Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1233
Peer-review started: November 1, 2016
First decision: December 2, 2016
Revised: December 13, 2016
Accepted: January 3, 2017
Article in press: January 3, 2017
Published online: February 21, 2017
To develop predictive markers in blood for colorectal cancer liver metastasis.
Twenty colorectal cancer patients were selected and divided into two groups. Group A consisted of 10 patients whose pathological TNM stage was IIIC (T3-4N2M0), while another 10 patients with synchronous liver metastasis (TNM stage IV) were recruited for group B. During the surgical procedure, a 10-mL drainage vein (DV) blood sample was obtained from the DV of the tumor-bearing segment prior to the ligation of the DV. At the same time, a 10-mL peripheral vein (PV) blood sample was collected via peripheral venipuncture. The serum levels of 24 molecules that are potentially involved in the mechanism of liver metastasis in both DV blood and PV blood were analyzed by using high-throughput enzyme-linked immunosorbent assay technology.
Univariate analysis revealed that platelet-derived growth factor AA (PDGFAA) in DV blood (dPDGFAA) (P = 0.001), PDGFAA in PV blood (pPDGFAA) (P = 0.007), and human epidermal growth factor receptor-2 in PV blood (pHER2) (P = 0.001), pMMP7 (P = 0.028), pRANTES (P = 0.013), and pEGF (P = 0.007) were significantly correlated with synchronous liver metastasis. Multivariate analysis identified dPDGFAA (HR = 1.001, P = 0.033) and pHER2 (HR = 1.003, P = 0.019) as independent predictive factors for synchronous liver metastasis. Besides, high peripheral HER2 level may also be a risk factor for metachronous liver metastasis, although the difference did not reach statistical significance (P = 0.06). Significant correlations were found between paired DV and PV blood levels for PDGFAA (r = 0.794, P < 0.001), but not for HER2 (r = 0.189, P = 0.424).
PDGFAA in tumor drainage and HER2 in PV blood may be useful predictive factors for synchronous liver metastasis of colorectal cancer.
Core tip: We investigated the serum levels of most commonly studied tumor growth factors that are known to be associated with the mechanism of liver metastasis not only in peripheral vein (PV) blood but also in tumor drainage vein (DV) blood. To our knowledge, this is one of few studies taking DV blood into analysis and comparing the differences in serum molecules between PV blood and DV blood. We found that platelet-derived growth factor AA in tumor drainage and human epidermal growth factor receptor 2 in PV blood may be useful predictive factors for synchronous liver metastasis of colorectal cancer.
- Citation: Pan HD, Peng YF, Xiao G, Gu J. High levels of serum platelet-derived growth factor-AA and human epidermal growth factor receptor-2 are predictors of colorectal cancer liver metastasis. World J Gastroenterol 2017; 23(7): 1233-1240
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1233.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1233
Liver metastasis occurs in almost 50% of patients with colorectal cancer and it is the leading cause of death from colorectal cancer[1]. Thus, developing useful predictive markers for screening patients at high risk for liver metastasis is of prime importance. Although many studies have reported predictive factors for liver metastasis[2-13], the fundamental pathogenesis remains unclear. The majority of previous studies mainly focused on clinicopathologic characteristics and features in tumor tissue specimens.
Several studies have revealed that circulating factors constitute significant predictors of metastasis in patients with colorectal cancer[2-13]. However, the prognostic value of factors in blood, especially in tumor drainage vein (DV) blood, has received relatively little attention. Theoretically, factors produced by the primary tumor must first go through the metabolism or undergo breakdown in the liver, lungs, and other organs before they can reach the peripheral veins (PV). Therefore, the levels of factors in a tumor DV could provide more accurate information about the primary tumor than assessments performed using PV blood.
This study was undertaken to develop predictive markers in blood for liver metastasis and improve our understanding of its prognosis. We investigated the serum levels of the most commonly studied tumor growth factors that are known to be associated with the mechanism of liver metastasis not only in PV blood but also in tumor DV blood.
This retrospective study was approved by the Institutional Review Board and written informed consent was obtained from each patient before the study. The data of patients who underwent surgery for colorectal cancer in the Department of Colorectal Surgery, Peking University Cancer Hospital, from January 2011 to May 2011 were identified from a surgical database. Patients pathologically diagnosed with colonic or rectal adenocarcinoma, with radiologically confirmed non-metastasis (M0) or synchronous liver metastasis (M1), were included. Patients with extrahepatic metastasis, patients who received prior chemotherapy or radiotherapy, or patients who had a history of cancer within 5 years were excluded from the study.
Twenty patients were selected for the study and they were divided into two groups. Group A consisted of 10 patients whose pathological TNM (tumor-node-metastasis) stage was IIIC (T3-4N2M0), while another 10 patients with synchronous liver metastasis (TNM stage IV) were recruited for group B. The diagnosis of synchronous liver metastasis was confirmed by intraoperative biopsy, liver magnetic resonance imaging, or positron emission tomography (PET)/computed tomography (CT). Abdominal CT and/or PET scans ruled out extrahepatic metastasis. The patients in group A received curative surgery for the primary colorectal cancer. In group B, nine patients received surgery for the primary tumor only and one patient underwent simultaneous liver resection with curative intent. All of the patients were recommended to receive postoperative chemotherapy.
Patients were followed every 3 mo during the first 2 years, and every 6 mo thereafter in the outpatient clinic. At each follow-up, physical examination, detection of serum tumor markers, routine blood tests, and serum chemistry profiling were performed. Proctoscopy, abdominal ultrasonography, abdomen and pelvis CT, and chest radiography were performed every 6-12 mo. The primary endpoints were metastasis and local recurrence.
We searched PubMed using the following keywords: colorectal adenocarcinoma, liver metastasis, and tumor growth factor. Then we reviewed the published relevant articles and selected molecules that could be detected in serum and that could be analyzed by enzyme-linked immunosorbent assay (ELISA) with commercially available antibodies. Twenty-four molecules that are potentially involved in the mechanism of liver metastasis were identified for further investigation, including fibroblast growth factor-b, hepatocyte growth factor, platelet-derived growth factor-BB (PDGFBB), vascular endothelial growth factor (VEGF), matrix metalloproteinase 2, VEGFD, CD30, myeloperoxidase, total plasminogen activator inhibitor 1, regulated on activation normal T cell expressed and secreted (RANTES), Resistin, tissue inhibitor of metalloproteinase (TIMP) 1, TIMP2, vascular cell adhesion molecule 1, epidermal growth factor (EGF), intercellular adhesion molecule 3, platelet-derived growth factor AA (PDGFAA), P-selectin, E-cadherin, osteopontin, MMP9, human epidermal growth factor receptor 2 (HER2), and MMP7.
Once the peritoneal cavity was opened, a 10-mL DV blood sample was obtained from the DV of the tumor-bearing segment prior to the ligation of the DV and removal of the tumor. While sampling the DV blood, a 10-mL PV blood sample was collected via peripheral venipuncture by a circuit nurse. Blood samples were instantly centrifuged and serum and cells were stored separately at -80 °C for later analysis. Measurement of serum levels of all 24 molecules was conducted by ELISA using commercially available kits (LightArray Biotech Co., Ltd) according to the manufacturer’s instructions.
Statistical analyses were performed using IBM SPSS Statistics for Mac, Version 20.0 (Armonk, New York: IBM Corp.). For continuous covariates, means were compared by the Student’s t-test, while nonparametric data were assessed by the Mann-Whitney U test (GraphPad Software Prism 5). Categorical variables were analyzed by the Pearson chi-squared or Fisher’s exact test, when appropriate. Variables with a P value less than 0.2 during univariate analysis were selected for multivariate analysis using a logistic regression model. Correlations of factor levels in tumor drainage and in PV blood were performed by the Spearman rank correlation analysis. All hypothesis tests were two-sided, with a P value less than 0.05 and 0.1 considered statistically significant in univariate and multivariate analysis, respectively.
The median follow-up period of the patients was 40 mo (range, 38-42 mo), with no patients lost to follow-up. The clinical and pathological characteristics were comparable between the two groups (Table 1). The median age of group A (68 years) was higher than that of group B (60 years), but the difference was not statistically significant (P = 0.150). In both groups A and B, 60% of the patients had pT3 disease, and the remaining 40% had pT4. All patients in group A had pN2, while 60% of patients in group B had pN2, but the difference did not reach statistical significance (P = 0.168). Location and histological differentiation of the tumor were not different between groups A and B. The preoperative CEA level was significantly higher in group B (1.74 ng/mL vs 9.64 ng/mL, P = 0.017). No perioperative mortality was reported in either group. Five patients in group A developed metachronous distant metastases, but no local recurrence was observed. One patient in group B who received simultaneous liver resection with curative intent developed a hepatic recurrence.
| Clinical variable | Group A (n = 10) | Group B (n = 10) | P value |
| mean age (yr) | 68 (41-79) | 60 (30-73) | 0.150 |
| Male | 6 (60) | 4 (40) | 0.625 |
| pT stage | 1.000 | ||
| pT3 | 6 (60) | 6 (60) | |
| pT4 | 4 (40) | 4 (40) | |
| pN stage | 0.168 | ||
| pN0 | 0 | 1 (10) | |
| pN1 | 0 | 3 (30) | |
| pN2 | 4 (40) | 6 (60) | |
| Location | 1.000 | ||
| Right colon | 4 (40) | 4 (40) | |
| Left colon/rectum | 6 (60) | 6 (60) | |
| Histology differentiation | 1.000 | ||
| Well | 2 (20) | 2 (20) | |
| Moderately | 4 (40) | 4 (40) | |
| Poorly/mucinous | 4 (40) | 4 (40) | |
| Lymphovascular invasion | 6 (60) | 4 (40) | 0.625 |
| Preoperative CEA (median, range) | 1.74 (1.18-11.0) | 9.64 (1.12-512.9) | 0.017 |
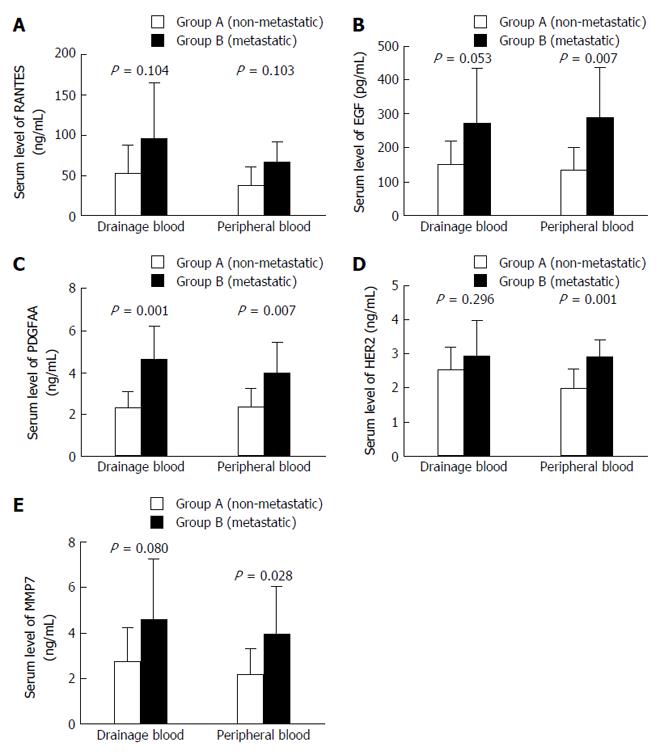
In the PV, serum levels of RANTES (36.34 ± 23.64 vs 66.60 ± 25.55, P = 0.013), EGF (135.16 ± 67.01 vs 289.36 ± 147.24, P = 0.007), PDGFAA (2.38 ± 0.85 vs 3.99 ± 1.46, P = 0.007), HER2 (2.01 ± 0.55 vs 2.91 ± 0.49, P = 0.001), and MMP7 (2.20 ± 1.08 vs 3.98 ± 2.10, P = 0.028) in group B were significantly higher than those in group A. In the DV, only serum level of PDGFAA in group B was significantly higher than that in group A (2.30 ± 0.80 vs 4.62 ± 16.00, P = 0.001) (Table 2). Other clinicopathological factors were also included in the univariate analysis; however, no significant difference was found (Figure 1).
| Drainage blood | Peripheral blood | |||||
| Group A (non-metastatic) | Group B (metastatic) | P value | Group A (non-metastatic) | Group B (metastatic) | P value | |
| RANTES (ng/mL) | 52.61 ± 35.37 | 95.17 ± 70.21 | 0.104 | 36.34 ± 23.64 | 66.60 ± 25.55 | 0.013 |
| EGF (pg/mL) | 150.09 ± 70.40 | 270.67 ± 163.70 | 0.053 | 135.16 ± 67.01 | 289.36 ± 147.24 | 0.007 |
| PDGFAA (ng/mL) | 2.30 ± 0.80 | 4.62 ± 1.60 | 0.001 | 2.38 ± 0.85 | 3.99 ± 1.46 | 0.007 |
| HER2 (ng/mL) | 2.54 ± 0.68 | 2.95 ± 1.02 | 0.296 | 2.01 ± 0.55 | 2.91 ± 0.49 | 0.001 |
| MMP7 (ng/mL) | 2.77 ± 1.47 | 4.57 ± 2.70 | 0.080 | 2.20 ± 1.08 | 3.98 ± 2.10 | 0.028 |
Variables with a P-value less than 0.2 in univariate analysis were selected for multivariate analysis. A logistic regression model was used to identify factors associated with synchronous liver metastasis. The levels of PDGFAA in the DV (HR = 1.001, P = 0.033) and HER2 (HR = 1.003, P = 0.019) in the PV were identified as independent predictive factors for synchronous liver metastasis.
Factors that showed statistical significance in univariate analysis were selected for correlation analysis using Spearman rank correlation analysis (Table 3). pEGF level was significantly correlated with the level of pPDGFAA (r = 0.887, P < 0.001). The levels of PDGFAA (r = 0.794, P < 0.001) and MMP7 (r = 0.863, P < 0.001) in tumor DV blood samples were highly correlated with those in paired PV blood samples. It was noteworthy that there was a very low correlation between paired tumor DV and PV blood levels for HER2 (r = 0.189, P = 0.424).
| Correlation | r | P value |
| dPDGFAA-pPDGFAA | 0.794 | < 0.001 |
| dHER2-pHER2 | 0.189 | 0.424 |
| dRANTES-pRANTES | 0.705 | 0.001 |
| dEGF-pEGF | 0.605 | 0.005 |
| dMMP7-mMMP7 | 0.863 | < 0.001 |
| pEGF-dPDGFAA | 0.678 | 0.001 |
| pEGF-pPDGFAA | 0.887 | < 0.001 |
| pEGF-pHER2 | 0.489 | 0.029 |
| pHER2-pMMP7 | 0.529 | 0.016 |
In the subgroup analysis of group A, we investigated factors correlated with metachronous liver metastasis by univariate and multivariate analyses; however, no factor was found to be associated with metachronous liver metastasis. The patients who developed metachronous metastasis had higher pHER2 levels than those who did not, but there was no statistical significance (2.33 ng/mL vs 1.70 ng/mL, P = 0.06).
This study is one of few to analyze serum molecules not only in PV blood but also in DV blood to develop predictive factors for colorectal cancer liver metastasis. Group A and group B consisted of patients with stage IIIC and stage IV colorectal cancer, respectively. Except for the TNM stage, other clinicopathological characteristics were comparable between the two groups.
There is a great difference in the 5-year overall survival between stage IIIC and IV colorectal cancer (28% vs 5.7% for colon cancer[14], and 33.4% vs 6.0% for rectal cancer[15]). Second only to stage IV, stage IIIC has the worst prognosis in locally advanced colorectal cancer. It is notable that although lymph node metastasis is present in stage IIIC cases, the disease is still confined to the local region rather than spreads to distant organs. Therefore, we propose the hypothesis that stage IV tumors may have stronger capabilities of proliferation, colonization, invasion, and angiogenesis than stage IIIC tumors. There might be some critical factors that trigger the final progression from locally advanced to systemic metastasis. The rationale behind this study is that if the difference between IIIC and IV can be identified, we may possibly clarify the underlying mechanism of colorectal cancer liver metastasis.
Many studies have investigated the molecules not only in PV blood, but also in tumor DV blood, and the relationship between recurrence or metastasis of colorectal cancer and those molecules has been explored. Tien et al[6] postulated that if the primary colorectal tumor is the exclusive or major source of tumor growth factors, the levels of serum tumor growth factors should be higher in tumor DV blood than in PV blood, and there should be a good correlation for the levels of all tumor growth factors between paired tumor DV and PV blood. After entering the circulation, molecules that are expressed and secreted by the primary tumor may be metabolized and broken down by the liver, lungs, or other organs before they reach the PV.
These authors[6] investigated the relationship between tumor stage and disease recurrence with the level of angiogenic factors in both the DV and PV in patients with colon cancer. The results showed that the level of VEGF in the tumor DV blood was an independent predictor of disease recurrence. A previous study by Min et al[5] demonstrated that colon cancer patients with high levels of VEGF and TIMP-1 in the DV had a high risk of metachronous liver metastasis and hepatic recurrence following the resection of synchronous liver metastasis.
The results of the present study have shown that the elevated levels of PDGFAA detected in the DV blood of the primary tumor, and HER2 in the PV blood, were significantly correlated with synchronous liver metastasis. Moreover, high peripheral HER2 level may also be a risk factor for metachronous liver metastasis, although the difference did not reach statistical significance (P = 0.06).
Platelet-derived growth factor (PDGF) is one of the numerous growth factors that regulate cell growth and division. In chemical terms, PDGF is a dimeric glycoprotein composed of two A (PDGFAA) or two B (PDGFBB) chains or a combination of the two (PDGFAB). In particular, it plays a significant role in angiogenesis and its overexpression leads to uncontrolled angiogenesis in the majority of solid organ cancers[16-18]. Several studies have shown that PDGF expression in tumor tissue is correlated with a poor prognosis of colorectal cancer[7,8]. However, there are few reports studying the relationship between serum PDGFAA levels and prognosis in colorectal cancer. Inanç et al[10] assessed the serum levels and prognostic role of tumor growth and angiogenic factors in patients with metastatic colorectal cancer treated with chemotherapy. These authors found that PDGFAA was significantly decreased in both patients with a partial response and stable disease.
HER2 (ErbB2) has been shown to play a role in the tumor growth process and it is an established therapeutic target in breast[19-21] and gastric cancers[22-24]. Nevertheless, the role of HER2 in colorectal cancer remains unclear, because conflicting data on the prevalence of HER2 expression have been reported[25-30]. Park et al[26] reported that 47.4% of patients with colorectal cancer were determined by immunohistochemistry to overexpress HER2. Patients with tumors with HER2 overexpression had a higher postoperative recurrence rate (39.3% vs 14.6%, P = 0.013), and its overexpression was associated with poorer 5-year survival rates (55.1% vs 78.3%, P < 0.05). However, the authors of the EXPERT-C trial reported that only 4.3% of patients had HER2 overexpression, and HER2 does not appear to represent a useful therapeutic target in high-risk rectal cancer[27]. Our previous study[28] showed that HER2 was overexpressed in 15% of rectal cancer patients who received neoadjuvant radiotherapy. Moreover, HER2 overexpression could be a predictive biomarker of distant metastasis in rectal cancer patients after preoperative radiotherapy. A recent study[29] assessed HER2-amplification/overexpression in 1914 stages II-III and IV CRC patients. The result showed that HER2 was not associated with overall survival or progression free survival. A higher proportion of HER2-overexpressing cases experienced recurrence, but the difference was not significant. Fusco et al[30] considered that HER2 status should be assessed as a putative biomarker of resistance to anti-EGFR therapy in KRAS wild-type patients.
In the correlation analysis, we found significant correlations between paired distal vein and PV blood levels for PDGFAA (r = 0.794, P < 0.001), but not for HER2 (r = 0.189, P = 0.424). These findings indicate that the primary tumor was the dominate source of PDGFAA. PDGFAA levels in tumor DV blood provided better prognostic information than those in PV blood. On the other hand, there may be some additional sources producing HER2 outside the primary tumor, perhaps the metastatic tumors in the liver, and therefore the HER2 level detected in the distal vein cannot faithfully reflect the characteristics of the tumor. In addition, a significant correlation was noted between pEGF and pPDGFAA levels, which indicated that EGF and PDGFAA have an interaction effect in the development of colorectal cancer liver metastasis.
The limitations of this study are obvious. The study only enrolled a small series of patients and these results require validation. Variations of the mesentery vein are very common, and exposure of the vein needs well-developed surgical skills. Blood from the tumor DV is difficult to obtain, and requires more effort than that from the PV. It is impossible to draw DV blood in non-surgical patients.
In conclusion, the present study analyzed the serum levels of 24 commonly studied tumor growth factors in both tumor drainage and PV blood from patients with colorectal cancer by using high-throughput ELISA technology. We found that PDGFAA in tumor drainage and HER2 in PV blood may be useful predictive factors for synchronous liver metastasis. PDGFAA levels in tumor DV blood provided better prognostic information than those in PV blood. On the contrary, HER2 levels in PV blood reflected tumor characteristics more accurately than those in tumor DV blood.
Liver metastasis occurs in almost 50% of patients with colorectal cancer and it is the leading cause of death. Developing useful predictive markers for screening patients at high risk for liver metastasis may optimize our therapy strategy. Previous studies mainly involved clinicopathologic characteristics and factors in tumor tissue specimens, however, the fundamental pathogenesis remains unclear.
Several studies have revealed that circulating factors constitute significant predictors of metastasis for patients with colorectal cancer. However, the prognostic value of factors in blood, especially in drainage venous (DV) blood, has received relatively little attention.
The authors investigated the serum levels of most commonly studied tumor growth factors that are known to be associated with the mechanism of liver metastasis not only in peripheral venous (PV) blood but also in tumor DV blood. To our knowledge, this is one of few studies taking DV blood into analysis and comparing the differences of serum molecules between PV blood and DV blood.
The present study provides us with a new angle of predicting liver metastasis of colorectal cancer. Platelet-derived growth factor AA (PDGFAA) in tumor drainage and human epidermal growth factor receptor 2 (HER2) in PV blood may constitute useful predictive factors for synchronous liver metastasis. PDGFAA levels in tumor DV blood provided better prognostic information than those in PV blood, while HER2 levels in PV blood reflected tumor characteristics more accurately than those in tumor DV blood.
Platelet-derived growth factor (PDGF) plays a significant role in blood vessel formation, the growth of blood vessels from already-existing blood vessel tissue. Uncontrolled angiogenesis is a characteristic of cancer. PDGF is a dimeric glycoprotein composed of two A (-AA) or two B (-BB) chains or a combination of the two (-AB). HER2 is a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family. Amplification or overexpression of this oncogene has been shown to play an important role in the development and progression of certain aggressive types of cancer.
This is an interesting manuscript about the predictors of colorectal cancer liver metastasis. In this study, twenty patients were selected for the study and they were divided into two groups. The serum levels of 24 molecules that are potentially involved in the mechanism of liver metastasis in both DV blood and PV blood were analyzed by using enzyme-linked immunosorbent assay technology.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jones G, Taylor ME S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 777] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 2. | Yoon SS, Kim SH, Gonen M, Heffernan NM, Detwiller KY, Jarnagin WR, D’Angelica M, Blumgart LH, Tanabe KK, Dematteo RP. Profile of plasma angiogenic factors before and after hepatectomy for colorectal cancer liver metastases. Ann Surg Oncol. 2006;13:353-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Ivankovics IG, Fernandes LC, Saad SS, Matos D. Peripheral and mesenteric serum levels of CEA and cytokeratins, staging and histopathological variables in colorectal adenocarcinoma. World J Gastroenterol. 2008;14:6699-6703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Kanellos I, Zacharakis E, Kanellos D, Pramateftakis MG, Tsahalis T, Altsitsiadis E, Betsis D. Prognostic significance of CEA levels and detection of CEA mRNA in draining venous blood in patients with colorectal cancer. J Surg Oncol. 2006;94:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Min BS, Kim NK, Jeong HC, Chung HC. High levels of serum VEGF and TIMP-1 are correlated with colon cancer liver metastasis and intrahepatic recurrence after liver resection. Oncol Lett. 2012;4:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Tien YW, Chang KJ, Chiu YF, Huang KW, Lee PH. Comparison of angiogenic factor levels in tumor drainage and peripheral venous blood from colorectal cancer patients. Ann Surg Oncol. 2006;13:1357-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Nakamura Y, Tanaka F, Yoshikawa Y, Mimori K, Inoue H, Yanaga K, Mori M. PDGF-BB is a novel prognostic factor in colorectal cancer. Ann Surg Oncol. 2008;15:2129-2136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Hamilton SR, Fidler IJ. Expression of activated platelet-derived growth factor receptor in stromal cells of human colon carcinomas is associated with metastatic potential. Int J Cancer. 2006;119:2567-2574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Braicu C, Tudoran O, Balacescu L, Catana C, Neagoe E, Berindan-Neagoe I, Ionescu C. The significance of PDGF expression in serum of colorectal carcinoma patients--correlation with Duke’s classification. Can PDGF become a potential biomarker? Chirurgia (Bucur). 2013;108:849-854. [PubMed] [Cited in This Article: ] |
| 10. | Inanç M, Er O, Karaca H, Berk V, Ozkan M, Saraymen R, Elmalı F. Prognostic value of tumor growth factor levels during chemotherapy in patients with metastatic colorectal cancer. Med Oncol. 2012;29:3119-3124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Toiyama Y, Fujikawa H, Kawamura M, Matsushita K, Saigusa S, Tanaka K, Inoue Y, Uchida K, Mohri Y, Kusunoki M. Evaluation of CXCL10 as a novel serum marker for predicting liver metastasis and prognosis in colorectal cancer. Int J Oncol. 2012;40:560-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Beştaş R, Kaplan MA, Işikdoğan A. The correlation between serum VEGF levels and known prognostic risk factors in colorectal carcinoma. Hepatogastroenterology. 2014;61:267-271. [PubMed] [Cited in This Article: ] |
| 13. | Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M, Filho AL. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genomics Proteomics. 2013;10:55-67. [PubMed] [Cited in This Article: ] |
| 14. | Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 15. | Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Yuge R, Kitadai Y, Shinagawa K, Onoyama M, Tanaka S, Yasui W, Chayama K. mTOR and PDGF pathway blockade inhibits liver metastasis of colorectal cancer by modulating the tumor microenvironment. Am J Pathol. 2015;185:399-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Liu C, Li J, Xiang X, Guo L, Tu K, Liu Q, Shah VH, Kang N. PDGF receptor-α promotes TGF-β signaling in hepatic stellate cells via transcriptional and posttranscriptional regulation of TGF-β receptors. Am J Physiol Gastrointest Liver Physiol. 2014;307:G749-G759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Holleran G, Hall B, O’Regan M, Smith S, McNamara D. Expression of Angiogenic Factors in Patients With Sporadic Small Bowel Angiodysplasia. J Clin Gastroenterol. 2014;49:831-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8204] [Cited by in F6Publishing: 7828] [Article Influence: 340.3] [Reference Citation Analysis (0)] |
| 20. | Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1128] [Cited by in F6Publishing: 1061] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 21. | Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-1684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4010] [Cited by in F6Publishing: 3810] [Article Influence: 200.5] [Reference Citation Analysis (0)] |
| 22. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 737] [Cited by in F6Publishing: 809] [Article Influence: 50.6] [Reference Citation Analysis (2)] |
| 23. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 868] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 24. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4615] [Cited by in F6Publishing: 4831] [Article Influence: 345.1] [Reference Citation Analysis (0)] |
| 25. | Ingold Heppner B, Behrens HM, Balschun K, Haag J, Krüger S, Becker T, Röcken C. HER2/neu testing in primary colorectal carcinoma. Br J Cancer. 2014;111:1977-1984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Park DI, Kang MS, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Han WK, Kim H. HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int J Colorectal Dis. 2007;22:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Sclafani F, Roy A, Cunningham D, Wotherspoon A, Peckitt C, Gonzalez de Castro D, Tabernero J, Glimelius B, Cervantes A, Eltahir Z. HER2 in high-risk rectal cancer patients treated in EXPERT-C, a randomized phase II trial of neoadjuvant capecitabine and oxaliplatin (CAPOX) and chemoradiotherapy (CRT) with or without cetuximab. Ann Oncol. 2013;24:3123-3128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Yao YF, Du CZ, Chen N, Chen P, Gu J. Expression of HER-2 in rectal cancers treated with preoperative radiotherapy: a potential biomarker predictive of metastasis. Dis Colon Rectum. 2014;57:602-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2016;238:562-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 30. | Fusco N, Bosari S. HER2 aberrations and heterogeneity in cancers of the digestive system: Implications for pathologists and gastroenterologists. World J Gastroenterol. 2016;22:7926-7937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |