Published online Apr 21, 2018. doi: 10.3748/wjg.v24.i15.1658
Peer-review started: March 10, 2018
First decision: March 29, 2018
Revised: April 2, 2018
Accepted: April 9, 2018
Article in press: April 9, 2018
Published online: April 21, 2018
To perform a systematic review and meta-analysis on platelet-to-lymphocyte ratio (PLR) as a risk factor for post-transplant hepatocellular cancer (HCC) recurrence.
A systematic literature search was performed using PubMed. Participants of any age and sex, who underwent liver transplantation for HCC were considered following these criteria: (1) studies comparing pre-transplant low vs high PLR values; (2) studies reporting post-transplant recurrence rates; and (3) if more than one study was reported by the same institute, only the most recent was included. The primary outcome measure was set for HCC recurrence after transplantation.
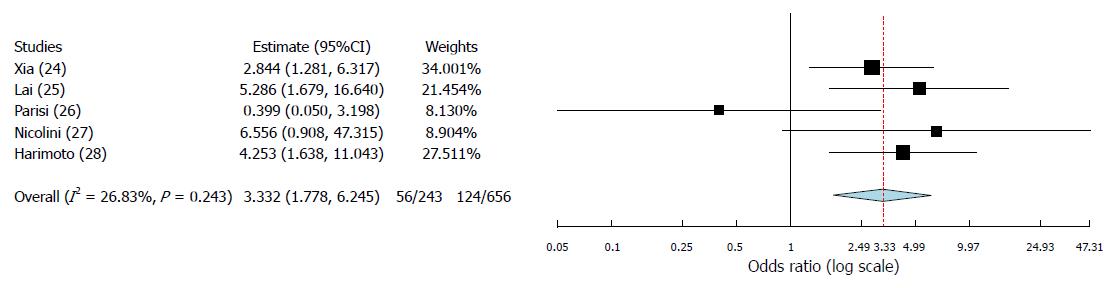
A total of 5 articles, published between 2014 and 2017, fulfilled the selection criteria. As for the quality of the reported studies, all the investigated articles presented an overall high quality. A total of 899 cases were investigated: 718 cases (80.0%) were males. Three studies coming from European countries and one from Japan presented HCV as the main cause of cirrhosis. On the opposite, one Chinese study presented a greater incidence of HBV-related cirrhotic cases. In all the studies apart one, the PLR cut-off value of 150 was reported. At meta-analysis, high PLR value was associated with a significant increase in recurrence after transplantation (OR = 3.33; 95%CI: 1.78-6.25; P < 0.001). A moderate heterogeneity was observed among the identified studies according to the Higgins I2 statistic value.
Pre-transplant high PLR values are connected with an increased risk of post-operative recurrence of hepatocellular cancer. More studies are needed for better clarify the biological mechanisms of this results.
Core tip: Poor data exist on the role of the inflammatory marker platelet-to-lymphocyte ratio (PLR) and hepatocellular cancer (HCC) recurrence after liver transplantation. This is the first systematic review and meta-analysis specifically investigating the role of PLR in the setting of liver transplant for HCC. Pre-transplant high PLR values confirmed their utility as predictors of recurrence, being connected with a 3.33-fold increased risk of post-transplant HCC recurrence.
- Citation: Lai Q, Melandro F, Larghi Laureiro Z, Giovanardi F, Ginanni Corradini S, Ferri F, Hassan R, Rossi M, Mennini G. Platelet-to-lymphocyte ratio in the setting of liver transplantation for hepatocellular cancer: A systematic review and meta-analysis. World J Gastroenterol 2018; 24(15): 1658-1665
- URL: https://www.wjgnet.com/1007-9327/full/v24/i15/1658.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i15.1658
Liver transplantation (LT) represents the best therapy for the treatment of hepatocellular cancer (HCC)[1]. However, LT represents a scarce resource. As a consequence, a careful selection of HCC patients must be done preoperatively, with the intent to minimize the risk of post-LT recurrence[2] It is, in fact, clear that transplanting too advanced tumors is connected with a higher risk of poor post-LT outcomes[3]. Moreover, an error in the selection process corresponds to a “futile transplant”, avoiding to transplant another patient in the waiting list[4].
After the introduction of the Milan Criteria (MC) in 1996, several other scores have been proposed in the last decades with the intent to refine the selection of HCC patients waiting for LT[5-8]. Apart from tumor morphology, also biology has been integrated into prognostic scores in the last years: Thus, the markers alpha-fetoprotein and des-gamma-carboxy-prothrombin, the radiological response after locoregional therapies or the tumor behaviour at PET scan have been largely investigated[9-13]. Recently, also systemic inflammation has been added as a possible value to add in the complex “mainframe” of HCC selection[14]. Among the different evaluated markers, the neutrophil-to-lymphocyte ratio (NLR) has been proposed as the most promising predictor of HCC recurrence[15,16]. NLR has been also integrated into several scores aimed at better select HCC patients waiting for LT[17,18]. However, another less intensely investigated ratio, namely the platelet-to-lymphocyte ratio (PLR), has also reported interesting results[19,20].
The main aim of the present study is to report a systematic review of the literature and a meta-analysis focused on investigating the role of PLR in the setting of liver transplantation as a useful predictor of HCC recurrence.
A systematic search was done in relation to relevant studies focusing on the role of PLR in HCC patients undergoing LT. The search strategy was done in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) guidelines, as well as PRISMA for abstracts[21]. A search of the electronic databases MEDLINE-PubMed, Cochrane Library and EMBASE was conducted using the following research terms: (liver transplant*[tw]) AND (platelet-to-lymphocyte ratio[tw] OR PLR[tw]). Text word [tw] was preferred respect to MeSH words with the intent to identify In Process citations. Studies published before March 6, 2018, were taken into consideration.
The present qualitative systematic review included a priori search criteria of journal articles among adult (age ≥ 18 years) human patients. Studies were limited to the English language. We defined as enrollable all the studies based on HCC patients having received LT in which pre-operative PLR values were correlated with the risk of post-LT HCC recurrence. Investigated time to recurrence was set at 5 years after LT.
Exclusion criteria were: (1) papers lacking sufficient details; (2) review articles; (3) nonclinical studies; (4) expert opinions; (5) letters; (6) conference summaries; and (7) case reports.
Two reviewers (QL and FM) independently screened the identified studies and their extracted data. In case of disagreement, the paper was discussed by all the authors.
Selected studies were reviewed based on the representativeness of the study population, comparability of cohorts, adequate assessment of outcomes, sufficient length of follow-up, adequacy of follow-up, and source of study funding. The quality of the papers was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS): studies with scores > 6 were defined as high-quality studies[22].
NOS details of each selected study were reported in Table 1. The characteristics coming from each study were collected in Table 2. The following features were collected: first author’s name, reference number, number of patients, patient age, patient gender, waiting time duration in months, model for end-stage liver disease, underlying liver pathology, the diameter of the major lesion and the number of tumors at the moment of LT, the MC-OUT status at the moment of LT, AFP value ≥ 200 ng/mL, any type of locoregional treatment (LRT), the PLR cut-off used in the article, the area under the receiver operator curve (ROC) for the diagnosis of recurrence, the number of post-LT recurrences and the 5-year tumor-free survival (TFS).
| Ref. | Selection | Comparability | Outcome | Quality score | |||||
| Case definition | Representativeness | Selection of controls | Definition of controls | Comparable for therapy | Comparable for etiology | Assessment of outcomes | Integrity of follow-up | ||
| Xia et al[24] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★ |
| Lai et al[25] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★ |
| Parisi et al[26] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★ |
| Nicolini et al[27] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★ |
| Harimoto et al[28] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★ |
| Ref. | n | Age | Male gender (%) | waiting time (mo) | MELD | Underlying disease | Major lesion diam (cm) | Number lesions | MC-OUT (%) | AFP ≥ 200 ng/mL (%) | LRT (%) | Cut-off | AUROC | > cut-off (%) | Recurr (%) | 5-yr TFS |
| Xia et al[24] | 343 | 49 ± 10 | 308 (90) | NA | 13 ± 6 | HBV = 320 | > 5:110 | > 3:91 | 199 (58) | 165 (48) | 222 (65) | 150 | 0.63 | 33 (10) | NA | < 150: 52 |
| Other = 23 | ≥ 150:25 | |||||||||||||||
| Lai et al[25] | 146 | 58 (54-63) | 116 (80) | 8 (3-10) | 11 (8-11) | HCV = 63 | 2.5 (1.7-3.5) | 1 (1-2) | 32 (22) | 8 (6) | 136 (93) | 150 | 0.66 | 28 (19) | 14 (10) | < 150:92 |
| HBV = 26 | ≥ 150:81 | |||||||||||||||
| Other = 57 | ||||||||||||||||
| Parisi et al[26] | 150 | 54 ± 7 | 125 (83) | 2 (0-12) | NA | HCV = 60 | 2.7 | 1 | 0 (-) | 13 (9) | 71 (47) | 150 | NA | 17 (11) | 19 (13) | NA |
| HBV = 34 | ||||||||||||||||
| Other = 56 | ||||||||||||||||
| Nicolini et al[27] | 70 | 57 (51-62) | 62 (89) | NA | 11 (7-15) | HCV = 41 | 1.3 (0.0-2.1) | 1 (0-2) | 12 (17) | 6 (9)1 | 70 (100) | 150 | NA | 5 (7) | 8 (11) | < 150:89 |
| HBV = 15 | ≥ 150:50 | |||||||||||||||
| Other = 14 | ||||||||||||||||
| Harimoto et al[28] | 190 | ≥ 59:97 | 107 (56) | NA | ≥ 15:60 | HCV = 134 | > 5:8 | > 3:41 | 66 (35) | 30 (16)2 | NA | 70.4 | 0.70 | 97 (51) | 28 (15) | < 70.4:95 |
| Other = 56 | ≥ 70.4:76 |
Different PLR cut-offs were observed among the identified studies. TFS end-point in the different studies corresponded to 5 years after LT. Summary measures were extracted from each study and used to generate a pooled odds ratio (OR). Higgins I2 statistic was used to assess heterogeneity. Higgins I2 statistic values of 0%-25%, 25%-50%, and > 50% were considered as indicative of homogeneity, moderate heterogeneity, and high heterogeneity, respectively. Only the random-effects model was used, starting from the assumption that a common OR was unreliable in the analyzed studies due to the broad eligibility criteria and the different used PLR cut-off values. OR was considered statistically significant when the P-value was < 0.05. OR and 95% confidence intervals (CI) > 1 revealed that the patients with high PLR values had poor prognoses (higher risk of recurrence), whereas a result < 1 had the opposite meaning. The analysis was performed using OpenMEE software (http://www.cebm.brown.edu/openmee/index.html).
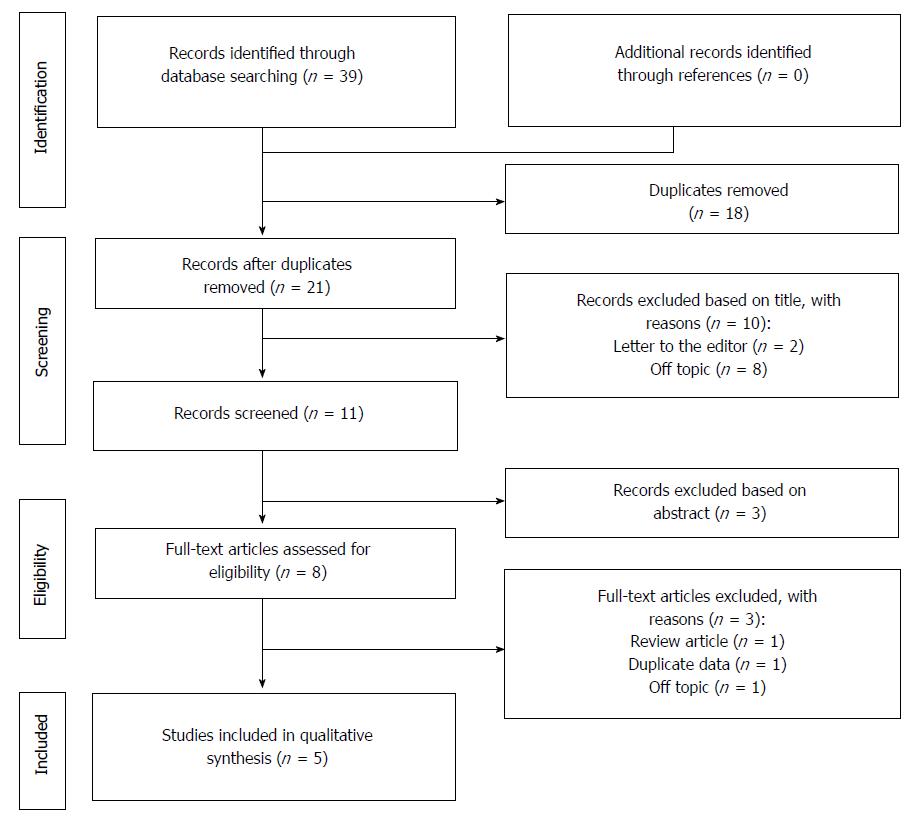
The selection process of the articles is explained in Figure 1.
As for the selection process according to the PRISMA guidelines, the various examined databases provided a total of 39 articles to screen. After removing the duplicates and reading the title and the abstract, 28 articles were removed. Of the remaining 11 papers, 6 were not considered eligible after full-text evaluation. Two studies coming from Hangzhou China were performed on the same population, so only one of these studies was selected for the last analysis[23,24].
Eventually, 5 articles were identified, with a total of 899 investigated cases (Table 2)[24-28].
As for the quality of the reported studies, all the investigated articles were retrospective cohort studies all presenting the excellent NOS value of eight, thus reporting the overall high quality of the studies focused on this topic (Table 1).
In the selected series, median/mean age ranged 49-58 years. As for patient gender, 718 cases (80.0%) were males. Three studies coming from European countries and one from Japan presented HCV as the main reason for underlying cirrhotic liver disease (298/556 cases; 53.6%). On the opposite, one Chinese study presented a greater incidence of HBV-related cirrhotic cases (320/343 cases; 93.3%).
Last radiology before LT was available in only three studies, showing a median diameter of the major lesion ranging 1.3-2.7 cm and a median single lesion (range 0-2). MC-OUT status was observed in 309 (34.4) patients: interestingly enough, a great discrepancy was observed among the reported studies, with one series coming from Europe showing no cases exceeding the MC, and the study coming from China presenting 58.0% of MC-OUT individuals. Different AFP cut-offs were reported in the studies (200/300/400 ng/mL). Also in this case, great discrepancies were observed among the study coming from Hangzhou and the other ones in terms of cases exceeding the reported threshold values (48.1% vs 5.5%-15.8%). When reported, LRT were performed in 47.3%-100.0% of cases.
Specifically investigating the PLR values, the cut-off of 150 was reported in all the series apart from the study from Fukuoka Japan, in which the median value of 70.4 was investigated. When the diagnostic power of PLR in terms of HCC recurrence was investigated, an area under the ROC curve of 0.63-0.70 was observed, showing an acceptable-to-good ability of this variable to diagnose post-transplant recurrence. A total of 180 (20.0%) cases exceeded the proposed threshold values, with a percentage ranging 7.1%-51.1% in the various series.
Five-year tumor-free survivals were reported in four studies. In all of them, patients presenting high PLR values had worse results, with a value ranging 81%-25% respect to the ones reported in patients with low PLR values (95%-52%).
The binary random-effects meta-analysis showed a strong relationship between poor TFS and elevated PLR values (OR = 3.33, 95%CI: 1.78-6.25; P < 0.001). Higgins I2 statistic presented a value = 26.8% (P = 0.24), showing a moderate heterogeneity among the examined studies (Figure 2).
Until now, few data have been reported on the predictive role of PLR as a risk factor for HCC recurrence after liver transplant. Indeed, only five studies have been identified in the present systematic review, the first of whom published in 2014[24-28]. However, although the number of reported cases is relatively limited (n = 899), the biological effect of PLR looks to be clear, with a strong correlation between high PLR values and a greater risk for recurrence. According to the results of the meta-analysis, subjects having pre-LT high PLR values present a 3.33-fold increased risk of experiencing HCC recurrence after LT.
Interestingly enough, the only study in which the PLR failed to be a prognostic tool for recurrence was the sole in which only patients meeting the MC were transplanted[26]. Such an evidence should represent a possible explanation for the observed results. It is, in fact, possible that a direct correlation may exist between higher PLR values and a progressively increasing tumor aggressiveness (i.e., higher AFP and greater tumor burden). As a possible confirmation of these data, all the series reported in the present study showed a gradient among PLR values, patients exceeding the MC and worse survival results.
This evidence has been observed also in an ITA.L.I.C.A. study in which a direct correlation between tumor dimension and the absolute number of platelets was reported[29]. Another study from Taiwan performed on more than 3000 HCC patients showed that platelets count efficaciously predicted extrahepatic metastases, even better than AFP did[30].
The link between HCC and platelets has been recently documented also in a Korean study only investigating absolute platelets count and HCC recurrence. A platelets value > 75 × 109/L was connected with higher 5-year recurrence rates (28.2% vs 13.2% in patients with lower count; P = 0.002). Similarly, at multivariable analysis, a significantly greater recurrence risk was confirmed in the high platelets group (HR = 1.90; 95%CI: 1.02-3.54; P = 0.04). Of interest, platelets count remained significant as a risk factor for recurrence even when it was introduced in a multivariable model comprehending aspects of tumor biology and morphology. Thus, we can postulate that platelets present an independent role in favouring tumor progression[31].
As a confirmation of this result, it has been well established that platelets are effector cells directly interacting with tumor cells in the metastatic cascade[32,33]. During total hepatectomy for LT, some tumor cells may be observed in the bloodstream due to HCC manipulation, only needing a few hours to days to complete the metastatic cascade[33]. Platelets favour some of the mechanisms required for metastatic dissemination: For example, they favour tumor cell surviving in the bloodstream, extravasation, initial seeding and tumor re-growth[34].
All these considerations present great repercussions from a clinical point of view: In fact, platelets might provide a potential therapeutic target for anti-hepatoma treatments. Of interest, sorafenib already represents an anti-HCC drug directly acting against cellular pathways mediated by platelet-derived growth factors (i.e., vascular endothelial growth factor and platelet-derived growth factor)[35]. Other therapies including cyclooxygenase inhibitors, protease-activated receptor inhibitors, and glycoprotein IIb/IIIa inhibitors may further play an underestimated role in this phenomenon[36].
However, although the reported data suggest an effective biological correlation between platelets and tumor aggressive behaviour, we should underline that further clinical studies trying to univocally demonstrate the biological role of platelets in the HCC oncogenesis are needed. Indeed, the present meta-analysis was in fact affected by several potential shortcomings. First, moderate heterogeneity was observed among the studies investigated, as clearly shown by the reported Higgins I2 statistic value (26.8%). Such a phenomenon was surely caused by the broad eligibility criteria for HCC and the different PLR cut-off values used in the different centers. It is, in fact, clear that a meta-regression weighted for the geographical area, HCV vs HBV as the main cause of liver failure, living-donor vs deceased-donor LT, and markers of tumor aggressiveness should represent a more accurate way for better clarify the role of PLR in this setting. Unfortunately, the limited number of cases reported did not consent us to perform more sophisticated analyses. Secondly, no information was reported in the different series on the presence and grade of portal hypertension, a very well known cause of thrombocytopenia in cirrhosis[37].
In conclusion, platelet-to-lymphocyte ratio is an easy and cheap value to use for selecting patients with hepatocellular cancer waiting for liver transplantation. A direct correlation between PLR values and tumor aggressiveness has been observed in several studies. High pre-transplant PLR values cause a 3.3-fold increased risk for post-transplant recurrence. More studies aimed at better understanding biological and clinical mechanisms of the link between PLR and HCC are needed.
Liver transplantation is the best curative therapy in case of hepatocellular cancer (HCC). However, it represents a scarce resource due to the reduced number of donors. Thus, a careful selection of HCC patients must be done preoperatively, with the intent to minimize the risk of futile transplants (i.e., post-operative cancer recurrence). As a consequence, new and easy-to-use predictors of recurrence are needed.
Recently, several biological aspects of HCC have been investigated, with the intent to identify scores aimed at improving the prediction of poor post-transplant outcomes. Among them, the inflammatory marker platelet-to-lymphocyte ratio (PLR) has been only marginally investigated, although it should represent a potentially excellent and cheap marker to use.
The main objective of the present study is to evaluate the role of PLR as a possible selection tool for the risk of HCC recurrence in the setting of liver transplantation.
A systematic review and a meta-analysis have been performed with the intent to evaluate the role of PLR. The PRISMA Guidelines have been used for performing the systematic research of studies focused on PLR, HCC and LT.
Five articles coming from Europe and Asia have been identified, with a total of 899 subjects investigated. At meta-analysis, high PLR value was associated with a significant increase in recurrence after transplantation (OR = 3.33; 95%CI: 1.78-6.25; P < 0.001).
A direct correlation between PLR values and tumor aggressiveness has been observed. High pre-transplant PLR values cause a 3.3-fold increased risk for post-transplant recurrence. Platelet-to-lymphocyte ratio is an easy and cheap value to use for selecting patients with hepatocellular cancer waiting for liver transplantation. PLR should be taken into account in the creation of new selection scores for HCC.
More studies aimed at better understanding biological and clinical mechanisms of the link between PLR and HCC are necessary.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boin IF, Gencdal G, Ramia JM, Zheng SS S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 4991] [Article Influence: 178.3] [Reference Citation Analysis (0)] |
| 2. | Lai Q, Lerut JP. Hepatocellular cancer: how to expand safely inclusion criteria for liver transplantation. Curr Opin Organ Transplant. 2014;19:229-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Mazzaferro V. Results of liver transplantation: with or without Milan criteria? Liver Transpl. 2007;13:S44-S47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Lai Q, Vitale A, Iesari S, Finkenstedt A, Mennini G, Spoletini G, Hoppe-Lotichius M, Vennarecci G, Manzia TM, Nicolini D. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology. 2017;66:1910-1919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44-S57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 6. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1594] [Cited by in F6Publishing: 1583] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 7. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1267] [Cited by in F6Publishing: 1421] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 8. | Gao T, Xia Q, Qiu DK, Feng YY, Chi JC, Wang SY, Xi ZF, Zhang JJ, Xu N, Chen SY. Comparison of survival and tumor recurrence rates in patients undergoing liver transplantation for hepatitis B-related hepatocellular carcinoma using Milan, Shanghai Fudan and Hangzhou criteria. J Dig Dis. 2013;14:552-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Lai Q, Avolio AW, Manzia TM, Sorge R, Agnes S, Tisone G, Berloco PB, Rossi M. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant. 2012;26:E125-E131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-994.e3; quiz e14-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 561] [Cited by in F6Publishing: 635] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 11. | Lai Q, Iesari S, Levi Sandri GB, Lerut J. Des-gamma-carboxy prothrombin in hepatocellular cancer patients waiting for liver transplant: a systematic review and meta-analysis. Int J Biol Markers. 2017;32:e370-e374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, Goffette P, Vogel W, Pitton MB, Lerut J; European Hepatocellular Cancer Liver Transplant Study Group. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, Sappler A, Habrecht O, Gottschild D, Settmacher U. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, Kubota K, Sharma R. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012;57:1013-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Halazun KJ, Hardy MA, Rana AA, Woodland DC 4th, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS Jr, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Xu ZG, Ye CJ, Liu LX, Wu G, Zhao ZX, Wang YZ, Shi BQ, Wang YH. The pretransplant neutrophil-lymphocyte ratio as a new prognostic predictor after liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. Biomark Med. 2018;12:189-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, Kato T, Verna EC, Emond JC, Brown RS Jr. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg. 2017;265:557-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 18. | Lai Q, Nicolini D, Inostroza Nunez M, Iesari S, Goffette P, Agostini A, Giovagnoni A, Vivarelli M, Lerut J. A Novel Prognostic Index in Patients With Hepatocellular Cancer Waiting for Liver Transplantation: Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) Score. Ann Surg. 2016;264:787-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Zheng J, Cai J, Li H, Zeng K, He L, Fu H, Zhang J, Chen L, Yao J, Zhang Y. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: a Meta-Analysis and Systematic Review. Cell Physiol Biochem. 2017;44:967-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Li X, Chen ZH, Xing YF, Wang TT, Wu DH, Wen JY, Chen J, Lin Q, Dong M, Wei L. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2015;36:2263-2269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4165] [Cited by in F6Publishing: 4134] [Article Influence: 459.3] [Reference Citation Analysis (0)] |
| 22. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8858] [Cited by in F6Publishing: 10617] [Article Influence: 758.4] [Reference Citation Analysis (0)] |
| 23. | Xia W, Ke Q, Guo H, Wang W, Zhang M, Shen Y, Wu J, Xu X, Yan S, Yu J. Expansion of the Milan criteria without any sacrifice: combination of the Hangzhou criteria with the pre-transplant platelet-to-lymphocyte ratio. BMC Cancer. 2017;17:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Xia W, Ke Q, Wang Y, Wang W, Zhang M, Shen Y, Wu J, Xu X, Zheng S. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World J Surg Oncol. 2015;13:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int. 2014;27:32-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Parisi I, Tsochatzis E, Wijewantha H, Rodríguez-Perálvarez M, De Luca L, Manousou P, Fatourou E, Pieri G, Papastergiou V, Davies N. Inflammation-based scores do not predict post-transplant recurrence of hepatocellular carcinoma in patients within Milan criteria. Liver Transpl. 2014;20:1327-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Nicolini D, Agostini A, Montalti R, Mocchegiani F, Mincarelli C, Mandolesi A, Robertson NL, Candelari R, Giovagnoni A, Vivarelli M. Radiological response and inflammation scores predict tumour recurrence in patients treated with transarterial chemoembolization before liver transplantation. World J Gastroenterol. 2017;23:3690-3701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Harimoto N, Yoshizumi T, Shimagaki T, Nagatsu A, Motomura T, Harada N, Okabe H, Itoh S, Ikegami T, Uchiyama H. Inflammation-based Prognostic Score in Patients with Living Donor Liver Transplantation for Hepatocellular Carcinoma. Anticancer Res. 2016;36:5537-5542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Carr BI, Guerra V, Giannini EG, Farinati F, Ciccarese F, Rapaccini GL, Di Marco M, Benvegnù L, Zoli M, Borzio F. Significance of platelet and AFP levels and liver function parameters for HCC size and survival. Int J Biol Markers. 2014;29:e215-e223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Lee CH, Lin YJ, Lin CC, Yen CL, Shen CH, Chang CJ, Hsieh SY. Pretreatment platelet count early predicts extrahepatic metastasis of human hepatoma. Liver Int. 2015;35:2327-2336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Han S, Lee S, Yang JD, Leise MD, Ahn JH, Kim S, Jung K, Gwak MS, Kim GS, Ko JS. Risk of posttransplant hepatocellular carcinoma recurrence is greater in recipients with higher platelet counts in living donor liver transplantation. Liver Transpl. 2018;24:44-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328:562-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 33. | Bihari C, Rastogi A, Shasthry SM, Bajpai M, Bhadoria AS, Rajesh S, Mukund A, Kumar A, Sarin SK. Platelets contribute to growth and metastasis in hepatocellular carcinoma. APMIS. 2016;124:776-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Liu D, Zhang Y, Wei Y, Liu G, Liu Y, Gao Q, Zou L, Zeng W, Zhang N. Activation of AKT pathway by Nrf2/PDGFA feedback loop contributes to HCC progression. Oncotarget. 2016;7:65389-65402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Nishida N, Kitano M, Sakurai T, Kudo M. Molecular Mechanism and Prediction of Sorafenib Chemoresistance in Human Hepatocellular Carcinoma. Dig Dis. 2015;33:771-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | 36 Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL. Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. Int J Oncol. 2012;40:1298-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Zhang Z, Zhang Y, Wang W, Hua Y, Liu L, Shen S, Peng B. Thrombocytopenia and the outcomes of hepatectomy for hepatocellular carcinoma: a meta-analysis. J Surg Res. 2017;210:99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |