3’ for 16S rDNA. qRT-PCR in a volume of 20 μL was performed using FastStart Universal SYBR Green Master. All samples were analyzed in triplicate, and the results were normalized to the expression of 16S rDNA. The results were expressed as 2−ΔΔCt.
Segments of colon were homogenized using radioimmunoprecicpitation assay buffer and protein inhibitor cocktail (1:10) (PhosSTOP ESAYpack; Roche). The homogenates were kept on ice for 30 min and centrifuged at 12000 g for 5 min at 4 °C. The protein concentration was determined using the bicinchoninic acid assay Protein Assay Kit (Beyotime, Shanghai, China). For ELISA, the supernatants were collected and subjected to IL-1β, TNF-α, and MPO assays. Results of IL-1β and TNF-α were expressed as pg/mg and MPO as U/g. For western blot analysis, proteins were electroblotted onto a polyvinylidene difluoride membrane following separation on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The immunoblot was then incubated with primary antibodies against occludin, TLR4, MyD88, TRAF6, and IκBα, or GAPDH. The chemiluminescence signals were analyzed using Quantity One (version 4.5.2; Bio-Rad Laboratories, Hercules, CA, United States) and Image J software.
Results were expressed as mean ± standard error of the mean (SEM). Differences between groups were determined using one-way analysis of variance followed by Tamhane multiple comparisons post-hoc tests using SPSS version 19.0 (IBM, Armonk, NY, United States). Graphs were analyzed using Graphpad Prism version 5.0 (Graphpad Software, La Jolla, CA, United States). Statistical significance was denoted as P < 0.05.
Compared with the DSS group, the DSS + UCB group exhibited less weight loss, diarrhea, and intestinal bleeding, as reflected by the significantly lower DAI scores (P < 0.001, Figure 1A and B). The two control groups that received regular drinking water or UCB alone did not develop signs of colitis throughout the experiment (all DAI score < 1 from 1 d to 8 d, Figure 1B). Furthermore, UCB treatment significantly reduced colon shortening and increased spleen weight (P < 0.001, Figure 1C and D).
Digestive proteases are believed to play a crucial role in the destruction of the intestinal barrier[24,25], and UCB has previously been demonstrated to inactivate digestive proteases activity[26]. Therefore, we tested the effects of UCB on trypsin and chymotrypsin activity in the feces of mice with DSS-induced colitis. Compared with the DSS group, the DSS + UCB group showed remarkably lower activities of fecal digestive proteases trypsin and chymotrypsin, and levels in the UCB alone group were similar to the control group (P < 0.01, Figure 2A and B).
DSS-induced colon tissue injury was demonstrated by epithelial destruction, intense inflammatory infiltration, and crypt distortion (Figure 3A) as well as increased histological scores for inflammation and crypt damage (Figure 3B). DSS with UCB treatment reduced neutrophil infiltration and crypt damage in the colon, leading to a decrease in colonic MPO activity (P < 0.001, Figure 3C), which is an inflammatory marker for colitis. The epithelial destruction, histological score, and MPO activity of the UCB alone group were similar to those in the control group.
Compared with the control group, expression of the tight junction protein occludin in the colon of the DSS group was decreased significantly (P < 0.001, Figure 4A), and serum level of D-lactate was significantly elevated (P < 0.001, Figure 4B). However, in the DSS + UCB group, expression of occludin was increased in the colon with a decrease in serum levels of D-lactate (P < 0.001, Figure 4A and B). Results in the UCB alone group were similar to those in the control group (Figure 4).
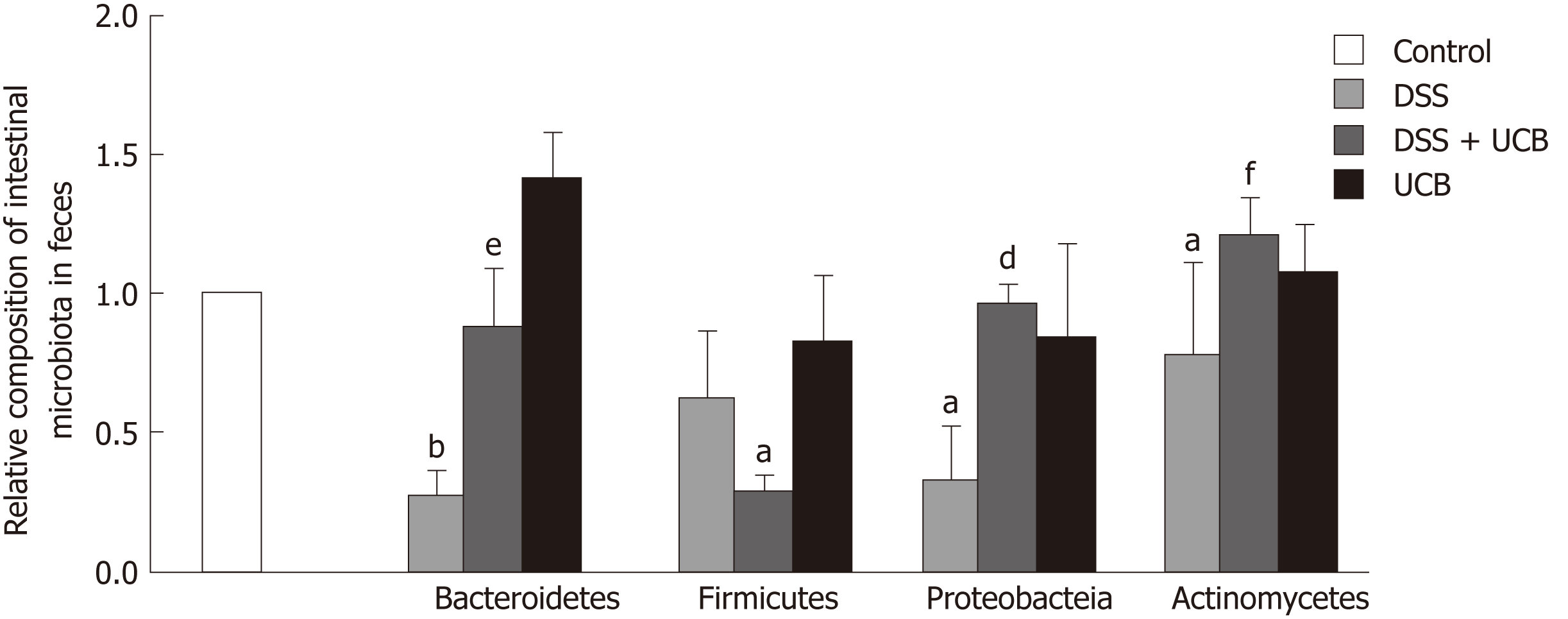
We assessed the change in fecal microbiota in mice at the phylum level. DSS induced an overall decrease in feces microbiota. Compared with the controls, Firmicutes and Actinomycetes were dominant in the intestinal flora (P < 0.05), while Bacteroidetes (P < 0.01) and Proteobacteria (P < 0.05) were less. The intestinal flora of UCB treated mice recovered approximately. However, Firmicutes (P < 0.01) were decreased and Actinomycetes (P < 0.001) were increased compared with the DSS group. Bacteroidetes in the UCB alone group were also higher than in the control group, but the difference was not significant (Figure 5).
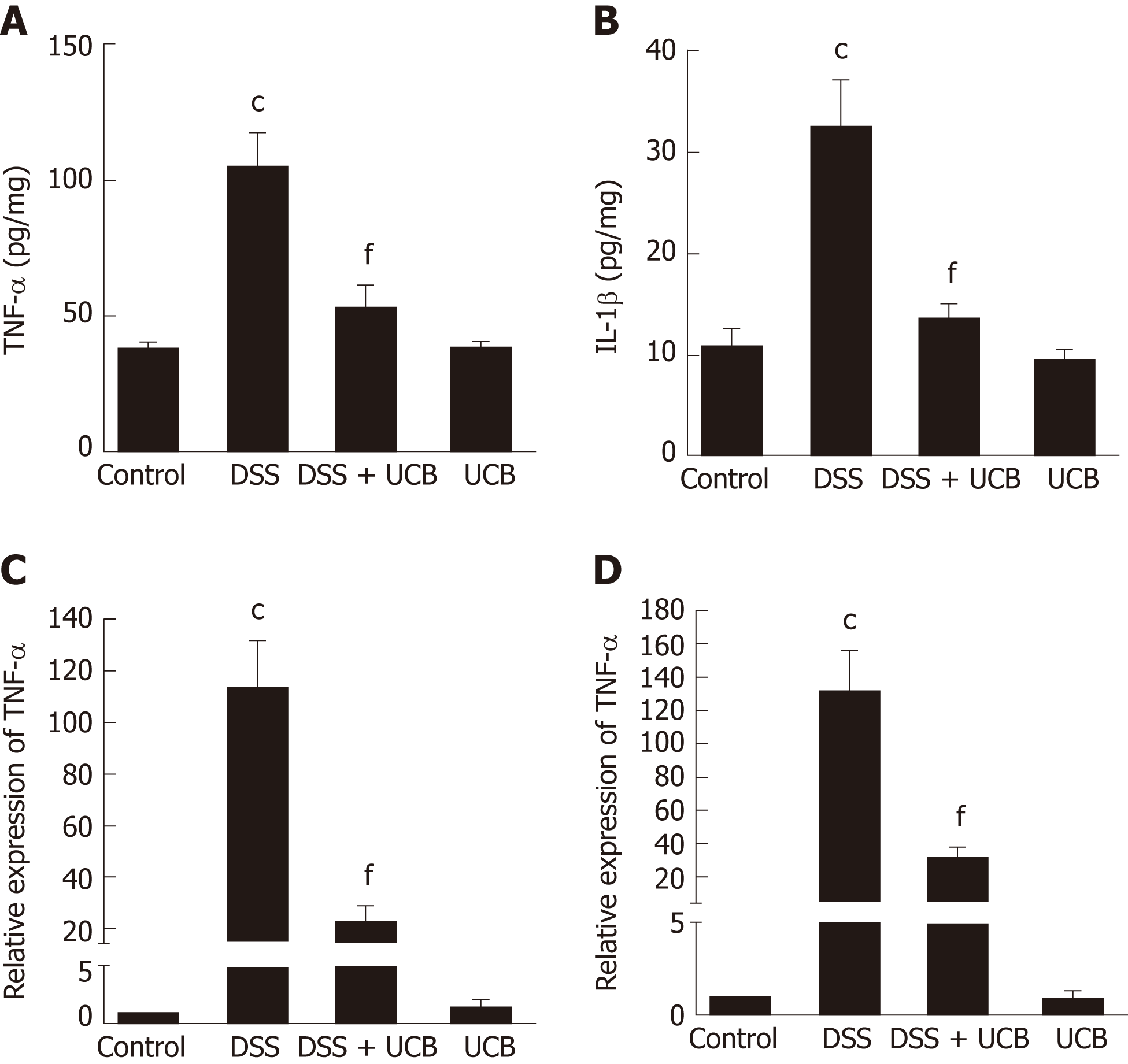
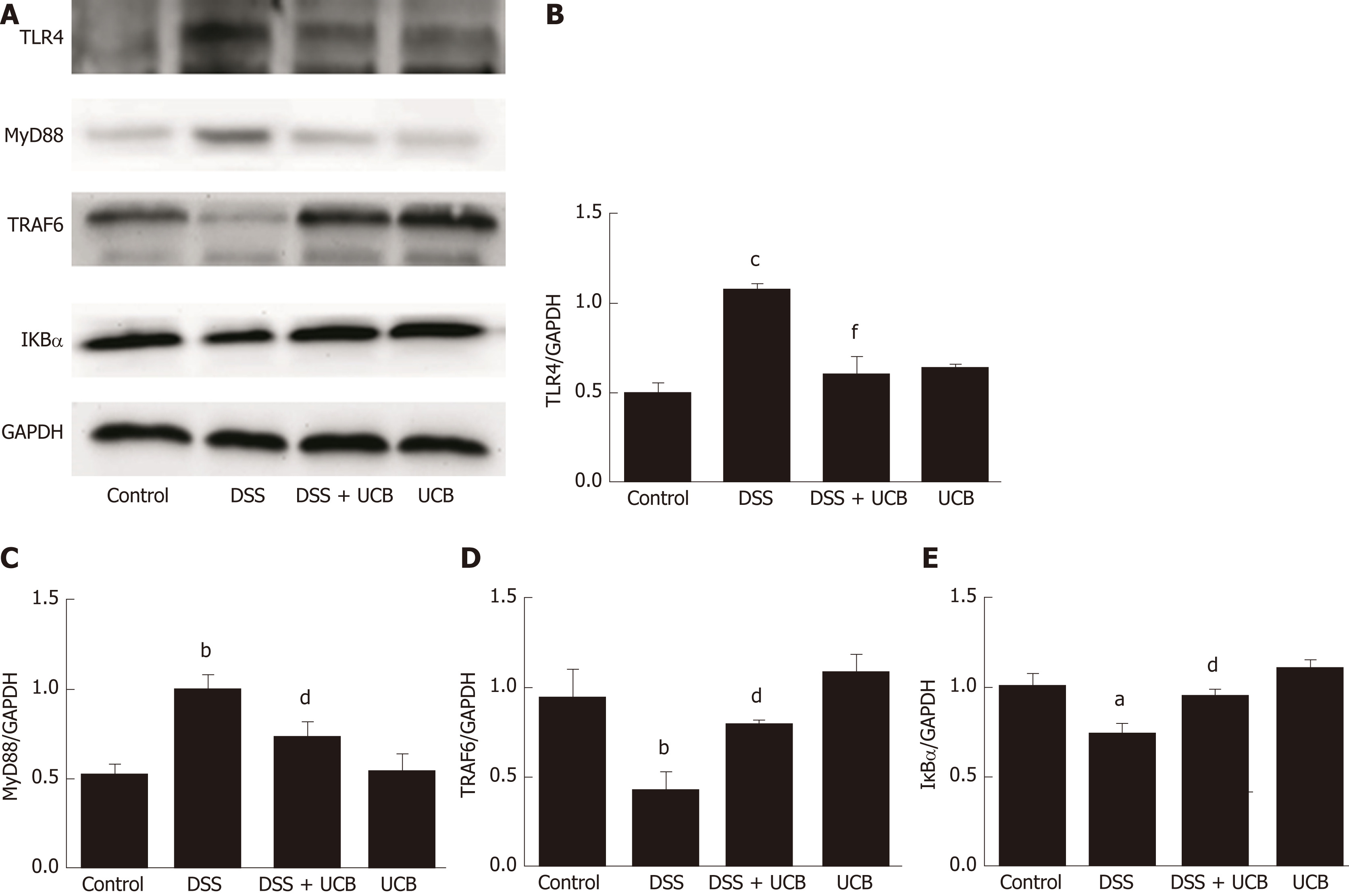
The mRNA and protein levels of the pro-inflammatory cytokines, TNF-α and IL-1β, in colon tissues of mice were determined. DSS-induced acute colitis was accompanied by a significant increase in TNF-α and IL-1β levels (P < 0.001), and treatment with UCB restored these cytokines to normal levels (P < 0.001, Figure 6A, B). TLR4 is a key immune receptor that plays an important role in the regulation of colonic inflammation[27]. Compared with the control group, expression of TLR4 (P < 0.01) and MyD88 (P < 0.05) was increased in the DSS group, and expression of TRAF6 (P < 0.01) and IκBα (P < 0.05) was decreased. In comparison, expression of these proteins was restored to near normal in the UCB-treated group. The results in the UCB only group were similar to those in the control group (Figure 7).
Previous observation on human evolution has shown that bilirubin-predominant species are often carnivores or omnivores, while biliverdin-predominant species are often herbivores[28], suggesting that bilirubin may have an important yet unknown effect on the human body. Significant increases in concentration of serum bilirubin in neonates (> 20 mg/dL, approximately 340 μM) can trigger nerve damage[29]; however, it is a powerful antioxidant and toxic in adults[11,30,31]. UC patients have reduced total serum bilirubin levels[32]. Numerous studies have suggested that UCB plays an important potential protective role in vascular endothelial function[33]; ameliorates allergic lung inflammation in a mouse model of asthma[18]; prevents murine colitis by inhibiting leukocyte infiltration and suppressing upregulation of inducible NO synthase[34]; or scavenges various reactive oxygen species[12].
Our previous work confirmed that UCB ameliorates the inflammation in trinitrobenzenesulfonic acid-induced colitis; an animal model of CD[35]. In this study, we further established the UC mouse model that has a different extent of ulceration and tissue edema. Our data suggest two potential mechanisms by which UCB exerts its protective effect on DSS-induced colitis: (1) Inactivation of digestive proteases to protect intestinal mucosal barrier and prevent intestinal flora translocation to the colon leading to an abnormal interaction; and (2) suppression of the TLR4/MyD88/TRAF6/NF-κB signaling pathway to inhibit immune inflammation and regulate intestinal microflora homeostasis.
The intestinal barrier is a complex multilayer system, consisting of an external anatomical barrier and an inner functional immunological barrier. The interplay of these two barriers maintains normal intestinal function and a stable intestinal environment[36]. We detected that the intestinal tract of UC mice contained elevated active digestive proteases, which can destroy the intestinal tissue structure and increase intestinal permeability. This provides favorable conditions for intestinal pathogens or toxins of microbiota invading the body. The large number of digestive proteases as vital factors contributes to the pathogenesis and development of IBD due to destruction of the intestinal barrier. UCB can be a specific inhibitor to inactivate digestive proteases to protect the physical barrier function that is the first line of defense against invasion of intestinal pathogens.
Another pivotal factor, intestinal microbes, participates in many important physiological processes, including nutritional absorption, substance metabolism, and immunity[37,38]. In order to investigate how UCB may specifically contribute to intestinal microbiota during DSS colitis, we tested the composition and abundance at the phylum level (Bacteroidetes, Firmicutes, Actinomycetes and Proteobacteria). Exposure to DSS reduced the diversity and abundance of intestinal flora. The ratio of Bacteroidetes to Firmicutes was decreased and that of Proteobacteria to Actinomycetes was increased. UCB treatment can reverse these phenomena; however, it dramatically reduces the content of Firmicutes and makes Bacteroidetes and Actinomycetes dominant in the flora. Actinomycetes have always produced many antibiotics[39]; therefore, we suspect that UCB can promote Actinomycetes to produce some secondary products to protect mice against colitis. Our study shows that UCB is beneficial to maintain the stability of intestinal flora, but its specific role needs to be examined through further research that our group has and will continue to be concerned.
TLRs are the recognition receptors of the innate immune system and are located on the surface of various immune cells and play an important role in defense against infection and regulation of immune responses. TLR4 expression is usually low under normal conditions, while in IBD, the intestinal flora lose tolerance and specific recognition of lipopolysaccharide, then produce excessive signal transduction, and finally activate TLR4 and cascade induce transcription of NF-κB and pro-inflammatory cytokines[40,41]. Our results demonstrate that UCB decreases expression of TLR4 and MyD88, and increases expression of TRAF6 and IκBα in the colon. In addition, UCB reduces levels of pro-inflammatory cytokines, TNF-α and IL-1β, suggesting it has an anti-inflammatory role in DSS-induced colitis.
UCB can suppress intestinal positive immune response and inflammation through the TLR4/NF-κB signaling pathway. Therefore, this appears to be an additional mechanism of UCB to regulate the relationship between intestinal flora and host immunity. UCB can improve intestinal inflammation, regulate homeostasis of bacterial flora, and maintain intestinal immune function; it is a way to resolve the serious problem of adverse effects and drug resistance of IBD. Perhaps low dose of UCB or in combination with other probiotics can develop healthcare to prevent clinical diseases. It is a novel therapeutic approach, not only for UC but also for other gastrointestinal diseases.
This study had some limitations. First, we found that UCB affected the intestinal flora but the change in genera was unclear. Second, we only induced acute UC, which may have different mechanisms from chronic UC. Third, this study shows that UCB protects intestinal barrier function and suppresses immunity and inflammation in DSS-induced colitis, but we did not study how UCB regulates intestinal immune cells and other potential pleiotropic roles of UCB. These issues will be studied in our future research.
In conclusion, our findings not only shed light on how UCB ameliorates UC but also offer a clue about the physiological function of UCB. This provides a noteworthy example of how UCB can serve as a “double-edged sword” in the human body. This is believed to be the first study to show that UCB ameliorates DSS-induced colitis via inactivation of digestive proteases to protect the intestinal barrier and regulate the intestinal flora and TLR4/NF-κB signaling pathway to suppress intestinal inflammation.
Clinical treatment of ulcerative colitis consists of drugs that are both expensive and have side effects. Unconjugated bilirubin (UCB) has gained recent prominence for its anti-inflammatory and antioxidant properties. How UCB influences UC remains unresolved.
Patients with UC require lifelong treatment, and drugs for UC are linked to many adverse effects. Therefore, there is an urgent need to develop effective and safe drugs for UC.
To investigate the significance of UCB in intestinal barrier function and immune inflammation of mice with dextran sodium sulfate (DSS)-induced colitis.
UC was induced by 3% (w/v) DSS in drinking water for 6 d followed by untreated water for 2 d. Concurrently, colitis mice were administered 0.2 mL UCB (400 μM) by intra-gastric gavage for 7 d. Disease activity index (DAI) was monitored daily. The length of the colon and weight of the spleen were recorded. Serum level of D-lactic acid, intestinal digestive proteases activity, and changes in gut flora were analyzed. In addition, colonic specimens were analyzed by histology and for expression of inflammatory markers and proteins.
UCB significantly relieved the severity of colitis, including lower DAI, longer colon length, and smaller spleen weight (P < 0.001). UCB inactivated digestive proteases (P < 0.01), increased expression of tight junction protein occludin (P < 0.001), decreased serum level of D-lactate (P < 0.001), and lowered histopathological score and activity of myeloperoxidase compared with those in colitis mice (P < 0.001). UCB also regulated the intestinal microbiota, inhibited expression of tumor necrosis factor (TNF)-α and interleukin-1β (P < 0.001), decreased expression of Toll-like receptor (TLR) 4 (P < 0.001) and myeloid differentiation primary response gene 88 (P < 0.05), and increased expression of TNF-receptor-associated factor 6 (P < 0.05) and IκBα (P < 0.05) in the colon.
UCB has a beneficial regulatory effect on intestinal barrier function and regulates normal intestinal homeostasis, and can suppress inflammation via the TLR4/NF-κB signaling pathway. This provides a theoretical basis for use of UCB as a clinical drug.
UCB plays a pivotal role in intestinal innate immunity and inflammation. Thus, the findings of this study indicate a novel potential mechanism by which UCB can treat UC. More studies are needed to investigate the effect of UCB on chronic UC or colon cancer.
We acknowledge Jin-An Zhou, Rong-Yan Li, and Yu Song for their excellent laboratory assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Madnani MA, Rath T, Touil-Boukoffa C S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152:313-321.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 566] [Cited by in F6Publishing: 645] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 2. | Kaplan GG. The global burden of IBD: From 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1157] [Cited by in F6Publishing: 1498] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 3. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL; Asia–Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 538] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 4. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 805] [Cited by in F6Publishing: 935] [Article Influence: 116.9] [Reference Citation Analysis (0)] |
| 5. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3061] [Cited by in F6Publishing: 3050] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 6. | Sipos F, Fűri I, Constantinovits M, Tulassay Z, Műzes G. Contribution of TLR signaling to the pathogenesis of colitis-associated cancer in inflammatory bowel disease. World J Gastroenterol. 2014;20:12713-12721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Soufli I, Toumi R, Rafa H, Touil-Boukoffa C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:353-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 210] [Cited by in F6Publishing: 219] [Article Influence: 27.4] [Reference Citation Analysis (5)] |
| 8. | Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010-7017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 917] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 9. | Tan Y, Zou KF, Qian W, Chen S, Hou XH. Expression and implication of toll-like receptors TLR2, TLR4 and TLR9 in colonic mucosa of patients with ulcerative colitis. J Huazhong Univ Sci Technolog Med Sci. 2014;34:785-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Toumi R, Soufli I, Rafa H, Belkhelfa M, Biad A, Touil-Boukoffa C. Probiotic bacteria lactobacillus and bifidobacterium attenuate inflammation in dextran sulfate sodium-induced experimental colitis in mice. Int J Immunopathol Pharmacol. 2014;27:615-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2595] [Cited by in F6Publishing: 2556] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 12. | Lee Y, Kim H, Kang S, Lee J, Park J, Jon S. Bilirubin Nanoparticles as a Nanomedicine for Anti-inflammation Therapy. Angew Chem Int Ed Engl. 2016;55:7460-7463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Hench PS. The analgesic effect of hepatitis and jaundice in chronic arthritis, fibrositis and sciatic pain. Ann Intern Med. 1934;7:1278-1294. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Mroz MS, Lajczak NK, Goggins BJ, Keely S, Keely SJ. The bile acids, deoxycholic acid and ursodeoxycholic acid, regulate colonic epithelial wound healing. Am J Physiol Gastrointest Liver Physiol. 2018;314:G378-G387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, Lu L, Wang D. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:802-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 434] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 16. | Wang WW, Smith DL, Zucker SD. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology. 2004;40:424-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Zhu B, Wang X, Luo L, Li P, Paty DW, Cynader MS. Bilirubin as a potent antioxidant suppresses experimental autoimmune encephalomyelitis: Implications for the role of oxidative stress in the development of multiple sclerosis. J Neuroimmunol. 2003;139:27-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Kim DE, Lee Y, Kim M, Lee S, Jon S, Lee SH. Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials. 2017;140:37-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 1160] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 20. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] [Cited in This Article: ] |
| 21. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 747] [Cited by in F6Publishing: 858] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 22. | Treetharnmathurot B, Ovartlarnporn C, Wungsintaweekul J, Duncan R, Wiwattanapatapee R. Effect of PEG molecular weight and linking chemistry on the biological activity and thermal stability of PEGylated trypsin. Int J Pharm. 2008;357:252-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Qin X. Inactivation of digestive proteases by deconjugated bilirubin: The possible evolutionary driving force for bilirubin or biliverdin predominance in animals. Gut. 2007;56:1641-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Giuffrida P, Biancheri P, MacDonald TT. Proteases and small intestinal barrier function in health and disease. Curr Opin Gastroenterol. 2014;30:147-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Biancheri P, Di Sabatino A, Corazza GR, MacDonald TT. Proteases and the gut barrier. Cell Tissue Res. 2013;351:269-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Zhou K, Jiang M, Qin X, Wang X. Role of bilirubin in digestive proteases inactivation in the lower intestine. Dig Liver Dis. 2015;47:438-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Cao AT, Yao S, Stefka AT, Liu Z, Qin H, Liu H, Evans-Marin HL, Elson CO, Nagler CR, Cong Y. TLR4 regulates IFN-γ and IL-17 production by both thymic and induced Foxp3+ Tregs during intestinal inflammation. J Leukoc Biol. 2014;96:895-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Mowat AP. Bile pigments and jaundice: molecular, metabolic, and medical aspects. Gut. 1987;28:365-366. [Cited in This Article: ] |
| 29. | Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage--mechanisms and management approaches. N Engl J Med. 2013;369:2021-2030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 30. | Rizzo AM, Berselli P, Zava S, Montorfano G, Negroni M, Corsetto P, Berra B. Endogenous antioxidants and radical scavengers. Adv Exp Med Biol. 2010;698:52-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Qaisiya M, Coda Zabetta CD, Bellarosa C, Tiribelli C. Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cell Signal. 2014;26:512-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Schieffer KM, Bruffy SM, Rauscher R, Koltun WA, Yochum GS, Gallagher CJ. Reduced total serum bilirubin levels are associated with ulcerative colitis. PLoS One. 2017;12:e0179267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Liu J, Wang L, Tian XY, Liu L, Wong WT, Zhang Y, Han QB, Ho HM, Wang N, Wong SL, Chen ZY, Yu J, Ng CF, Yao X, Huang Y. Unconjugated bilirubin mediates heme oxygenase-1-induced vascular benefits in diabetic mice. Diabetes. 2015;64:1564-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Zucker SD, Vogel ME, Kindel TL, Smith DL, Idelman G, Avissar U, Kakarlapudi G, Masnovi ME. Bilirubin prevents acute DSS-induced colitis by inhibiting leukocyte infiltration and suppressing upregulation of inducible nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol. 2015;309:G841-G854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Zhou JA, Jiang M, Yang X, Liu Y, Guo J, Zheng J, Qu Y, Song Y, Li R, Qin X, Wang X. Unconjugated bilirubin ameliorates the inflammation and digestive protease increase in TNBS-induced colitis. Mol Med Rep. 2017;16:1779-1784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Antoni L, Nuding S, Wehkamp J, Stange EF. Intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2014;20:1165-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 248] [Cited by in F6Publishing: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 37. | Bakker GJ, Zhao J, Herrema H, Nieuwdorp M. Gut Microbiota and Energy Expenditure in Health and Obesity. J Clin Gastroenterol. 2015;49 Suppl 1:S13-S19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1428] [Cited by in F6Publishing: 1832] [Article Influence: 261.7] [Reference Citation Analysis (0)] |
| 39. | Mahajan GB, Balachandran L. Antibacterial agents from actinomycetes - a review. Front Biosci (Elite Ed). 2012;4:240-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Szebeni B, Veres G, Dezsõfi A, Rusai K, Vannay A, Mraz M, Majorova E, Arató A. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin Exp Immunol. 2008;151:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: Upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56:267-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |