Published online Mar 14, 2018. doi: 10.3748/wjg.v24.i10.1084
Peer-review started: January 11, 2018
First decision: January 25, 2018
Revised: January 31, 2018
Accepted: February 9, 2018
Article in press: February 9, 2018
Published online: March 14, 2018
To study sex disparity in susceptibility to hepatocellular carcinoma (HCC), we created a transgenic mouse model that expressed the full hepatitis B virus (HBV) genome with the W4P mutation.
Transgenic mice were generated by transferring the pHY92-1.1x-HBV-full genome plasmid (genotype A2) into C57Bl/6N mice. We compared serum levels of hepatitis B surface antigen (HBsAg), interleukin (IL)-6, and the liver enzymes alanine aminotransferase (ALT) and aspartate transaminase (AST), as well as liver histopathological features in male and female transgenic (W4P TG) mice and in nontransgenic littermates of 10 mo of age.
W4P TG males exhibited more pronounced hepatomegaly, significantly increased granule generation in liver tissue, elevated HBsAg expression in the liver and serum, and higher serum ALT and IL-6 levels compared to W4P TG females or littermate control groups.
Together, our data indicate that the W4P mutation in preS1 may contribute to sex disparity in susceptibility to HCC by causing increased HBV virion replication and enhanced IL-6-mediated inflammation in male individuals. Additionally, our transgenic mouse model that expresses full HBV genome with the W4P mutation in preS1 could be effectively used for the studies of the progression of liver diseases, including HCC.
Core tip: With the development of hepatitis B virus (HBV) vaccine, the rate of chronic HBV infection has dramatically declined worldwide. However, the incidence of hepatocellular carcinoma (HCC), which is characterized by poor prognosis and low survival rate, is on the rise. Predominance in males is a representative global epidemiological characteristic of HCC. Recently, we introduced the novel W4P substitution into the preS1 region, which associated with HCC and notably occurred exclusively in male patients. Our study in the nude mouse xenograft model indicated that the W4P mutation likely contributed to IL-6-dependent HCC progression, particularly in male individuals. Here, to gain further insight into the role of this mutation in HBV-induced liver inflammation, we created transgenic mice carrying the full HBV genome with this mutation. Of note, our data showed that W4P transgene males of 10 mo of age, but not W4P transgene females, spontaneously developed liver damage due to IL-6-mediated liver inflammation, further supporting the previous finding regarding the contribution of the W4P mutation to sex disparity in susceptibility to HCC. Furthermore, our results prove the utility of the developed W4P transgene mouse model for research into the mechanisms of HBV-caused liver diseases.
- Citation: Lee SA, Lee SY, Choi YM, Kim H, Kim BJ. Sex disparity in viral load, inflammation and liver damage in transgenic mice carrying full hepatitis B virus genome with the W4P mutation in the preS1 region. World J Gastroenterol 2018; 24(10): 1084-1092
- URL: https://www.wjgnet.com/1007-9327/full/v24/i10/1084.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i10.1084
Hepatitis B virus (HBV) infection causes a wide range of chronic infectious diseases, including chronic hepatitis, liver cirrhosis and hepatocellular carcinoma (HCC). In 2010, the number of patients in which HBV infection was the main cause of death was reported to be 786000 worldwide[1,2].
The incidence of chronic HBV infection in children has been considerably decreased by the successful development of antiHBV vaccine[3-5]. Nevertheless, the high risk of liver cirrhosis (LC) and HCC is still a problem in adult HBV carriers. The five-year cumulative risk of HCC progression is approximately 10%-17% in LC patients, and disease progression from chronic hepatitis B to LC is expected in 12%-20% of patients in 5 years[6-8]. HBV genotype C2, which is predominant in Asia, is associated with a particularly significant risk of HCC compared to that conferred by HBVs of other genotypes[9-11]. The correlation between HBV infection and sex disparity in susceptibility to HCC has been well documented. However, the mechanism by which HBV causes cancer development is still unresolved. Premature termination of HBV X protein (HBx), which results in truncated hepatitis B surface antigen (HBsAg), or mutations, particularly deletions, in the preS region of large-surface proteins (LHBs) have been reported to be associated with HCC progression[12-15].
Prevalence in males is one of the remarkable global epidemiological characteristics of HCC, as approximately 3-5 times more cases of HCC are observed in men than in women[16-18]. The sex disparity is more prominent in HBV-related HCC than in hepatitis C virus-related HCC, suggesting the presence of an HBV infection-related factor that determines HCC male predominance[19,20]. It has been reported that high expression levels of both androgen and active androgen receptor gene alleles increase the risk of HCC in male patients with chronic hepatitis B due to the interaction between HBx and androgen axis[21-23].
HCC development is likely affected not only by the HBx-androgen axis interactions but also by a tumor-protective effect of estrogen. In particular, it has been suggested that taking contraceptives or postmenopausal hormone therapy associated with long-term exposure to estrogen reduces the risk of HCC in female patients[24]. In addition, it has been reported that estrogen receptor α-mediated inhibition of interleukin (IL)-6 production had an essential role in inhibiting carcinogenesis in a mouse model of HCC induced chemically by diethylnitrosamine[25,26].
On the basis of differential time courses of HCC development and disease severity in wild-type (WT) individuals and in individuals with LHB mutations, it has been proposed that mutated LHBs lead to carcinogenesis by inducing endoplasmic reticulum stress pathway or by altering transactivating capacity of hepatocytes[27-29].
In a molecular epidemiological study, we have previously found that the W4P mutation in the preS1 start region is associated with HCC development in male but not female patients[30]. In addition, our further cell-based and nude mouse xenograft model studies supported the notion that the W4P mutation likely induced HCC progression in an IL-6-dependent manner in male patients[31]. Here, to gain further insight into the role of this mutation in the sex disparity of HBV-induced liver inflammation, we created transgenic (TG) mice carrying the full HBV genome with the W4P mutation and evaluated HBV virion replication and IL-6-mediated inflammation in male and female TG and WT individuals.
The mutant full-length HBV genome construct carrying the W4P mutation in the preS1 region (hereafter, pHY92-W4P) was generated by site-directed mutagenesis of the WT pHBV-1.1x vector (hereafter, pHY92-WT) (Genotype A, GenBank No. AF305422), which was kindly provided by Yang et al[32]. The mutagenesis was performed using the forward primer W4P-F (5’- AACAAGAGCTACGCATGGGAGGTCCGT CATCAAAACCTC-3’) and the reverse primer W4P-R (5’-GAGGTTTTGATGACGGACCT CCCATGCTGTAGCTCTTGTT-3’) located from 2473 bp and 2513 bp. Site-directed mutagenesis of the full HBV genome was performed as described[33].
To generate W4P TG mice, fertilized C57BL/6N embryos and HBV full genome with the W4P mutation were co-microinjected into one-cell embryo in accordance with the standard microinjection procedures for TG mouse production (Macrogen, Seoul, Korea). Genotyping of TG mice was conducted by PCR and viral DNA samples obtained from tail vein bleeds were screened using the primers PreS-F (5’-GGGTCACCATATTCTTGGGAA-3’) and PreS-R (5’-CGAATGCTCCCRCTCCTAC-3). The mice were housed in a specific pathogen-free laboratory animal center. The TG mice were crossed with B6D2F1/J mice (The Jackson Laboratory, Bar Harbor, ME, United States) and the HBV-expressing offspring mice, as well as their littermates, were used in this study. All animal experiments were conducted following United States’ National Institutes of Health guidelines for housing and care of laboratory animals and in accordance with the protocol approved by the Institutional Animal Care and Use Committee (IAUAC) of the Seoul National University College of Medicine (Protocol No. SNU-111025).
Serum HBsAg levels in male and female W4P TG mice and their WT littermates were determined by enzyme-linked immunosorbent assay (ELISA) using a commercial Bioelisa HBsAg color kit (Biokit, Barcelona, Spain) according to the procedures provided by the manufacturer. The amount of secreted IL-6 was determined by a mIL-6 ELISA kit (eBioscience, San Diego, CA, United States). Serum levels of alanine aminotransferase (ALT) and aspartate transaminase (AST) were determined at the Seoul National University Hospital Biomedical Research Institute facility.
Liver samples were fixed with 4% paraformaldehyde in phosphate-buffered saline and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin at the Seoul National University Hospital Biomedical Research Institute facility. Immunohistochemical staining with an anti-preS1 monoclonal antibody (Aprogen, Daejeon, South Korea) was also performed. Deparaffinized sections were heated in citrate buffer (Zytomed, Berlin, Germany) to accomplish antigen retrieval. Endogenous peroxidase was blocked with peroxidase blocking solution (Zytomed). An anti-preS1 antibody was applied as the primary antibody followed by the application of the avidin-biotin complex method to detect the primary antibody. Peroxidase activity was visualized by a 3,3’-diaminobenzidine substrate kit (Zytomed) with hematoxylin (Wako, Osaka, Japan) as counterstain.
All ELISA assays in this study were repeated at least three times, and the results were expressed as the mean percentage ± standard deviation, or as the median (± range). For continuous variables, separate one-way analyses of variance were used to determine if there was a significant difference by using the Bartlett’s test. All statistical analyses were conducted with a significance level of α = 0.05 (P < 0.05).
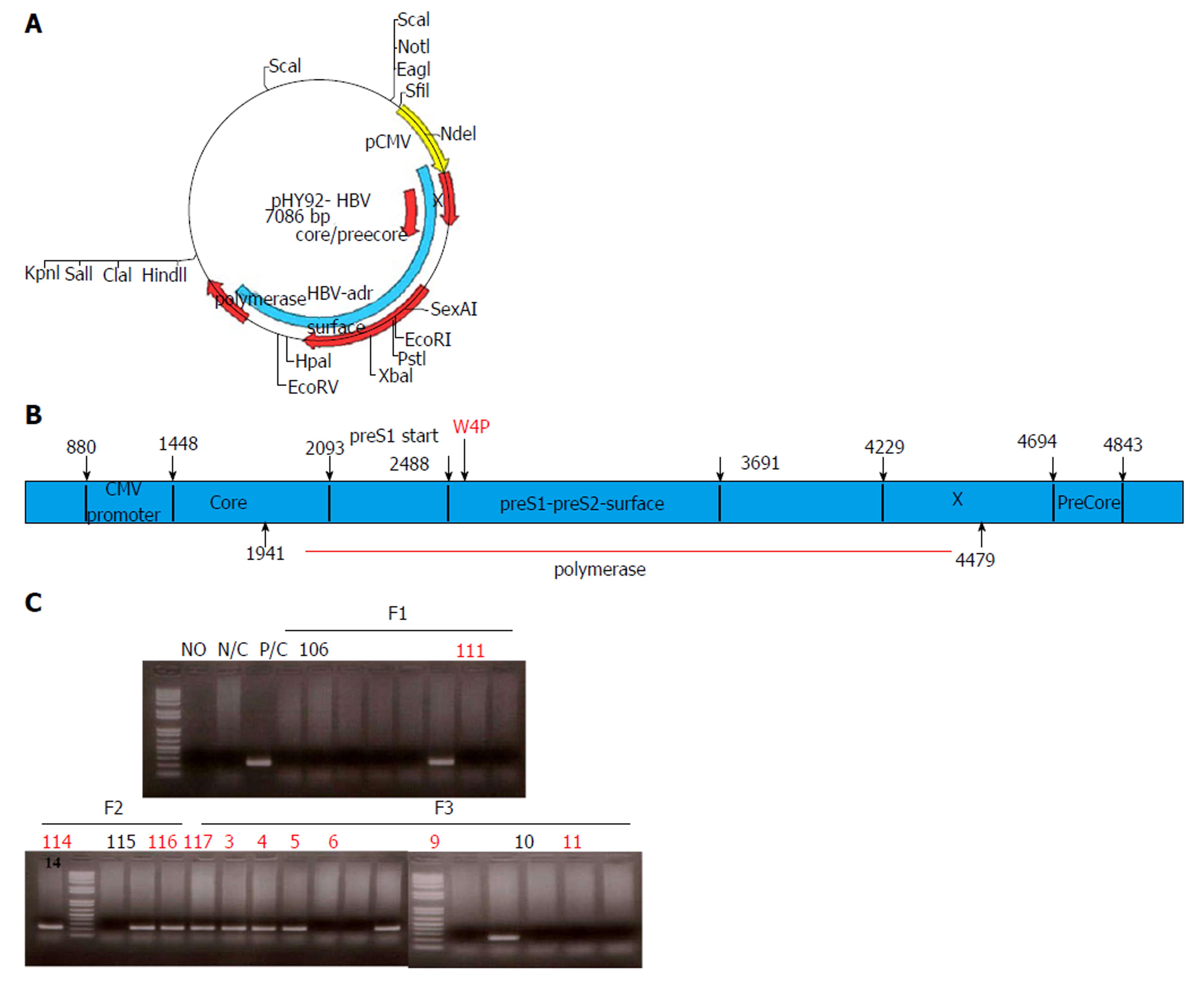
TG mice generated on B6D2F1/J background expressed the full-length HBV genome with the W4P mutation in preS1 under the control of the cytomegalovirus (CMV) promoter. For this purpose, we used site-directed mutagenesis of the pHY92 vector containing a copy of the 1.1x-unit length HBV genome under the control of the CMV promoter (genotype A, serotype adw, HBV strain identical to GenBank AF305422), which was provided by Yang et al[32], and generated a missense mutation, changing tryptophan to proline (TGG to CCG) at the fourth codon of preS1 (Figure 1A). Comparison of WT and W4P mutant LHB region sequences is shown in Supplementary Figure 1.
To confirm whether TG mice harbored the full HBV genome, the presence of virion DNA and secreted HBsAg in the serum or liver was checked by PCR and ELISA, respectively (Figure 1A).
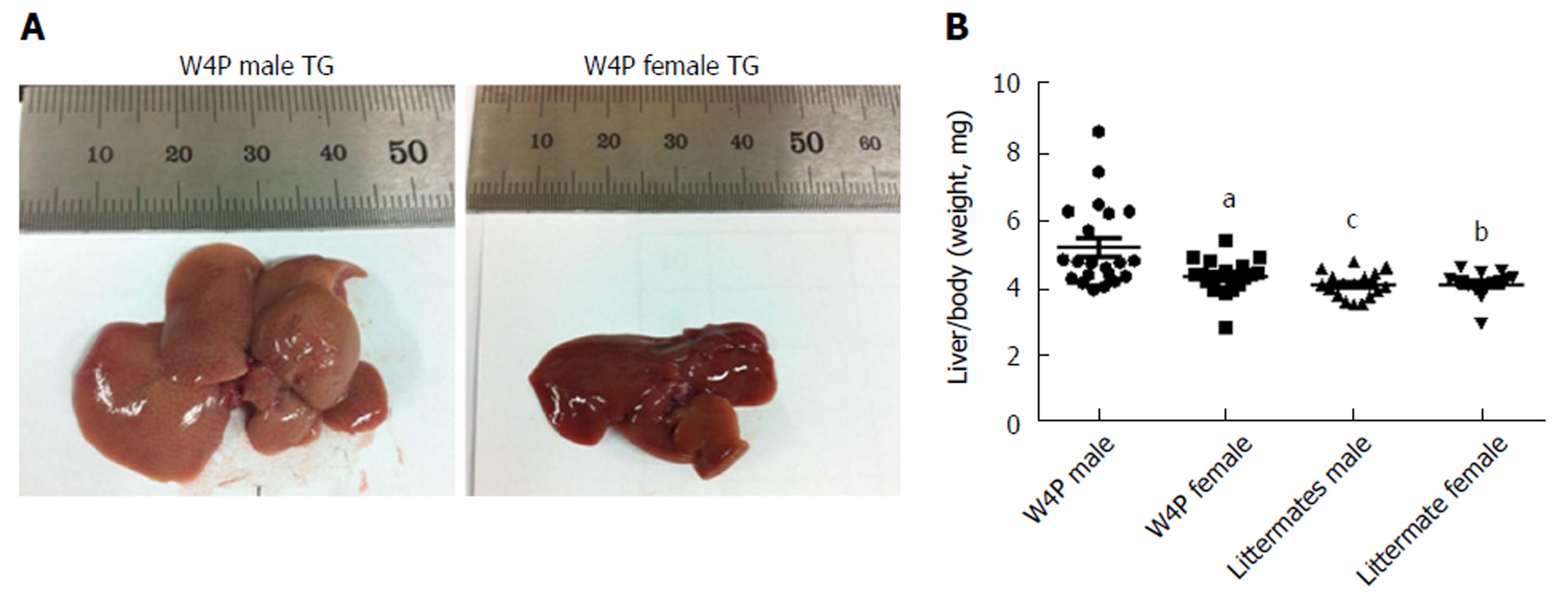
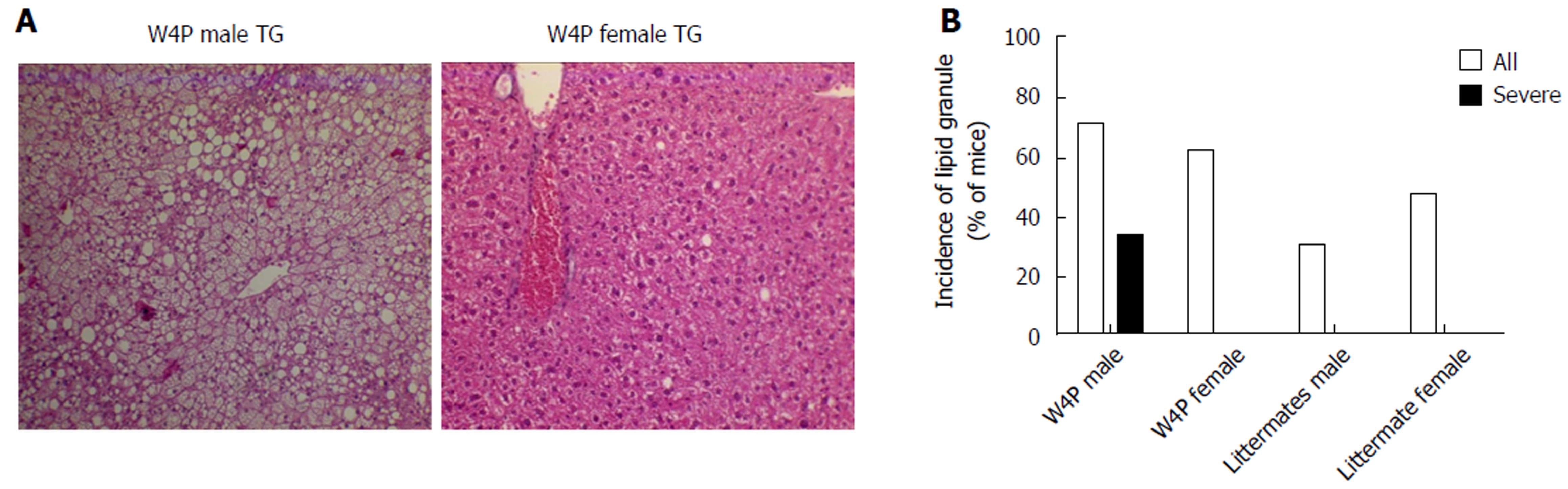
To check whether there was sex disparity in hepatomegaly, we examined the ratio of the liver weight to total body weight between W4P TG mice (24 males, 18 females) and their nonTG littermates (17 males, 15 females) at 10 mo of age. W4P TG male mice showed a significantly higher liver to total body weight ratio compared to that in mice of the three other groups, including W4P TG female mice and nonTG littermates (male and female mice) (Figure 2). Examination of histological samples stained with hematoxylin and eosin revealed that the incidence of mice generating lipid granules was higher in W4P male mice compared to that in W4P TG female mice and nonTG littermates (Figure 3).
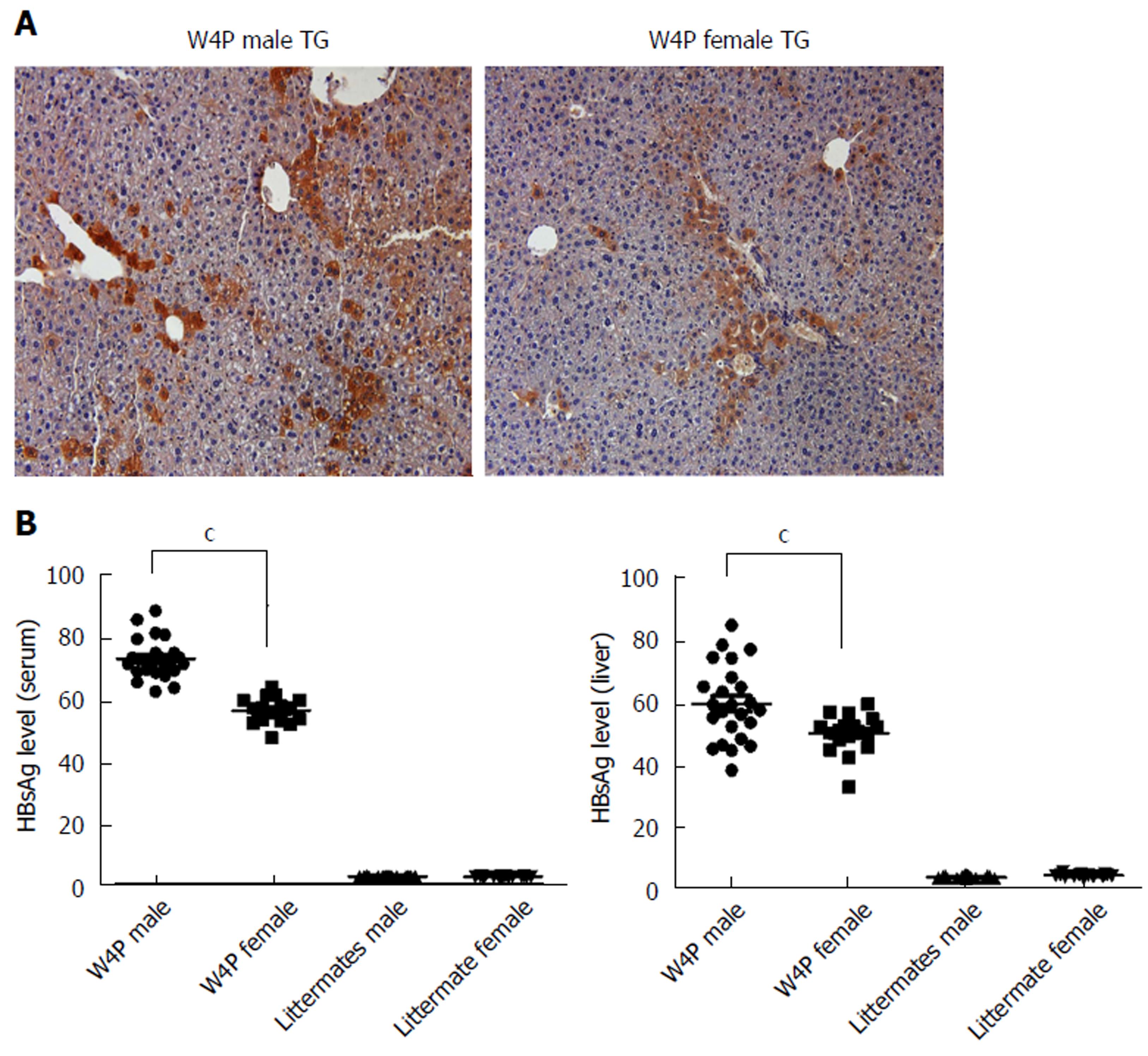
Next, to check whether there was sex disparity in HBV production, we determined HBsAg levels in the serum and LHB levels in the livers of W4P TG mice (24 males, 18 females) and their nonTG littermates (17 males, 15 females) at 10 mo of age. W4P TG male mice showed a significantly higher level of HBsAg in the serum compared to that in mice from the other three groups. Immunohistochemical staining of the liver samples using an anti-preS1 antibody also showed increased LHB production in W4P TG male mice (Figure 4).
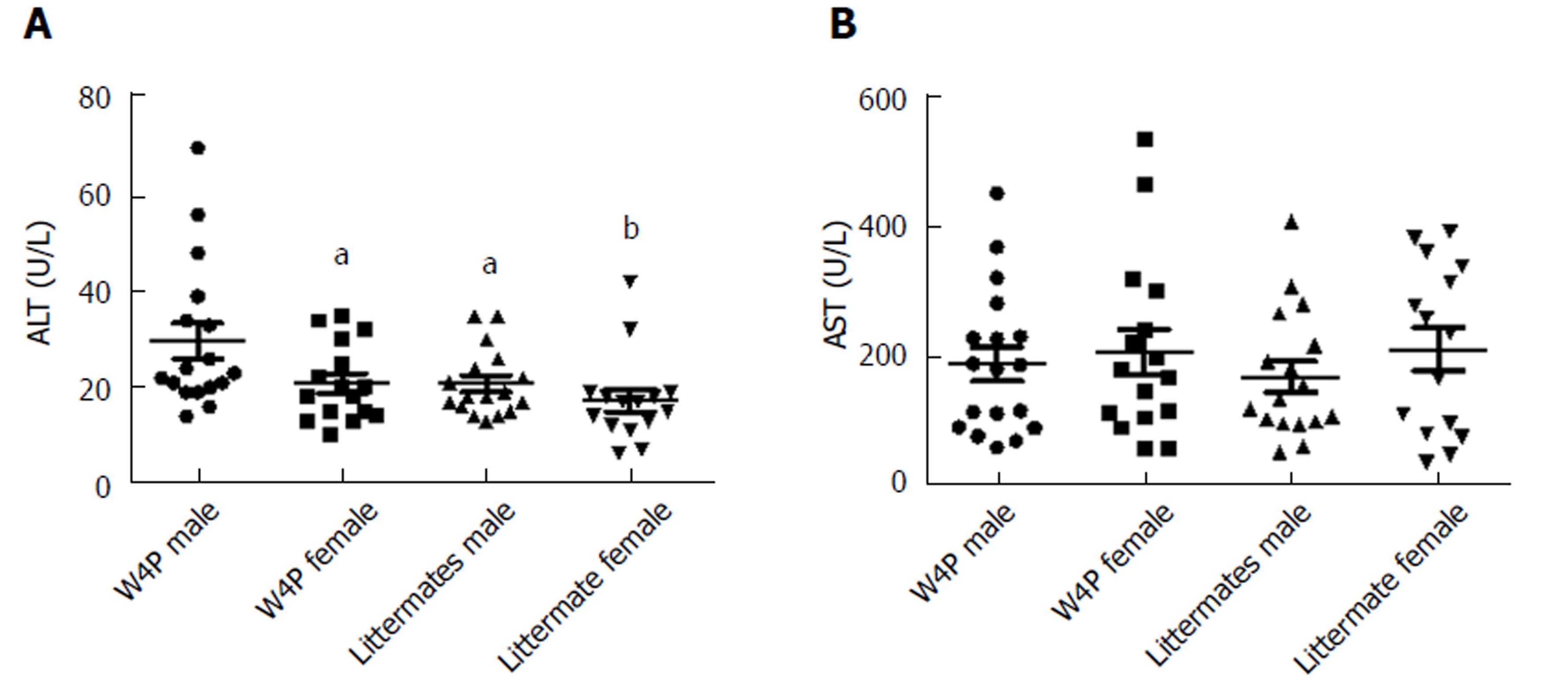
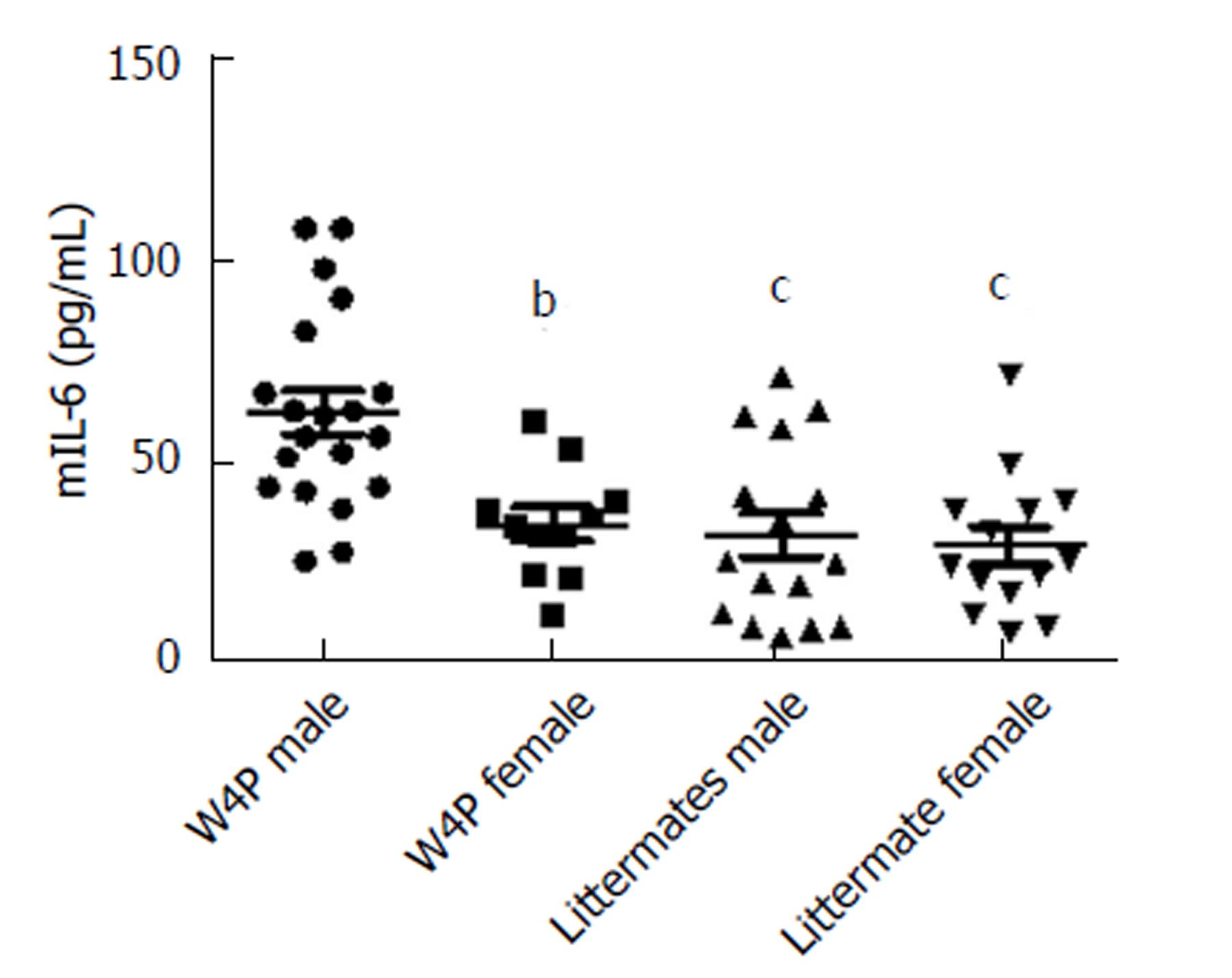
It has been reported previously that the presence of the W4P mutation in the preS1 region sex-dependently affected IL-6 production in the xenograft nude mouse model system, which could be one of the reasons for increased male susceptibility to HCC[31]. Thus, to check whether there was sex disparity in the induction of IL-6-mediated inflammation, we next examined serum IL-6 levels in W4P TG mice (24 males, 18 females) and their nonTG littermates (17 males, 15 females) at 10 mo of age. W4P TG male mice showed significantly higher serum IL-6 levels than did mice of the other three groups (Figure 5). We also checked the levels of liver enzymes in the serum as indicators of liver damage in the four groups of mice. We found that W4P TG male mice had significantly higher serum levels of ALT than mice from the other three groups. However, serum AST levels were not significantly different in the four groups of mice (Figure 6).
Increasing evidence has shown sex disparity in the incidence of HBV-associated HCC in a sex hormone-dependent manner. Sex hormones, including androgen and estrogen, likely affect the progression of HBV infection and development of HBV-related HCC via their actions on receptor-mediated cell signaling[24-26]. To date, of all HBV proteins, HBx has been most extensively studied as the predominant virus interactor with host cell sex hormone-mediated signaling[22,23]. However, in our recent molecular epidemiologic and cell-based studies, we have demonstrated that LHB harboring the W4P mutation in preS1 could also contribute to the sex disparity of HBV-associated HCC in an IL-6-dependent manner[30,31]. In the present study, we constructed W4P TG mice that expressed the full HBV genome, which can help us to study sex disparity of the progression of liver diseases, including chronic hepatitis, steatohepatitis, cirrhosis and HCC, following chronic HBV infection.
We identified three noteworthy findings supporting the contribution of the W4P mutation in preS1 to liver disease progression in male patients. First, by using the W4P TG mouse model of chronic HBV infection, we found that male W4P TG mice exhibited higher levels of secreted HBsAg and liver LHBs, which was indicative of higher HBV replication than in female W4P TG mice (Figure 4) and is one of the known HCC risk factors[34]. Second, we found that male W4P TG mice showed increased incidence of hepatomegaly and lipid droplets (Figure 3), reflecting the imbalance of metabolic liver homeostasis, which could drive liver pathogenesis, including fatty liver and steatohepatitis, and further promote tumorigenesis. Third, we found that male W4P TG mice had increased IL-6-related liver inflammation and higher serum ALT levels (Figure 5), which were indications of liver damage, compared to those seen in female W4P TG mice.
IL-6 is one of the core stimulators that lead to persistent HBV infection and development of HBV-related HCC. It is also a key cytokine that may be a link to preferential male susceptibility to HCC[25,31]. A previous study that used diethylnitrosamine to evoke HCC showed that estrogen prevented HCC generation in female mice by inhibiting IL-6 production in a Myd88-dependent manner. That observation suggested that inhibition of IL-6 production in liver Kupffer cells by estrogen and estrogen receptor-mediated signaling pathways could be a major molecular mechanism that underlies sex disparity in HBV-associated liver diseases, including HCC[25,26]. Furthermore, increased hepatic IL-6 production also likely plays a pivotal role in the development of nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and insulin resistance, which are the leading causes of HCC[35-40]. Thus, our W4P TG model showing increased hepatic IL-6 production could provide a novel insight into the relationships between IL-6 production due to an infection caused by an HBV variant on the one hand, and development of nonalcoholic steatohepatitis, type 2 diabetes or HCC on the other hand.
Our study had some limitations. Unfortunately, we did not prove predominant carcinogenesis in males in our W4P TG mice. Therefore, further studies are necessary to demonstrate higher male susceptibility to liver carcinogenesis in our W4P TG mouse model and clarify its mechanism in the future. In addition, the relationships between increased hepatic production of IL-6 in mice expressing HBV genome with the W4P mutation and fat accumulation, increased liver weight and HCC development also remain to be elucidated in the future.
The phenotypes of male W4P TG mice, namely higher levels of IL-6 and ALT in the serum, could provide a technical advantage in drug screening protocols, as it will be possible to analyze not only the inhibition of HBV replication but also the antiinflammatory activity. To the best of our knowledge, this possibility is currently not available in other related TG mouse models.
In conclusion, we created W4P TG mice that constitutively express the full HBV genome with the W4P mutation in preS1 in the present study. Our data using W4P TG mice indicate that this mutation likely contributes to sex disparity in the susceptibility to liver disease, including HCC, leading to increased HBV virion replication and enhanced IL-6-mediated inflammation in male individuals. Additionally, the developed TG mouse model system carrying the full HBV genome with the W4P mutation in preS1 could be effectively used not only in basic research into the mechanisms of liver disease progression in HCC but also for the screening of antiHBV or antiinflammatory drugs.
A remarkable global epidemiological feature of hepatocellular carcinoma (HCC) is its higher incidence in males. Recently, we identified the novel W4P substitution in the preS1 region of hepatitis B virus (HBV) related to HCC that occurs exclusively in male patients. We have also shown that the W4P mutation likely contributed to HCC development, particularly in male patients, in an interleukin (IL)-6-dependent manner.
Studies of sex disparity in the susceptibility to HCC in vivo have mainly utilized the chemical agent diethylnitrosamine to induce HCC in mice. However, no transgenic (TG) mouse model system expressing the full HBV genome has yet been available for the study of sex disparity in HBV-related liver diseases.
To gain further insight into the role of the W4P mutation in the preS1 region of HBV on sex disparity of HBV-induced liver inflammatory manifestations, we created a TG mouse that carried the full HBV genome with this mutation and evaluated HBV virion replication and IL-6-mediated inflammation in mutant and wild-type (WT) mice of both sexes.
TG mice were generated by transferring the pHY92-1.1x-HBV-full genome plasmid (genotype A2) into C57Bl/6N mice. We compared serum levels of hepatitis B surface antigen (HBsAg), IL-6, and the liver enzymes alanine aminotransferase (ALT) and aspartate transaminase (AST), as well as liver histopathology features in male and female W4P TG mice and their WT littermates.
Our data showed significantly increased hepatomegaly, enhanced granule generation in liver tissue, higher HBsAg expression in the liver and serum, and higher serum ALT and IL-6 levels in W4P TG males compared to the values of these parameters in W4P TG females or littermate control groups.
This is the first study that used TG mice to uncover the role of the W4P mutation in HBV preS1 in sex disparity of liver disease progression due to concomitantly increased HBV virion replication and greater IL-6-mediated inflammation in male individuals.
The obtained results suggest that W4P TG mice developed in this study could be effectively used not only for the basic research into the mechanisms of HBV-associated liver diseases, including HCC, but also for screening antiHBV and antiinflammatory drugs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bramhall S, Huang C, Kai K, Tarantino G, Tomizawa M S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1728] [Cited by in F6Publishing: 1678] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 2. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9500] [Cited by in F6Publishing: 9071] [Article Influence: 755.9] [Reference Citation Analysis (0)] |
| 3. | Chang MH, Chen TH, Hsu HM, Wu TC, Kong MS, Liang DC, Ni YH, Chen CJ, Chen DS; Taiwan Childhood HCC Study Group. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res. 2005;11:7953-7957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention Suppl 1. J Clin Virol. 2005;34:S1-S3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 334] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Reprint of: Epidemiological serosurvey of Hepatitis B in China--declining HBV prevalence due to Hepatitis B vaccination Suppl 9. Vaccine. 2013;31:J21-J28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1734] [Cited by in F6Publishing: 1694] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 7. | Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK; Hong Kong Liver Fibrosis Study Group. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 929] [Cited by in F6Publishing: 936] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 9. | McMahon BJ. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatol Int. 2009;3:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 10. | Schaefer S. Hepatitis B virus taxonomy and hepatitis B virus genotypes. World J Gastroenterol. 2007;13:14-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 243] [Cited by in F6Publishing: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Croagh CM, Desmond PV, Bell SJ. Genotypes and viral variants in chronic hepatitis B: A review of epidemiology and clinical relevance. World J Hepatol. 2015;7:289-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Wang C, Teng Z, Zhu Y, Zhao AZ, Sun C. Associations between pre-S deletion mutation of hepatitis B virus and risk of hepatocellular carcinoma in the Asian population: a meta-analysis. Med Sci Monit. 2015;21:1072-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Mun HS, Lee SA, Jee Y, Kim H, Park JH, Song BC, Yoon JH, Kim YJ, Lee HS, Hyun JW. The prevalence of hepatitis B virus preS deletions occurring naturally in Korean patients infected chronically with genotype C. J Med Virol. 2008;80:1189-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Pollicino T, Cacciola I, Saffioti F, Raimondo G. Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications. J Hepatol. 2014;61:408-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 15. | Iwamoto M, Watashi K, Tsukuda S, Aly HH, Fukasawa M, Fujimoto A, Suzuki R, Aizaki H, Ito T, Koiwai O. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem Biophys Res Commun. 2014;443:808-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 16. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 17. | Prieto J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J Hepatol. 2008;48:380-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 832] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 19. | Liu WC, Liu QY. Molecular mechanisms of gender disparity in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2014;20:6252-6261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Wang SH, Chen PJ, Yeh SH. Gender disparity in chronic hepatitis B: Mechanisms of sex hormones. J Gastroenterol Hepatol. 2015;30:1237-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM, Chen CJ. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst. 2001;93:1644-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang CJ, Chen PJ, Yang WJ, Chen DS. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci. 2007;104:2571-2578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Bouchard MJ, Wang L, Schneider RJ. Activation of focal adhesion kinase by hepatitis B virus HBx protein: multiple functions in viral replication. J Virol. 2006;80:4406-4414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Lam CM, Yong JL, Chan AO, Ng KK, Poon RT, Liu CL, Lo CM, Fan ST. Better survival in female patients with hepatocellular carcinoma: oral contraceptive pills related? J Clin Gastroenterol. 2005;39:533-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1362] [Cited by in F6Publishing: 1400] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 26. | Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010;78 Suppl 1:172-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 27. | Wang HC, Huang W, Lai MD, Su IJ. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Sci. 2006;97:683-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, Chang WT, Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023-2032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Caselmann WH, Meyer M, Kekulé AS, Lauer U, Hofschneider PH, Koshy R. A trans-activator function is generated by integration of hepatitis B virus preS/S sequences in human hepatocellular carcinoma DNA. Proc Natl Acad Sci. 1990;87:2970-2974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Lee SA, Kim KJ, Kim DW, Kim BJ. Male-specific W4P/R mutation in the pre-S1 region of hepatitis B virus, increasing the risk of progression of liver diseases in chronic patients. J Clin Microbiol. 2013;51:3928-3936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Lee SA, Kim H, Won YS, Seok SH, Na Y, Shin HB, Inn KS, Kim BJ. Male-specific hepatitis B virus large surface protein variant W4P potentiates tumorigenicity and induces gender disparity. Mol Cancer. 2015;14:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Yang H, Westland C, Xiong S, Delaney WE 4th. In vitro antiviral susceptibility of full-length clinical hepatitis B virus isolates cloned with a novel expression vector. Antiviral Res. 2004;61:27-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Zheng L, Baumann U, Reymond JL. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 2004;32:e115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 835] [Cited by in F6Publishing: 872] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 34. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1691] [Cited by in F6Publishing: 1665] [Article Influence: 83.3] [Reference Citation Analysis (2)] |
| 35. | Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 426] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 36. | Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006;26:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am. 2007;91:1125-1149, ix. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70 Suppl 1:i104-i108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 405] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 39. | Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 683] [Cited by in F6Publishing: 710] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 40. | Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217-9228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 37] [Reference Citation Analysis (1)] |