Published online Dec 7, 2018. doi: 10.3748/wjg.v24.i45.5179
Peer-review started: August 20, 2018
First decision: October 14, 2018
Revised: October 18, 2018
Accepted: November 16, 2018
Article in press: November 16, 2018
Published online: December 7, 2018
To assess the incremental benefit of narrow band imaging (NBI) and white light endoscopy (WLE), randomizing the initial technique for the detection of residual neoplasia at the polypectomy scar after an endoscopic piecemeal mucosal resection (EPMR).
We conducted an observational study in an academic center to assess the incremental benefit of NBI and WLE randomly applied 1:1 (NBI-WLE or WLE-NBI) in the follow-up of a post-EPMR scar by the same endoscopist.
A total of 112 EPMR scars were included. The median baseline polyp size was 20 mm (interquartile range: 14-30). At first review, NBI and WLE showed good sensitivity (85.0% vs 78.9%), specificity (77.1% vs 84.2%) and overall accuracy (80.0% vs 82.5%). NBI after WLE (WLE-NBI group) improved accuracy, but this difference was not statistically significant [area under the curve (AUC): 86.8% vs 81.6%, P = 0.15]. WLE after NBI (NBI-WLE group) did not improve accuracy (AUC: 81.4% vs 81.1%, P = 0.9). Overall, recurrence was found in 39/112 (34.8%) lesions.
Although no statistically significant differences were found between the two techniques at the first post-EPMR assessment, the use of NBI after WLE may improve residual neoplasia detection. Nevertheless, biopsy is still required in the first scar review.
Core tip: Endoscopic mucosal resection of colon polyps in a piecemeal fashion requires a first close follow-up to detect residual neoplasia. There are limited data on the optimal approach to reviewing polypectomy scars with narrow band imaging (NBI). In this prospective observational study, which randomized the initial technique for the detection of residual neoplasia, NBI was slightly more accurate than white light endoscopy. To improve the assessment of polypectomy scars, high-definition endoscopes with NBI should be the rule. However, biopsies are still required at the first follow-up, even if there are no macroscopically evident lesions.
- Citation: Riu Pons F, Andreu M, Gimeno Beltran J, Álvarez-Gonzalez MA, Seoane Urgorri A, Dedeu JM, Barranco Priego L, Bessa X. Narrow band imaging and white light endoscopy in the characterization of a polypectomy scar: A single-blind observational study. World J Gastroenterol 2018; 24(45): 5179-5188
- URL: https://www.wjgnet.com/1007-9327/full/v24/i45/5179.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i45.5179
Resection of non-pedunculated polyps, usually large sessile colonic polyps, increases technical difficulty and often requires fragmented resection or endoscopic piecemeal mucosal resection (EPMR)[1]. EPMR has been associated with lesion recurrence in 16%-27% of cases[2-4]. Consequently, clinical guidelines recommend endoscopic follow-up between 3 mo and 6 mo after piecemeal resection of colorectal polyps to check for residual neoplasia[5,6].
Narrow band imaging (NBI) improves visibility and identification of the surface and vascular structures of colon polyps. This technique uses optical interference filters to spectrally narrow the bandwidths used in conventional white light, providing more visual detail to superficial mucosal structures, and enhancing visualization of the superficial mucosal capillaries in neoplastic tissue[7]. In contrast to conventional chromoendoscopy, NBI is easy to activate by pressing a button on the handle of the endoscope.
Virtual or conventional chromoendoscopy is applied during polyp resection, defining the edge of the lesion. There have been few studies using conventional chromoscopy[8] or NBI in the examination of the EPMR scar. In 2011, Rogart et al[9] compared the accuracy of NBI with that of standard white light for the detection of residual neoplasia at the resection site in 60 discrete lesions (from the upper gastrointestinal tract to colon). In 27 out of 43 (63%) lesions detected, the extension of the residual scar was greater with NBI than with white light. However, this finding does not reveal whether the use of this technique could improve the detection of residual tumor after piecemeal polypectomy, avoiding complications, time and the costs of biopsy and histological analysis.
In this context, the European Society of Gastrointestinal Endoscopy (ESGE) published the first guideline on advanced endoscopic imaging for the detection and differentiation of colorectal neoplasia and recommends conventional or virtual chromoendoscopy such as NBI in the evaluation of patients with a piecemeal polypectomy scar (a strong recommendation, but with low quality evidence)[10]. A recent paper by Desomer et al[11] was the first to describe a standardized imaging protocol with high-definition WLE and sequential NBI for post-endoscopic mucosal resection (EMR) scar assessment, showing that this protocol is highly accurate in the endoscopic detection of residual or recurrence adenoma in the EMR scar, however, both techniques were always applied sequentially in the same order (WLE plus NBI) precluding to conclude the global accuracy depending on the order of scar exploration of which technique at first is relevant.
Therefore, the present study was designed to assess the incremental benefit of NBI and WLE, randomizing the initial technique for the detection of residual neoplasia at the polypectomy scar after EPMR.
An observational study was conducted from May 2015 to May 2016 at the Endoscopy Unit of Hospital del Mar in Barcelona (Spain) to compare the accuracy of both NBI and high-definition WLE in detecting residual neoplastic tissue after EPMR of a colonic polyp.
The study protocol, in compliance with the ethical guidelines of the 1975 Declaration of Helsinki, was approved by the institutional review board of Parc de Salut Mar, 21 April 2015 (protocol number: 2015/6152/I) and registered at ClinicalTrials.gov (NCT02448693). Written informed consent was obtained from all patients included in the study.
We included from the electronic database of the Endoscopy Unit consecutive patients with a minimum age of 18 years who had undergone a baseline colonoscopy in the last 12 mo with one or more sessile or flat polyp removed in a piecemeal fashion regardless of size. All baseline colonoscopies were performed by general gastroenterologists or expert endoscopists. Patients were excluded from follow-up colonoscopy if an advanced colorectal cancer (CRC) was found at the baseline colonoscopy, or if they did not attend follow-up or did not provide informed consent, or if they had a high risk of complications due to sedation -including patients with high comorbidity (American Society of Anesthesiologists, ASA grade IV and higher)- or had inadequate bowel preparation defined by the Boston bowel preparation score[12].
Follow-up colonoscopies were performed by five experienced senior endoscopists (experience of > 4000 colonoscopies) using high-definition colonoscopes with NBI (EVIS EXERA III CV-190; Olympus Inc., Tokyo, Japan)[13]. All colonoscopies fulfilled the best standards of quality (cecal intubation, bowel preparation cleanliness and endoscope time withdrawal). All patients underwent bowel preparation using split-dose 4 L of oral polyethylene glycol-based solution. Level of consciousness was monitored by propofol alone or combined with midazolam and fentanyl at the discretion of the endoscopist. An anesthesia specialist was consulted in individual cases.
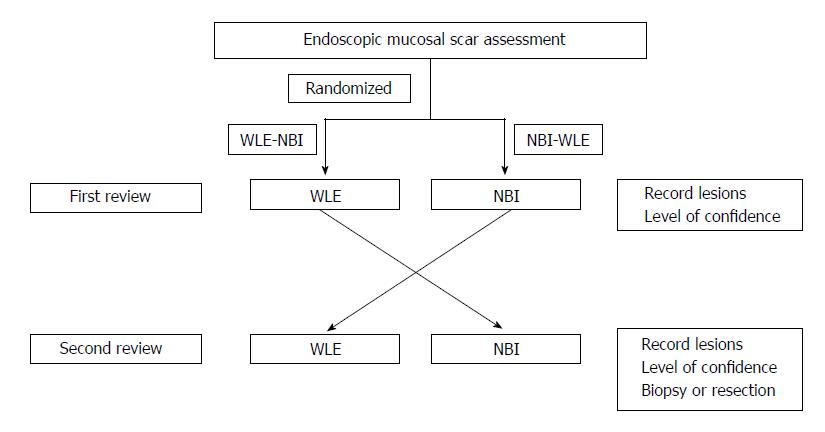
Each procedure was performed by the same endoscopist. Randomization was done before the initiation of the procedure. Allocation concealment of the first technique was computer-generated by the biomedical research consulting service of Hospital del Mar Medical Research Institute (IMIM), Barcelona. In addition, three specialized pathologists were blinded to the study protocol and samples were received as scar polypectomy for assessment. Colon inspection was done with WLE during withdrawal. At the proximity of the scar or scars, if there was multiplicity, WLE and NBI were used randomly one after the other (WLE-NBI or NBI-WLE group). If NBI was the first technique used, it was switched prior to scar detection, avoiding, as far as possible, a glance with WLE (Figure 1).
At first scar review, any macroscopically suspicious lesion or nodularity (evaluation site) was assigned a level of confidence, recorded on a data sheet by the endoscopist. The level of confidence represented a prediction of visual residual neoplasia graded as positive: High confidence of diagnostic certainty or negative: Low confidence or normal appearing scar. The morphology of each evaluation site was described as flat or nodular elevated. Residual neoplasia was defined for any adenoma or serrated tissue that was confirmed on histopathological analysis. After the first examination, the endoscopist switched to the second endoscopic technique and reviewed the polypectomy scar again. Any newly detected or suspicious lesions were classified by making another prediction with a level of confidence and recorded separately from the first review on a data sheet. For newly suspected irregularities considered as positive, the first review was being graded as negative. After both evaluations, all of the sites were biopsied (up to three), including an apparently normal scar. If detected lesions were larger than 5 mm, the endoscopist could use any additional therapeutic arsenal to destroy the residual tissue by means of cold snare, diathermy, EMR or argon plasma coagulation. The rest of the colon was inspected following conventional standards with WLE.
Data from the baseline colonoscopy included: lesion size and location, Paris classification[14], NBI International Colorectal Endoscopic (NICE) classification[15], histology, ASA classification, use of clips, use of endoscopic tattooing to mark the lesion, number of resected specimens from EPMR (categorized as 2-5 or > 5 specimens)[16], endoscopists who performed EPMR, and median time from baseline colonoscopy to evaluation.
The primary outcome was the accuracy of NBI and WLE in detecting residual neoplasia in a polypectomy scar, with pathological analysis as the gold standard.
Continuous variables were compared using the t-test, if normally distributed, and the Mann-Whitney test if not. Categorical variables were compared using the chi-square test or Fisher’s exact test. The accuracy of each technique in detecting residual neoplasia was assessed globally for each scar. Receiver operating characteristic (ROC) curve analyses were performed to reveal the relationship between the sensitivity and specificity of NBI and WLE. Kappa statistics were used to assess interobserver agreement between endoscopists for each technique and with histology. The threshold values of kappa defined by Landis and Koch[17] are: 0.0 agreement no greater than chance alone, 0.01-0.2 slight, 0.21-0.4 fair, 0.41-0.6 moderate, 0.61-0.8 substantial and 0.81-1.0 near perfect agreement. P values < 0.05 were considered to be statistically significant. Statistical analyses were performed using STATA/IC 13.1 (StataCorp, College Station, TX, United States).
On the basis of previous studies[9], we aimed to improve sensitivity in the detection of residual tissue after an EPMR (WLE vs NBI) from 70% to 85%. A sample size of 120 lesions (60 in each of the two groups) achieved 80% power to detect a difference, with alpha = 0.05.
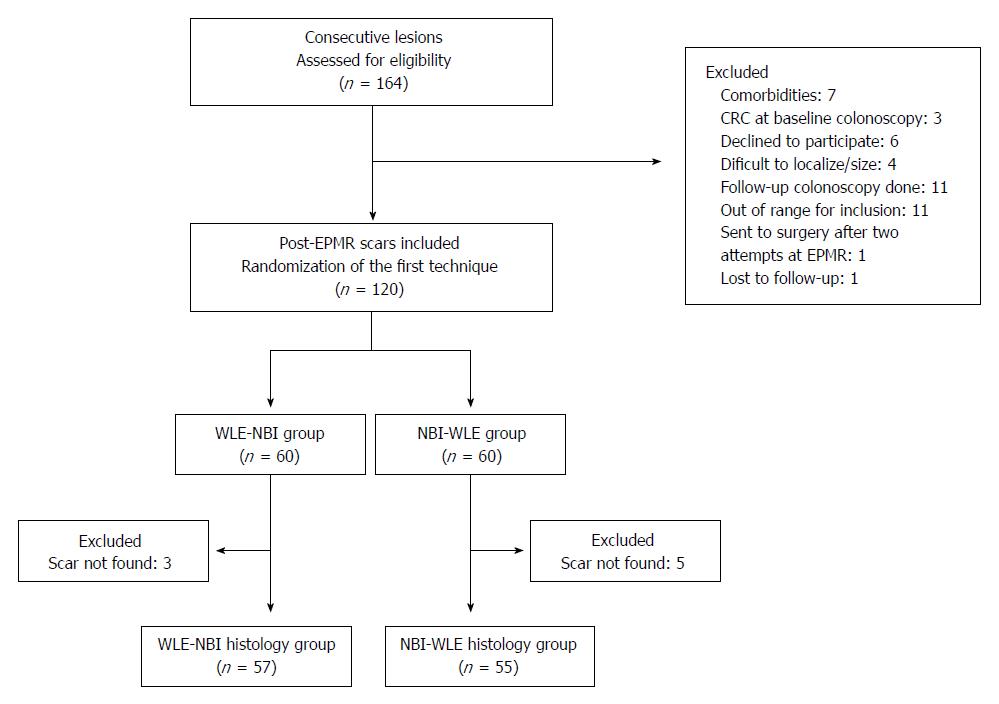
A total of 164 EPMR in 156 patients were assessed for eligibility. One hundred twenty lesions from 111 patients were included in this study and 44 were excluded as shown in Figure 2. The enrolled patients were randomly divided into two groups (WLE-NBI or NBI-WLE). After randomization, 8 lesions were excluded because the scar was not found, and therefore 112 scars were finally included to the analysis.
The patients’ baseline characteristics of one hundred twelve scars are depicted in Table 1. Male gender was more prevalent (57.1% males) and the mean age was 67.7 years (SD: 10.1) at the time of the EPMR procedure. The median size of the lesion was 20 mm (interquartile range: 14-30). Lesions were most frequently located proximal from the cecum to the transverse colon (78.6%). Polyp morphology was sessile 0-Is (42.9%), superficial elevated 0-IIa (44.6%), or other non-polypoid combinations (12.5%), with predominantly adenoma with low-grade dysplasia (47.3%). The resection technique was using saline injection with indigo carmine and optionally diluted adrenaline.
| Total number of lesions | 112 |
| Mean age, years (SD) | 67.7 (10.1) |
| Sex ratio, % male | 57.1 |
| Weight (Kg, SD) | 74.7 (15.4) |
| BMI (Kg/m2, SD) | 27.6 (5.1) |
| Family History of CRC | 27 (24.1) |
| Personal History of CRC | 7 (6.3) |
| Current smoking | 20 (17.9) |
| Diabetes | 19 (17.0) |
| Lesion size, mm (median, interquartile range) | 20 (14-30) |
| Categorical size | |
| 10-19 mm | 47 (42.0) |
| 20-39 mm | 52 (46.4) |
| ≥ 40 mm | 13 (11.6) |
| Location of the lesion | |
| Cecum | 15 (13.4) |
| Ascending | 53 (47.3) |
| Transverse | 20 (17.9) |
| Descending | 4 (3.6) |
| Sigmoid | 10 (8.9) |
| Rectum | 10 (8.9) |
| ASA classification | |
| ASA I | 18 (16.1) |
| ASA II | 56 (50.0) |
| ASA III | 38 (33.9) |
| Paris classification | |
| 0-Is | 48 (42.9) |
| 0-IIa | 50 (44.6) |
| Other combinations (IIa + Is/IIc) | 14 (12.5) |
| NICE classification | |
| NICE I | 17 (15.2) |
| NICE II | 94 (83.9) |
| NICE III | 1 (0.9) |
| Number of resected pieces | |
| 2-5 pieces | 46 (41.1) |
| > 5 pieces | 66 (58.9) |
| Baseline histology | |
| Hyperplastic | 4 (3.6) |
| Adenoma with LGD | 53 (47.3) |
| Adenoma with HGD | 39 (34.8) |
| Sessile serrated adenoma (SSA) | 11 (9.8) |
| Traditional serrated adenoma (TSA) | 2 (1.8) |
| Adenocarcinoma (pT1 stage) | 3 (2.7) |
| Use of clips | 59 (52.7) |
| Clips per lesion (median, interquartile range) | 3 (1-3) |
| Use of clips | |
| Prophylactic | 56 (94.9) |
| Intraprocedural bleeding | 2 (3.4) |
| Suspicion of deep mural injury | 1 (1.7) |
| Tattooed lesion after EPMR | 38 (33.9) |
| Endoscopist who performed piecemeal EMR | |
| Expert endoscopists | 98 (87.5) |
| General gastroenterologists | 14 (12.5) |
| Median time to review (months, interquartile range) | 3.9 (3.0-5.3) |
Endoscopic clips were used in 59 of 112 lesions (52.7%), with a median of three clips (interquartile range: 1-3) per session. The reasons for clipping were mostly for prophylactic measures in 56 patients (94.9%), intraprocedural bleeding in two patients (3.4%) and suspicion of deep mural injury in one patient (1.7%). All the complications were successfully managed endoscopically with no clinically significant post-procedural bleeding or late complications.
The median time from initial resection to scar review was 3.9 mo (interquartile range: 3.0-5.3). The characteristics of WLE-NBI and NBI-WLE are shown in Table 2. Both groups were similar, including the presence of clips that remained at the polypectomy scar, except for the median size of baseline polyps, which were larger in the WLE-NBI group.
| WLE-NBI (n = 57) | NBI-WLE (n = 55) | P value | |
| Sex (male, %) | 54.4 | 60.0 | 0.36 |
| Age (yr) | 67.3 | 68.2 | 0.65 |
| BMI (mean) | 28.2 | 27.0 | 0.23 |
| Baseline polyp size | 0.002 | ||
| 10-19 mm | 16 (28.1) | 31 (56.4) | |
| 20-39 mm | 30 (52.6) | 22 (40.0) | |
| ≥ 40 mm | 11 (19.3) | 2 (3.6) | |
| Location, right sided (%) | 79.0 | 78.2 | 0.92 |
| Paris Classification | 0.34 | ||
| 0-Is | 26 (45.6) | 22 (40.0) | |
| 0-IIa | 22 (38.6) | 28 (50.9) | |
| Other Combinations | 9 (15.8) | 5 (9.1) | |
| NICE Classification | 0.90 | ||
| NICE I | 8 (14.0) | 9 (16.4) | |
| NICE II | 48 (84.2) | 46 (83.6) | |
| NICE III | 1 (1.8) | 0 (0) | |
| Baseline histology | 0.78 | ||
| Hyperplastic | 2 (3.5) | 2 (3.6) | |
| Adenoma with LGD | 26 (45.6) | 27 (49.1) | |
| Adenoma with HGD | 22 (38.6) | 17 (30.9) | |
| Sessile Serrated Adenoma (SSA) | 5 (8.8) | 6 (10.9) | |
| Traditional serrated adenoma | 0 (0) | 2 (3.6) | |
| Adenocarcinoma (pT1 stage) | 2 (3.5) | 1 (1.8) | |
| Morphology of scar on site 1 | 0.12 | ||
| Flat | 29 (50.9) | 20 (36.4) | |
| Nodular elevated | 28 (49.1) | 35 (63.6) | |
| Presence of clips on scar | 3 (5.3) | 8 (14.6) | 0.12 |
| Residual neoplasia on histology assessment | 0.74 | ||
| Negative | 38 (66.7) | 35 (63.6) | |
| Positive | 19 (33.3) | 20 (36.4) | |
| Polyps resected (mean, SD) | 2.4 (2.7) | 2.4 (2.7) | 0.92 |
| Mean time baseline EPMR colonoscopy (min) | 55.9 | 51.9 | 0.38 |
Among the 112 lesions, a minimum of one biopsy per scar was assessed, two different biopsies in 25 scars, and up to three in three scars. When comparing the two techniques with histology as the gold standard, we analyzed those lesions as a whole scar.
In the WLE-NBI group, a first inspection with WLE obtained 78.9% sensitivity, 84.2% specificity, 71.4% positive predictive value (PPV) and 88.9% negative predictive value (NPV). The addition of a second review with NBI slightly increased sensitivity to 89.5% and NPV to 94.1%, without modifying specificity (84.2%) or PPV (73.9%).
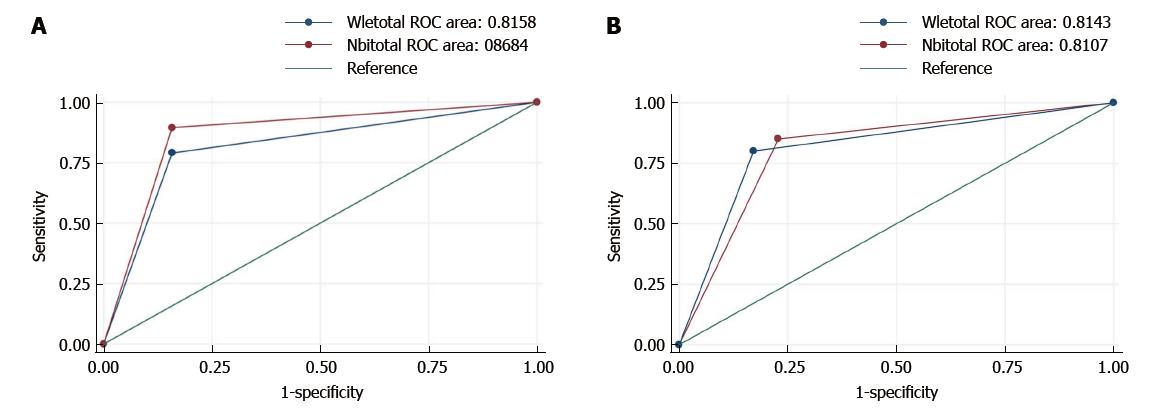
Similar findings were demonstrated by the area under the ROC curve for a global assessment. As shown in Table 3, the addition of NBI was followed by a slight but not significant increase in accuracy: WLE 81.6% (95%CI: 70.5%-92.7%) vs NBI 86.8% (95%CI: 77.6%-96.0%, P = 0.15, Figure 3).
| WLE-NBI group | NBI-WLE group | |||||
| WLE (95%CI) | NBI (95%CI) | NBI (95%CI) | WLE (95%CI) | |||
| Sensitivity | 78.9% (56.7-91.5) | 89.5% (68.6-97.1) | 85.0% (64.0-94.8) | 80.0% (58.4-91.9) | ||
| Specificity | 84.2% (69.6-92.6) | 84.2% (69.6-92.6) | 77.1% (61.0-87.9) | 82.9% (67.3-91.9) | ||
| False positive | 15.8% (7.4-30.4) | 15.80% (7.4-30.4) | 22.9% (12.1-39.0) | 17.1% (8.1-32.7) | ||
| False negative | 21.1% (8.5-43.3) | 10.50% (2.9-31.4) | 15.00% (5.2-36.0) | 20.0% (8.1-41.6) | ||
| PPV | 71.4% (50.0-86.2) | 73.9% (53.5-87.5) | 68.0% (48.4-82.8) | 72.7% (51.8-86.8) | ||
| NPV | 88.9% (74.7-95.6) | 94.1% (80.9-98.4) | 90.0% (74.4-96.5) | 87.9% (72.7-95.2) | ||
| LR + | 5 (2.3-10.8) | 5.7 (2.7-12.0) | 3.7 (2.0-7.0) | 4.7 (2.2-10.0) | ||
| LR - | 0.3 (0.1-0.6) | 0.1 (0.03-0.5) | 0.2 (0.1-0.6) | 0.2 (0.1-0.6) | ||
| Global accuracy | 82.5% (70.6-90.2) | 86.0% (74.7-92.7) | 80.0% (67.6-88.4) | 81.8% (69.7-89.8) | ||
| AUC | 81.6% (70.5-92.7) | 86.8% (77.6-96.0) | P = 0.15 | 81.1% (70.4-91.8) | 81.4% (70.4-92.4) | P = 0.9 |
In the NBI-WLE group, in which the first review was thorough NBI examination, we obtained 85.0% sensitivity, 77.1% specificity, 68.0% PPV and 90.0% NPV. Adding a second review with WLE did not increase sensitivity (80.0%) but improved specificity (82.9%) and PPV (72.7%) with similar NPV (87.9%).
As shown in Table 3, the AUC was similar for each technique: NBI 81.1% (95%CI: 70.4%-91.8%) vs WLE 81.4% (95%CI: 70.4%-92.4%, P = 0.9, Figure 3).
There was also a high degree of interobserver agreement between WLE and NBI (Kappa value, 0.91) and good concordance with histology (WLE: Kappa, 0.62 and NBI: Kappa, 0.65).
None of the patients had major complications due to the colonoscopy assessment.
Of the 112 scars detected at the endoscopic follow-up, 39 (34.8%) had residual neoplastic lesions on histologic assessment (Figure 4): 1 (2.6%) hyperplastic polyp, 29 (74.4%) adenomas with low-grade dysplasia, 5 (12.8%) adenomas with high-grade dysplasia, 3 (7.7%) sessile serrated polyps and 1 (2.6%) carcinoma. The latter case, considered a high-grade dysplastic adenoma at the baseline piecemeal polypectomy, was a flat depressed (0-IIc) unresectable lesion on colonoscopy follow-up.
We found that NBI and WLE showed high sensitivity, specificity and very good accuracy in detecting residual neoplasia. Of importance, in the first review with both techniques, the global assessment was almost equivalent (AUC: 82%). Sensitivity and NPV were improved only by NBI after WLE but this difference was not statistically significant.
Current ESGE guidelines of advanced colonoscopic imaging suggest the use of conventional or virtual chromoendoscopy at the piecemeal polypectomy scar, but there is scarce evidence[10]. In 183 lesions, Desomer et al[11] demonstrated that NBI achieves higher sensitivity and specificity than WLE alone (sensitivity: 93.3% vs 66.7%; specificity: 94.1% vs 96.1%). However, a ROC curve for global assessment would have been desirable to discriminate statistically significant differences. Despite the higher accuracy of WLE+NBI, the false negative result, meaning to give a negative diagnosis but was actually positive on histology, was 6.7%, indicating that a surveillance and histology protocol need to be implemented before biopsies can be omitted.
Globally, we identified 34.8% of patients with residual neoplasia, which is higher than values reported in the literature[2-4]. In most of the patients, the residual neoplastic tissue was treated endoscopically. However, due to the primary outcome of the study, we did not undertake a second follow-up to determine the persistence of residual neoplasia subsequently. Knabe et al[18] reported a similarly high recurrence of 31.7% at the first follow-up, and pointed out that 7% of macroscopically inconspicuous polypectomy scars were found to have occult residual adenoma. This highlights that a substantial proportion of large EPMR confers incomplete resection and some scars can harbor late recurrence. In our study, the use of NBI after WLE yielded a false negative rate of 11%. These lesions could have been missed had we not taken biopsies from the scar.
Of note, almost half of the lesions were clipped, mostly as a prophylactic measure. Their use or presence at the scar did not influence characterization or recurrence. This type of closure makes a nodularity of elevated normal mucosa or a granulation tissue, also called the clip artifact[19]. However, even though we did not evaluate the morphology of the clip artifact, the presence of a nodule or irregularity should be meticulously inspected and, if there is inconclusive focal change, biopsies or excision should be performed[20].
There are some explanations that plausibly strengthen our findings. First, for this study, we used high-definition colonoscopes (series 190 from Olympus). The vascularity or the pit pattern of the polypectomy scar seems to be well defined even with WLE. Second, the strength of randomization involves the assignment of a grade of suspicion each time the endoscopist detects irregularities from the normal or fibrotic mucosa. Our results suggest that switching the filter to NBI or WLE does not alter the first impression (near perfect agreement with a kappa of 0.91).
Our study has some limitations. First, this is a single-center study and the observed results should be compared with those of other centers with different level of expertise. In addition, we excluded patients with high comorbidity who had undergone EPMR to avoid losses or other complications related to sedation at follow-up colonoscopy.
Second, randomization of the two techniques in which the introduction of the colonoscope was on WLE could have biased the results for the NBI-WLE group. However, the results of NBI as the first evaluation were similar to those of WLE as the first (AUC: 81.1% vs 81.6%, respectively) and were slightly better than those for the WLE-NBI group (AUC: 86.8%). Other possible scenarios would have been insertion by a first endoscopist and assessment by a second endoscopist, or two different endoscopists at the same time for each technique. This would have introduced other bias by having two endoscopists in the same room, and the results would be less homogeneous between endoscopists.
Third, the median baseline polyp size was statistically significant between the two groups at the follow-up colonoscopy to evaluate the scar. We assume this non-intentional distribution was due to randomization of a small sample. However, there was no difference in the scar morphology or the number of residual tissue in the histology sample between the two groups.
Finally, the median time to review was short, which did not allow recurrence to be distinguished from residual tissue. Moreover, we did not carry out surveillance after 6 mo, which could have increased the proportion of late recurrences detected. Due to the primary outcome in our study, we could not extrapolate the risk factors for recurrence. There is robust evidence from a meta-analysis of potential predictors of local recurrence, and the recurrence risk can be stratified by using the Sydney EMR recurrence tool[21,22].
In conclusion, this prospective observational study of recurrence at endoscopic resection scars assessed by expert observers did not show a difference in the detection of recurrence at the EPMR scar depending on whether the initial technique was NBI or WLE but there was a slight accuracy improvement in the WLE-NBI group although non-significant. Biopsies are still required in the first review of the scar in all cases, either when there is any suspicious nodularity or clip artifact, even if no macroscopically evident lesion is observed. Although non-targeted forceps biopsy is an imperfect gold standard, larger lesions resected in a piecemeal fashion should be monitored at 3-6 mo to detect and resect residual tissue.
Endoscopic mucosal resection of colon polyps in a piecemeal fashion (EPMR) requires a first close follow-up at 3-6 mo. In addition, the European Society of Gastrointestinal Endoscopy recommends the use of advanced endoscopic imaging for the detection and differentiation of residual neoplasia at the polypectomy scar, but with low quality of evidence.
There are limited data on the best approach with the use of narrow band imaging (NBI) compared with white light endoscopy (WLE) to review a polypectomy scar.
This study was designed to assess the incremental benefit of NBI and WLE, randomizing the initial technique for the detection of residual neoplasia at the polypectomy scar after an EPMR.
We conducted an observational study of 120 polypectomy scars in 111 patients who had undergone a baseline colonoscopy with piecemeal polyp resection regardless of size and prospectively assigned to follow-up colonoscopy with random application of NBI and WLE 1:1 at the proximity of the scar. Patients were distributed in two groups (NBI-WLE or WLE-NBI). Five experienced endoscopists used Olympus 190 series for the assessment. Any macroscopically suspicious lesion was recorded as positive, with high confidence of a definitive diagnosis, or as negative. After the first examination, the endoscopist switched to the second technique and reviewed the polypectomy scar again, making a second prediction. After both evaluations, all of the sites were biopsied, including apparently normal scars. All resected specimens were blinded to the three specialized pathologists. The primary outcome was the accuracy of NBI and WLE in detecting residual neoplasia in the polypectomy scar, with pathological analysis as the gold standard. Receiver operating characteristic (ROC) curve analyses were performed to reveal the relationship between the sensitivity and specificity of NBI and WLE, and Kappa statistics were used to assess interobserver agreement between endoscopists for each technique and with histology.
After randomization, 8 lesions were excluded from the final assessment because the scar was not found, and therefore 112 scars were finally included to the analysis. In the WLE-NBI group, a first inspection with WLE showed 78.9% sensitivity, 84.2% specificity, 71.4% positive predictive value (PPV) and 88.9% negative predictive value (NPV). The addition of a second review with NBI slightly increased sensitivity to 89.5% and NPV to 94.1%, without modifying specificity (84.2%) or PPV (73.9%). The addition of NBI was followed by a slight but non- significant increase in accuracy, shown by the area under the ROC curve (AUC): WLE 81.6% vs NBI 86.8% (P = 0.15). In the NBI-WLE group, which underwent the first review with NBI, the results in terms of sensitivity and specificity were almost equivalent. There were no differences in the AUC with NBI (81.1%) vs WLE (81.4%) (P = 0.9). There was a high degree of interobserver agreement between WLE and NBI (Kappa value, 0.91) and good concordance with histology (WLE: Kappa 0.62 and NBI: Kappa, 0.65). Of the 112 scars detected at the endoscopic follow-up, 39 (34.8%) had residual neoplastic lesions on histologic assessment.
We found that NBI and WLE showed high sensitivity, specificity and very good accuracy in detecting residual neoplasia. Sensitivity and NPV were improved only when NBI was performed after WLE but this difference was not statistically significant. In our study we identified a higher rate of patients with residual neoplasia. Due to the primary outcome of the study, we did not undertake a second follow-up. Despite the use of NBI after WLE, we found a false negative rate of 11%. These lesions could have been missed if we had not taken biopsies from the scar. For this reason, we believe that biopsies are still required in the first review of the scars in all cases, even if there are no macroscopically evident lesions, although we recognize that this is an imperfect gold standard. Monitoring EPMR at 3 to 6 mo is mandatory to detect and resect residual tissue.
The future direction in this field will probably focus on the use of optical magnification or other digital improvements in image enhancing techniques.
This study was awarded as the best poster in the XXVI Catalan Society of Digestology Congress. Lleida (Spain) January 2017.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aoki T, Fei BY, Tsuji Y S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Burgess NG, Bahin FF, Bourke MJ. Colonic polypectomy (with videos). Gastrointest Endosc. 2015;81:813-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 3. | Khashab M, Eid E, Rusche M, Rex DK. Incidence and predictors of “late” recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc. 2009;70:344-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc. 2012;76:255-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Atkin WS, Valori R, Kuipers EJ, Hoff G, Senore C, Segnan N, Jover R, Schmiegel W, Lambert R, Pox C; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Colonoscopic surveillance following adenoma removal. Endoscopy. 2012;44 Suppl 3:SE151-SE163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1367] [Cited by in F6Publishing: 1377] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 7. | Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 359] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, Drew K, Lobo AJ. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004;53:1334-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Rogart JN, Aslanian HR, Siddiqui UD. Narrow band imaging to detect residual or recurrent neoplastic tissue during surveillance endoscopy. Dig Dis Sci. 2011;56:472-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Kamiński MF, Hassan C, Bisschops R, Pohl J, Pellisé M, Dekker E, Ignjatovic-Wilson A, Hoffman A, Longcroft-Wheaton G, Heresbach D. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2014;46:435-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Desomer L, Tutticci N, Tate DJ, Williams SJ, McLeod D, Bourke MJ. A standardized imaging protocol is accurate in detecting recurrence after EMR. Gastrointest Endosc. 2017;85:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 789] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 13. | ASGE Technology Committee. High-definition and high-magnification endoscopes. Gastrointest Endosc. 2014;80:919-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-S43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1117] [Cited by in F6Publishing: 1198] [Article Influence: 57.0] [Reference Citation Analysis (3)] |
| 15. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 384] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 16. | Sakamoto T, Matsuda T, Otake Y, Nakajima T, Saito Y. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol. 2012;47:635-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43944] [Cited by in F6Publishing: 38750] [Article Influence: 824.5] [Reference Citation Analysis (0)] |
| 18. | Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109:183-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Sreepati G, Vemulapalli KC, Rex DK. Clip artifact after closure of large colorectal EMR sites: incidence and recognition. Gastrointest Endosc. 2015;82:344-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Pellisé M, Desomer L, Burgess NG, Williams SJ, Sonson R, McLeod D, Bourke MJ. The influence of clips on scars after EMR: clip artifact. Gastrointest Endosc. 2016;83:608-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 22. | Tate DJ, Desomer L, Klein A, Brown G, Hourigan LF, Lee EY, Moss A, Ormonde D, Raftopoulos S, Singh R. Adenoma recurrence after piecemeal colonic EMR is predictable: the Sydney EMR recurrence tool. Gastrointest Endosc. 2017;85:647-656.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |