Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8465
Peer-review started: June 6, 2017
First decision: June 22, 2017
Revised: October 31, 2017
Accepted: November 28, 2017
Article in press: November 28, 2017
Published online: December 28, 2017
To provide new insights in treatment of colitis and ischemia and reperfusion in rats using stable gastric pentadecapeptide BPC 157.
Medication [BPC 157, L-NAME, L-arginine (alone/combined), saline] was bath at the blood deprived colon segment. During reperfusion, medication was BPC 157 or saline. We recorded (USB microscope camera) vessel presentation through next 15 min of ischemic colitis (IC-rats) or reperfusion (removed ligations) (IC + RL-rats); oxidative stress as MDA (increased (IC- and IC + RL-rats)) and NO levels (decreased (IC-rats); increased (IC + RL-rats)) in colon tissue. IC + OB-rats [IC-rats had additional colon obstruction (OB)] for 3 d (IC + OB-rats), then received BPC 157 bath.
Commonly, in colon segment (25 mm, 2 ligations on left colic artery and vein, 3 arcade vessels within ligated segment), in IC-, IC + RL-, IC + OB-rats, BPC 157 (10 μg/kg) bath (1 mL/rat) increased vessel presentation, inside/outside arcade interconnections quickly reappeared, mucosal folds were preserved and the pale areas were small and markedly reduced. BPC 157 counteracted worsening effects induced by L-NAME (5 mg) and L-arginine (100 mg). MDA- and NO-levels were normal in BPC 157 treated IC-rats and IC + RL-rats. In addition, on day 10, BPC 157-treated IC + OB-rats presented almost completely spared mucosa with very small pale areas and no gross mucosal defects; the treated colon segment was of normal diameter, and only small adhesions were present.
BPC 157 is a fundamental treatment that quickly restores blood supply to the ischemically injured area and rapidly activates collaterals. This effect involves the NO system.
Core tip: We rescued rat ischemic colitis. The gastric pentadecapeptide BPC 157, which has been used in clinical trials for ulcerative colitis, exerted rapid cytoprotective endothelium rescue against the disabled left colic artery and vein after blood deprivation via two ligations and during reperfusion (ligations removed). By bypassing obstructions, quickly rescuing blood supply, rapidly activating collaterals, and restoring arcade interconnections, as a new integrative beneficial effect, BPC 157 prevented the occurrence of pale lesions without mucosal folds and normalized the levels of NO and MDA, two oxidative stress markers, in tissues. BPC 157 showed effectiveness over the NO-system background, immobilized (L-NAME + L-arginine), (over)stimulated (L-arginine) or blocked (L-NAME). Likewise, later application of BPC 157 in a bath treatment to rats with pertinently obstructed vessels that underwent additional colon obstruction for three days produced a similar beneficial effect.
- Citation: Duzel A, Vlainic J, Antunovic M, Malekinusic D, Vrdoljak B, Samara M, Gojkovic S, Krezic I, Vidovic T, Bilic Z, Knezevic M, Sever M, Lojo N, Kokot A, Kolovrat M, Drmic D, Vukojevic J, Kralj T, Kasnik K, Siroglavic M, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J Gastroenterol 2017; 23(48): 8465-8488
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8465
The colon is particularly susceptible to insufficient vascular perfusion[1]. In this work, we focused on the prototype cytoprotective anti-ulcer peptide stable gastric pentadecapeptide BPC 157, which has been used in trials for ulcerative colitis and now for multiple sclerosis, in the treatment of colitis and ischemia in rats[2-8], seeking new insights into ischemic colitis (IC), ischemia and reperfusion and therapy. The harmful events were on the left colic artery and vein, such as two ligations (IC rats) or injuries from removed ligations (RL) (IC + RL rats) (there was always gross vessel presentation failure), and the combination of two obstructions, ligation of the vessels and additional colon obstruction (OB) (IC + OB rats). IC was assessed at short (minutes) (IC rats, IC + RL rats) or more prolonged (3- and 10-d) intervals (IC + OB rats).
The main focus of the intervention was that BPC 157 rapidly activates collaterals due to its particular direct and rapid effect on vessel presentation, the bypassing of one or more of the vascular obstructions and thereby achieving a therapeutic effect.
The next focus was on NO-system, the effect of NO system agents in IC rats, NOS blocker L-NAME and/or NOS substrate, L-arginine[2-8]; in colon tissue, assessing NO levels and oxidative stress (MDA levels) (as result of the lysis of endothelial cells[9,10]) and ischemia/reperfusion injury, both as a spontaneous course [when blood supply was deprived via ligation (IC rats)] and a more exaggerated course (after ligation removal (IC + RL rats)) in immediate post-ligation time. Notably, although the NO system is largely implicated in stomach cytoprotection and colitis lesions[2-4], the application of L-NAME (a vasoconstrictor)[11] and/or L-arginine (a vasodilator)[11] has not been investigated with respect to the immediate presentation of the blood vessels after a segment of left colic artery and vein was occluded by two ligations. By contrast, BPC 157 largely interacts with the NO system in various models and species, as shown in cytoprotection studies, in particular, studies using both L-NAME and L-arginine as individual agents or in combination[2-4].
The shared essential effect of cytoprotective agents, which was originally noted in the stomach, is regarded as a rapid endothelial protective mechanism that can be used to prevent and resolve adjacent ischemic mucosal lesions[12-16]. It may be possible to extend the rapidly occurring beneficial cytoprotective effect of these agents and to use them to prevent ischemic colitis lesions, in which specific activation of the collateral circulation can circumvent obstructions and reestablish the continuity of blood flow. This effect must be a long-lasting effect that is exerted within the immediate post-injury time period (a factor that has not investigated in vascular studies of ischemic colitis[17-19]); it must also be applicable in the later period, even after a considerable period of additional colon obstruction has occurred.
Unlike standard cytoprotective agents that exhibit only prophylactic effectiveness (shared limitation of activity)[9,10,12,13,15,16,20], BPC 157 represents a prototype of a more effective class of cytoprotective agents with both prophylactic and therapeutic ability[7,16]. BPC 157, as a novel mediator of Robert’s cytoprotection[2-8,20], is native and stable in human gastric juice and maintains gastrointestinal mucosal integrity[2-8]. BPC 157 additionally maintains the gross presentation of stomach blood vessels under harmful conditions[21,22] when these vessels would otherwise disappear. In addition to its beneficial effect, BPC 157 counteracts the vessels’ disappearance[21,22]. Furthermore, this additional rapid cytoprotective vascular recovery and presentation leads to a consequent strong angiogenic effect in subsequent days[2,22-28]. Its angiogenic response[2,22-28] in combination with its healing effects and its interaction with several molecular pathways[11,25-29] is more profound than the angiogenesis of standard anti-ulcer agents[23].
Previously, BPC 157 also counteracts colitis (in various models of colitis[30-35]) and its complications, such as fistulas[36-38], failed healing of anastomoses[30,34,38-40] and other gastrointestinal lesions that were otherwise poorly healed[2-8], given parenterally or per-orally. For further clarification and to show a direct beneficial effect, medication was given once as a bath to the segment of left colic artery and vein obstructed by two ligations. Consequently, beneficial effects can be directly related to the reversal of obstructive injury outcome consequences, in both early (IC rats; IC + RL rats) and later (IC + OB rats) periods, and may be triggered shortly after injury initiation [IC rats (vessels ligation)]; IC + RL rats (ligations removed, exaggerated reperfusion)) or later, with an already-advanced injury course (IC+OB rats). Then, to focus on the initial ligation-course (blood vessel presentation), pentadecapeptide BPC 157, L-NAME, L-arginine were given to in IC rats alone and/or in combination. Alternatively, BPC 157 was given post-ligation. At these points, colon tissue levels of MDA and NO were assessed, and results showed NO colon tissue levels and oxidative stress (MDA) and ischemia/reperfusion injury during the ligation course; these effects were even worse after ligation removal and post-ligation. Likewise, as a therapeutic effect that is also applicable in the later period, BPC 157 was given to the rats with ligated left colic artery and vein, and they underwent additional colon obstruction for three days (IC + OB rats).
Male Albino Wistar rats, 200 g b.w., were used in the experiments. The animals were randomly assigned to groups of at least 6 rats per group. The experiments were approved by the local ethics committee. The surgical procedure was performed in rats that had food and water ad libitum before the procedure and until the end of the experiment, and assessments were performed by an observer unaware of the treatments used.
The pentadecapeptide Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val, M.W. 1419, named BPC 157, which is a part of the sequence of human gastric juice protein, coded BPC, freely soluble in water at pH 7.0 and in saline, was prepared (Diagen, Ljubljana, Slovenia) as described before[2-7,34]. L-NAME and L-arginine were commercially purchased (Sigma, United States).
The purpose of the experiments was to counteract both immediate and late consequences of vessel ligation injury in blood-deprived colon segments and to produce and assess rapid cytoprotective recovery.
Initial assessment in rats with two ligations at the left colic artery and vein for 15 min (IC rats) and in rats that had two ligations at the left colic artery and vein for 15 min, and then were reperfused for the next 15 min (IC + RL rats)
The surgery was conducted in deeply anesthetized rats. A segment of the left colic artery and vein was occluded by 2 ligations (Premilene 7/0, Braun), 3 arcade vessels within the ligated segment, the 25-mm blood-flow-deprived descending colon segment. Medication (/kg, 1 mL bath/rat) applied to the 25-mm blood-flow-deprived colon segment, included BPC 157 (10 μg), the NOS blocker L-NAME (5 mg), the NOS substrate L-arginine (100 mg) alone or in combination or a saline bath of equal volume (control) at 1 min of ligation time. In rats that received two ligations of the left colic artery and vein for 15 min, the ligations were removed, and the area was then reperfused for next 15 min (IC + RL rats), medication at that colon segment during reperfusion included BPC 157 (10 μg) and saline bath of equal volume (control) at 1 min of reperfusion time.
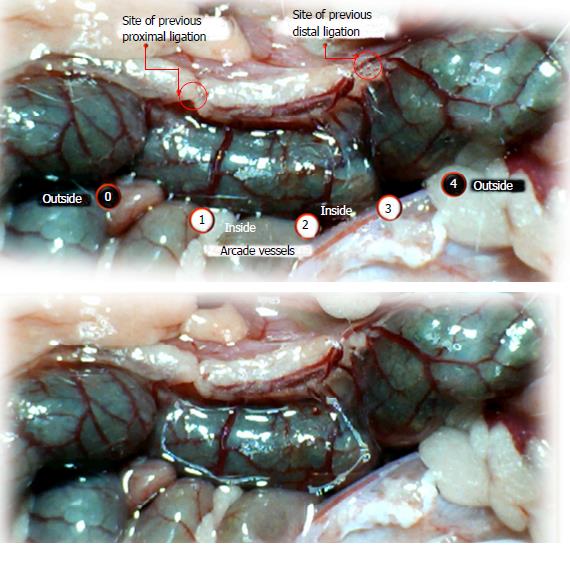
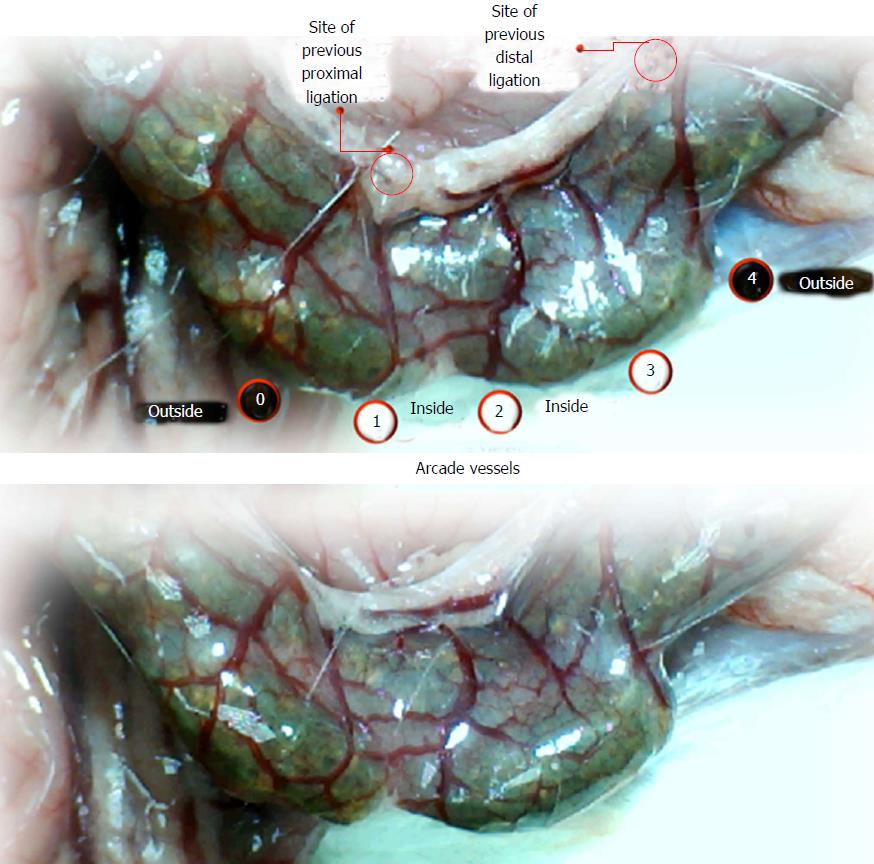
Using a camera attached to a USB microscope (Veho discovery VMS-004 deluxe), we recorded the vessel presentation (fulfilled/appearance or cleared out/disappearance) between the arcade vessels on the ventral and dorsal sides throughout the following 15 min [with regard to the point immediately before therapy (as 100%)] at selected time points before and after therapy [see the assessment shown in Figure 1 (IC rats)]. The extent of pale areas without mucosal folds was recorded upon colon opening and after sacrifice as % of total area of the deprived colon segment (IC rats) or as % of total area of the reperfused colon segment (IC + RL rats). Oxidative stress was assessed by quantifying thiobarbituric acid (TBA) reactivity as malondialdehyde (MDA) equivalents and by determining the NO levels in the colon tissue. Representative tissue sections were processed for further histological analysis as described previously[2-7,34].
At the end of the experiment and at 15 min of ligation time or at 15 min reperfusion time, oxidative stress in the collected tissue samples was assessed by quantifying thiobarbituric acid-reactive species (TBARS) as malondialdehyde (MDA) equivalents. The tissue samples were homogenized in PBS (pH 7.4) containing 0.1 mmol/L butylated hydroxytoluene (BHT) (TissueRuptor, Qiagen, United States) and sonicated for 30 s in an ice bath (Ultrasonic bath, Branson, United States). Trichloroacetic acid (TCA, 10%) was added to the homogenate, the mixture was centrifuged at 3000 rpm for 5 min, and the supernatant was collected. Then, 1% TBA was added, and the samples were boiled (95 °C, 60 min). The tubes were then kept on ice for 10 min. Following centrifugation (14000 rpm, 10 min), the absorbance of the mixture at the wavelength of 532 nm was determined. The concentration of MDA was read from a standard calibration curve plotted using 1,1,3,3’-tetraethoxy propane (TEP). The extent of lipid peroxidation was expressed as MDA using a molar extinction coefficient for MDA of 1.56 × 105 mol/L/cm. The protein concentration was determined using a commercial kit. The results are expressed in nmol per mg of protein.
At the end of the experiment and at 15 min ligation time or at 15 min reperfusion time, we determined the NO levels in colon tissue samples using the Griess reaction (Griess Reagent System, Promega, United States). Sulfanilamide was added to the homogenized tissue, the mixture was incubated, and N-1-naphthylethylenediamine dihydrochloride was added. The Griess reaction is based on the diazotization reaction in which acidified nitrite reacts with diazonium ions and, in a further step, is coupled to N-1-naphthylethylenediamine dihydrochloride, forming a chromophoric azo derivative. Absorbance was measured at 540 nm, using sodium nitrite solution as a standard. NO levels are reported in μmol/mg protein. Protein concentrations were determined using a commercial kit (BioRad Protein DR Assay Reagent Kit, United States).
In deeply anesthetized rats, in addition to the left colic artery and vein obstruction by two ligations performed as described above, which resulted in a 25-mm blood-deprived colon segment, an additional colon obstruction was made close to the distal ligation using a plastic ring 4 mm in diameter. The same BPC 157 protocol (/kg, 1 mL bath/rat) was applied at the 25-mm blood-flow-deprived colon segment after the adhesions were gently removed. BPC 157 (10 μg) or a saline bath of equal volume for the controls was applied on day 3 immediately after the ring was removed. Using a USB camera (Veho discovery VMS-004 deluxe) attached to a microscope, we recorded the vessel presentation for the 15 min immediately following treatment, as described above. In a separate group of rats, the colon was opened and the extent of pale areas without mucosal folds was recorded as described previously. At day 7 after ring removal and bath-therapy application, the deprived colon segment diameter, the colon was opened, and pale areas without mucosal folds were assessed as % of total area of the deprived colon segment and number of gross mucosal defects. Representative tissue sections were processed for further histological analysis as described previously[2-7,34]. The severity of adhesions was described as previously[34,37,39,40] and scored on a scale of 0 to 2 (0-no adhesion; 1 - fatty tissue adhering to colon; 2 - small intestine adhering to colon).
Statistical analysis was performed by parametric one-way ANOVA with post hoc Newman-Keuls test and non-parametric Kruskal-Wallis and subsequent Mann-Whitney U-test to compare groups. Values are presented as the mean ± SD and as the minimum/median/maximum. P < 0.05 was considered statistically significant.
We investigated the maintenance of vessel function upon the first innate reperfusion (i.e., during the initial recovery after blood supply was interrupted by the placement of two ligations on the left colic artery and vein (Figure 1) in IC rats. This method was also used much later in animals that had been subjected to the additional bowel obstruction (IC + OB rats) and, subsequently, upon massive reperfusion occurring after the removal of the vascular obstruction(s) (IC + RL rats).
In these experiments, ischemic colitis therapy was administered once at early ligation time (IC rats) or alternatively at the early post-ligation time (IC + RL rats). In other experiments, the therapy was administered once at a later ligation time after additional colon obstruction (IC + OB rats).
In general, we obtained results that showed the presence of increasing/decreasing collateralization between the arcade vessels inside and outside ligations or previous ligations. Subsequently, we demonstrated a markedly attenuated course of ischemic colitis in BPC 157-treated rats, IC rats, IC + RL rats, and IC + OB rats; conversely, an aggravated course of ischemic colitis was observed in L-NAME-treated rats, L-arginine-treated rats, and in IC rats (Tables 1-3 and Figures 1-25).
| Medication (/kg, 1-ml bath/rat) at the 25-mm blood-flow-deprived colon segment | Pale areas without mucosal folds as a percentage of the area of the blood-deprived colon segment, mean ± SD |
| 0.9% NaCl (control) | 46 ± 8 |
| BPC 157 (10 µg) | 7 ± 2a |
| L-NAME (5 mg) | 80 ± 10a |
| L-arginine (100 mg) | 65 ± 6a |
| L-NAME (5 mg) + L-arginine (100 mg) | 50 ± 8 |
| L-NAME (5 mg) + BPC 157 (10 µg) | 10 ± 4a |
| L-arginine (100 mg) + BPC 157 (10 µg) | 6 ± 2a |
| L-NAME (5 mg) + L-arginine (100 mg) + BPC 157 (10 µg) | 8 ± 2a |
| Medication (/kg, 1 ml bath/rat) applied to the 25-mm blood-flow-deprived and then reperfused colon segment | Pale areas without mucosal folds as a percentage of the area of the blood-flow-deprived and then reperfused colon segment (mean ± SD) |
| 0.9% NaCl (control) | 86 ± 8 |
| BPC 157 (10 µg) | 10 ± 2a |
| Medication (/kg, 1 ml bath/rat) at the 25-mm blood-flow- deprived colon segment | Pale areas without mucosal folds as a percentage of the entire area of the blood-deprived colon segment means ± SD | Number of gross mucosal defects,mean ± SD | Colon diameter,mean ± SD | Adhesion severityscored 0-2,Min/Med/Max |
| 0.9% NaCl (control) | 125 ± 10 | 4 ± 1 | 36 ± 8 | 2/2/2002 |
| BPC 157 (10 µg) | 10 ± 2a | 0 ± 0a | 9 ± 1a | 0/1/1a |
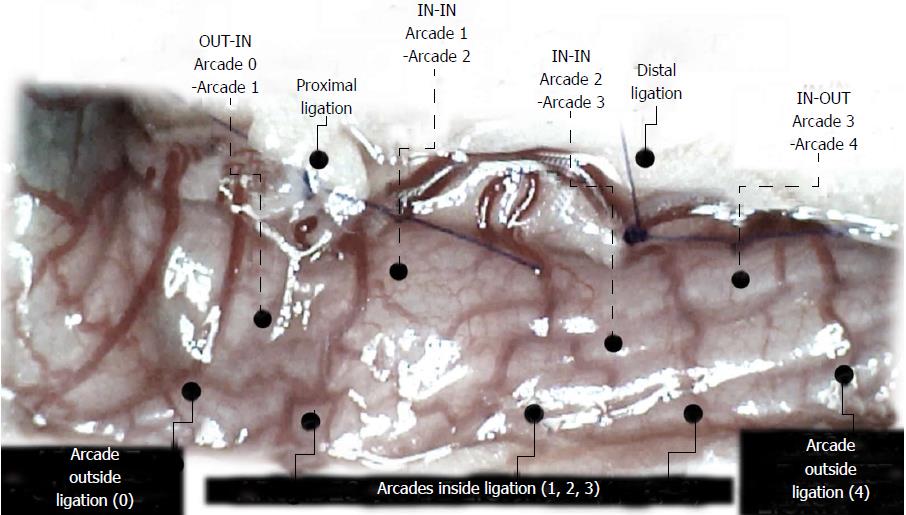
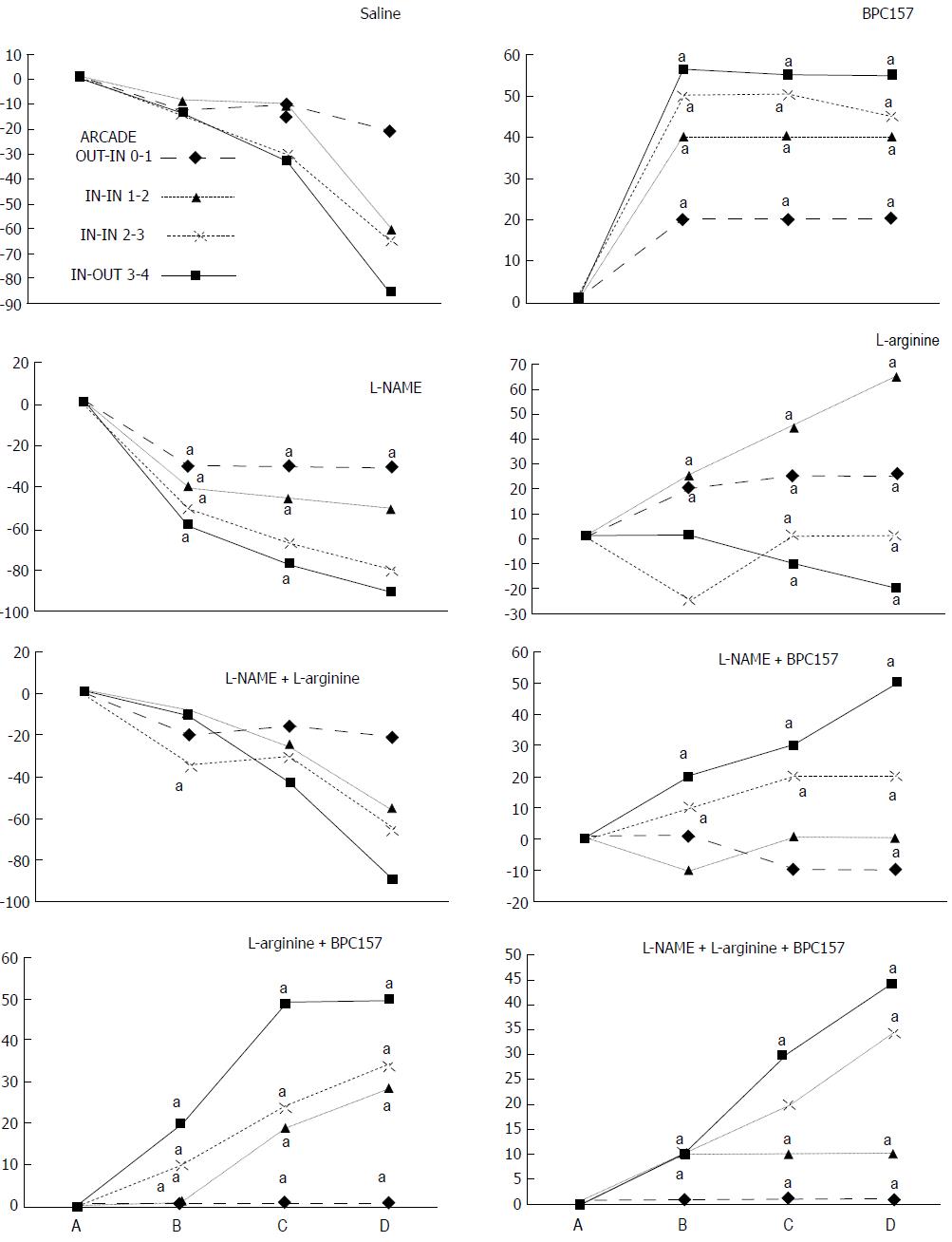
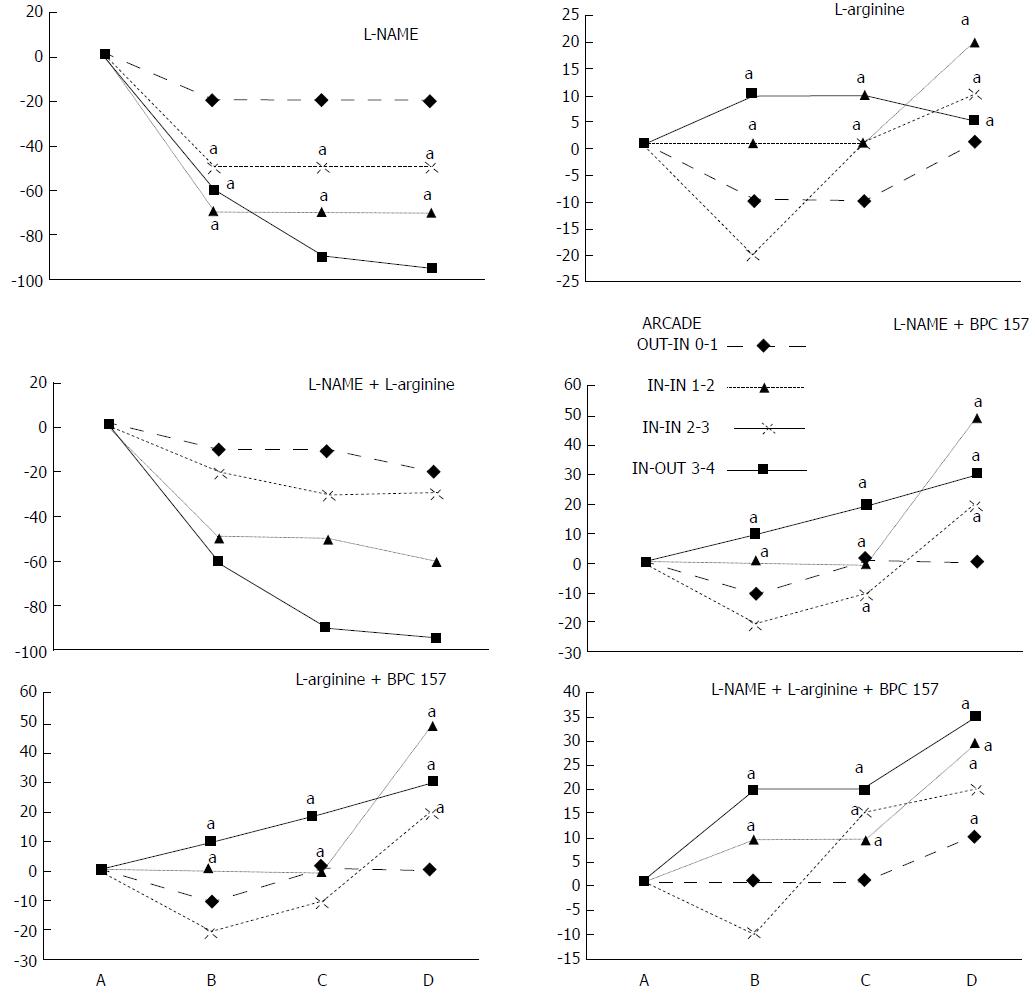
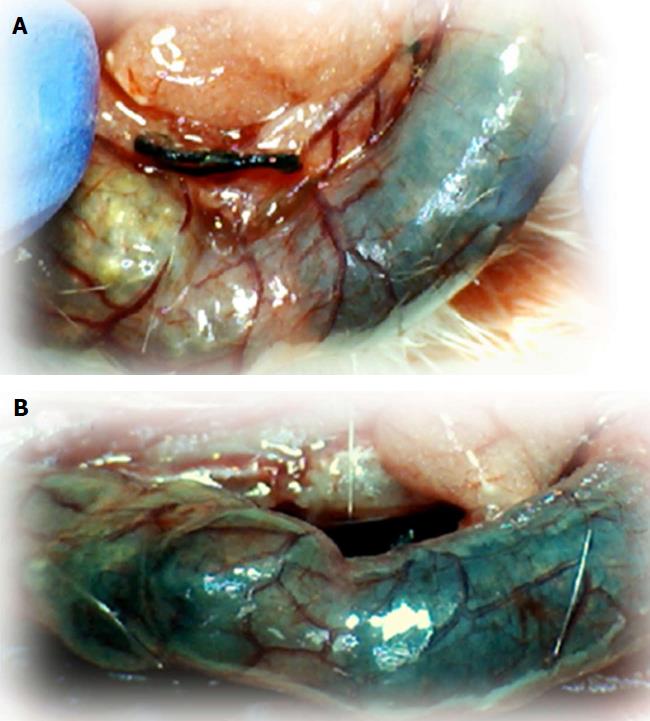
We focused on the 25-mm blood-deprived segment of the descending colon. Within this ligated area, there were three major arcades. Thus, it was possible for both the proximal and distal obstructions to be bypassed by an additional vessel network running between the arcades (outside-inside ligation, outside-inside, inside-inside and inside-outside) at both the ventral and dorsal sides of the colon. The particular arcade vessel presentation at specific subsequent time points [A (1 min before therapy), B (the first 5 min following therapy), C (the next 5 min, until the 10th min), D (until the end of the 15th min)] demonstrates how the obstructions in the tissues were bypassed. However, the alternative arcade pathway operated poorly at best in the blood-deprived colon segment. Nevertheless, the alternative arcade pathway was found to respond quickly to therapy with BPC 157 vs L-NAME and L-arginine.
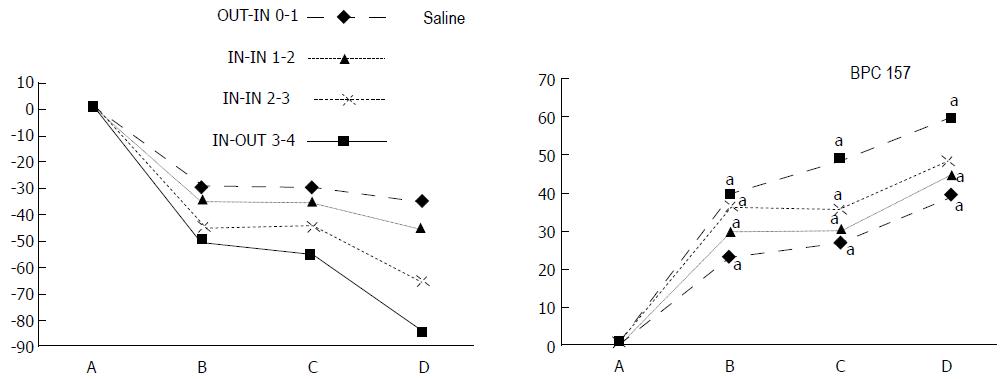
The appearance of the vessels was assessed (see also Figure 1) between (1) the last arcade proximal to first ligation (arcade 0) and the first arcade distal to the ligation (or to the previous ligation) (arcade 1) (0-1, Figure 2, Figures 5-8, and Figures 21 and 22); (2) from arcade 1 to the next arcade (arcade 2), middle arcade inside ligations (or previous ligation) (1-2, Figures 2, 5-8, 21 and 22); (3) from arcade 2 to the next arcade (arcade 3) to the last arcade inside the ligations (or the previous ligation) (2-3, Figures 2, 5-8, 21 and 22); and finally, (4) from the first arcade distal to the second ligation (or the previous ligation) (arcade 4) (3-4, Figures 2, 5-8, 21 and 22).
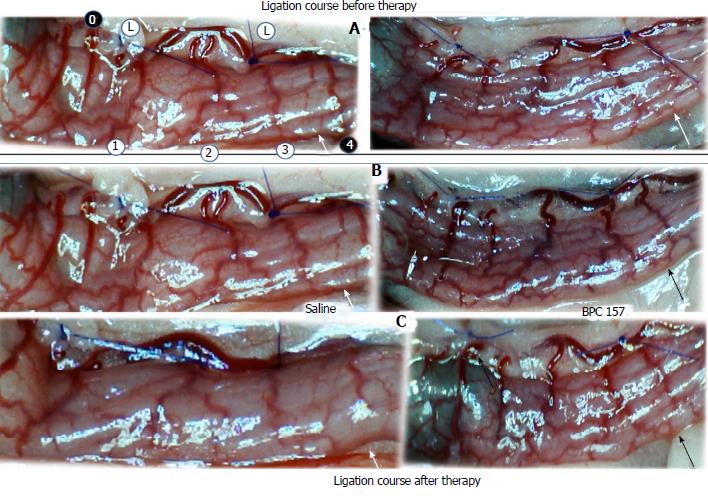
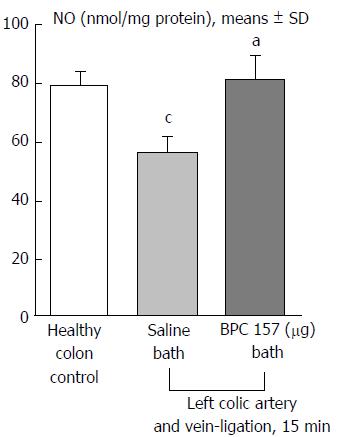
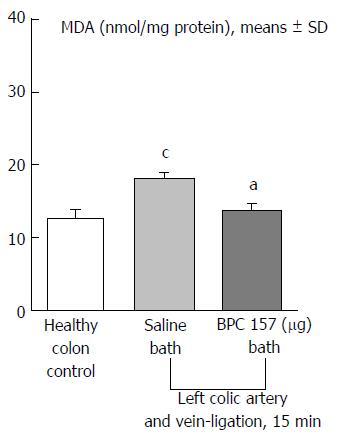
Regular course (saline bath, control): Frequently, once major vessels were obstructed, the alternative arcade only partially appeared in the blood-deprived colon segment (Figures 2, 3, 5 and 6). After a saline bath was applied to the colon segment that had been deprived of its blood supply, arcade vessels gradually disappeared from the proximal to the distal arcades. Loose connections between inside-outside blood vessels were quickly seen at the dorsal side. As a result, there was a rapid appearance of pale flat areas and a rapid disappearance of mucosal folds (Table 1 and Figures 2-4). This dorsal/ventral side distinction may be related to the relatively scarce dorsal side arcade collateral network; thus, once the final connection disappeared, it could not be easily reestablished (Figures 2 and 5). By contrast, at the ventral side, which displayed a much more abundant collateral network, despite a considerable decrease in inside-inside contact, inside-outside contact persisted (Figures 3 and 6). Along with the appearance of a considerable number of endothelial lesions following ligation, an initial blood flow deprivation and a presumably inadequate response, decreased NO levels (Figure 7) and increased MDA levels (Figure 8) were observed in the colon tissue.
BPC 157 bath: By contrast, blood vessels propagated toward the injury obstruction, bypassing it, interconnecting collaterals between arcades (Figures 2, 3, 5 and 6), recovering blood flow and thereby attenuated/counteracted ischemia/reperfusion injury[9,10]. Mucosal folds were present, and there were markedly fewer pale areas (Table 1, Figure 4). In particular, these effects all appeared in conjunction with BPC 157 application.
Notably, in animals that received the BPC 157 bath, vessel presentation was markedly increased at the re-established inside-outside connective point in particular (arcade 3-arcade 4) (Figures 2, 3, 5 and 6); in this way, blood flow was quickly restored. In support of this finding, increased MDA levels and decreased NO levels in colon tissue were found to be normal in rats that received BPC 157 bath application (Figures 13 and 14).
NO agents, L-NAME bath, L-arginine bath: After the application of NO agents, particular responses were observed; however, neither of the responses to the agents was effective with respect to the extent of the pale areas (Table 1, Figures 3, 5 and 6).
The application of L-NAME appeared to worsen the already debilitated vascular response. A larger proportion of the blood vessel network disappeared, in particular at the dorsal side (Figures 3, 5 and 6). Consequently, the pale areas and areas of mucosal fold disappearance were markedly larger (Table 1).
L-arginine increased vessel presentation but did not re-establish the broken inside-outside connection (Figures 5 and 6). Pale areas and mucosal fold disappearance were again increased (Table 1).
Combination studies, L-NAME + L-arginine bath; BPC 157 + L-NAME bath; BPC 157 + L-arginine bath; BPC 157 + L-NAME + L-arginine bath: In combination, we have mutual counteraction (L-NAME + L-arginine) or presentation similar to BPC 157-treated rats (BPC 157 + L-NAME; BPC 157 + L-arginine; BPC 157 + L-NAME + L-arginine) (Table 1, Figures 3, 5 and 6).
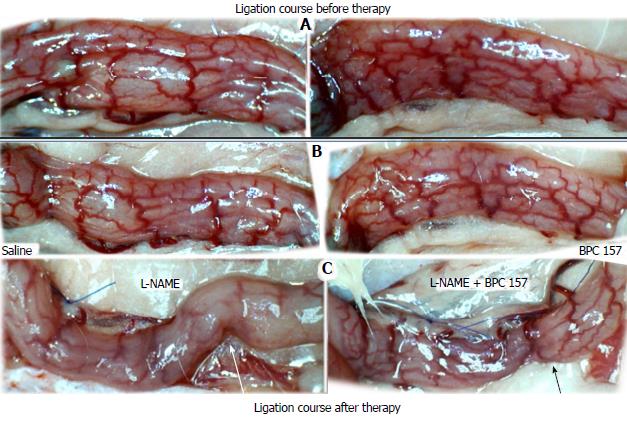
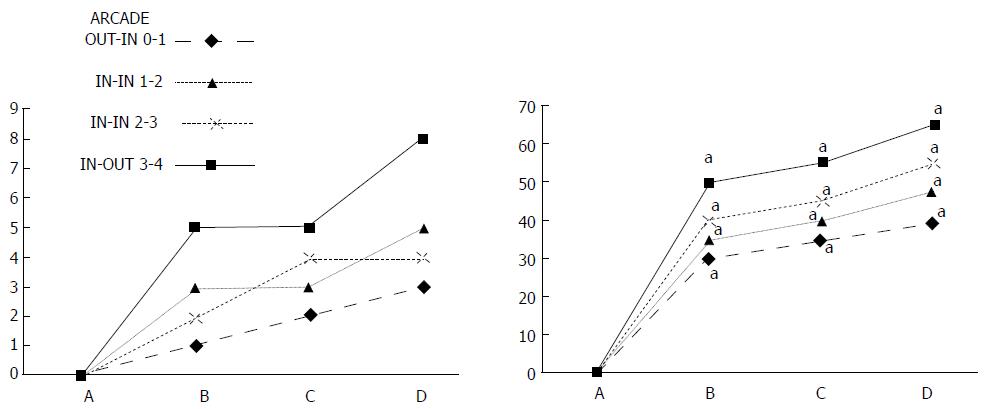
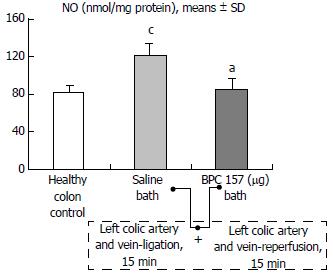
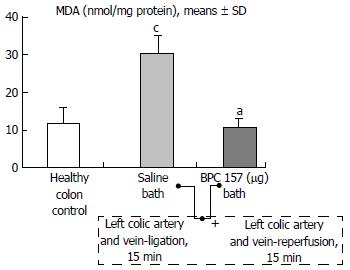
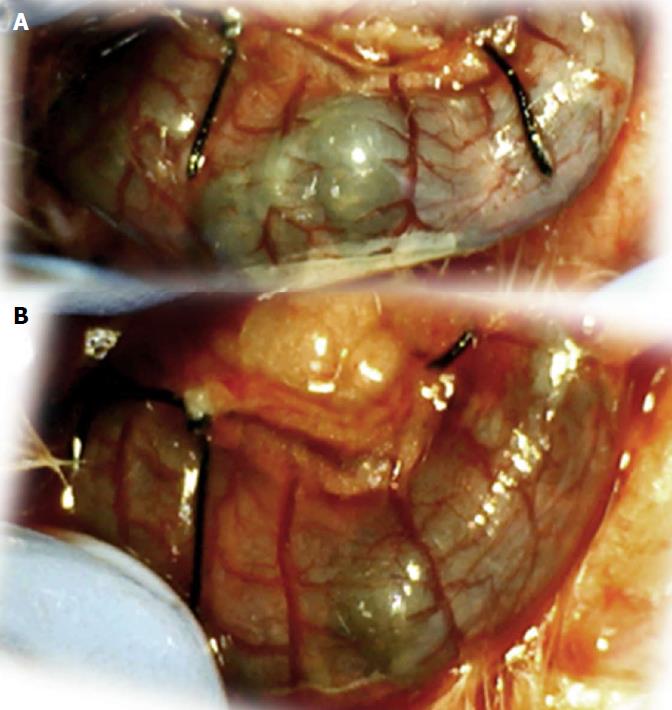
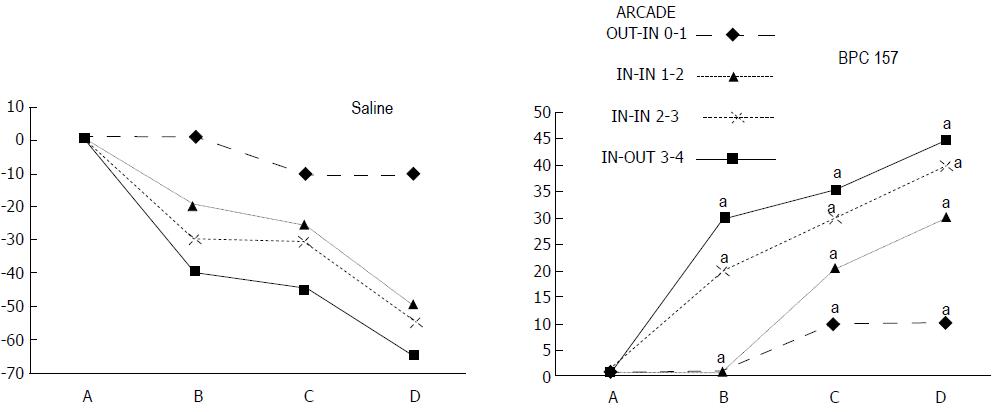
Regular course (saline bath, control): After a saline bath was applied to the reperfused colon segment, the arcade vessels were poorly established from proximal to distal arcades. The connections between inside-inside and inside-outside blood vessels that had been lost were poorly reestablished (Figures 7-9 and 11). As a result, there was an additional rapid appearance of pale flat areas and a disappearance of mucosal folds (Table 2, Figure 11).
BPC 157 bath: The application of BPC 157 after the initiation of full reperfusion with both ligations removed resulted in increased vessel presentation and arcade interconnections; the mucosal folds were recovered, and the pale areas were small and markedly reduced in area (Figures 7, 8, 10 and 12). Consistently, the otherwise increased MDA and NO levels in colon tissue were found to be normal in rats that received BPC 157 bath treatment (Figures 15 and 16).
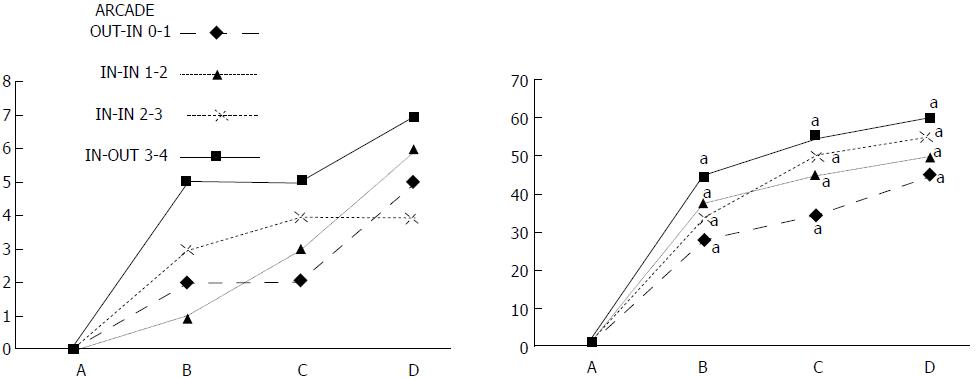
In these experiments, treatments were administered to blood-deprived colon segments that had additionally been challenged with ring obstruction. Notably, ligation of the left colic artery and vein caused extensive damage, and additional obstruction of the colon for three days led to even more severely disabled rats in which an extremely large pale flat area without mucosal folds occupied the entire ligated segment; thus, an advanced level of disability was present before therapy could be applied (Figure 19). If not reversed, this procedure consistently results in a perilous outcome (Table 3, Figures 17 and 19-22). We used the same BPC 157 protocol that was shown above to be effective when applied to the 25-mm blood-flow-deprived colon segment shortly after blood vessel obstruction by two ligations. We demonstrated that the BPC 157 treatment protocol was also efficacious when it was applied on day 3, immediately after the constricting ring was removed (Table 3, Figures 18 and 20-22), as shown by its subsequent rapid effect on the appearance of the arcade vessels. An extended beneficial effect was also apparent one week later, indicating rescue from ischemic colitis.
Immediate effect (day 3): Whereas the controls exhibited an increasing disappearance of arcade vessels, particularly at the dorsal side of the colon (Figures 9, 13 and 14), after BPC 157 bath treatment the arcade vessels vigorously reappeared together with interconnected collaterals between arcades, in particular, quickly rescuing blood flow (a finding especially seen at the dorsal side of the colon) (Figures 18, 21 and 22).
Final effect (day 10): On day 10, the blood-deprived colon segment contained extremely large pale areas without mucosal folds that occupied the entire area between the ligations (Table 3, Figure 20). These pale areas were present even outside the area that was ligated, and many gross mucosal defects were present in the enlarged colon segment (Table 2, Figure 20). Adhesions to the small intestine were commonly noted (Table 3). By contrast, the areas that had been treated with BPC 157 presented almost completely spared mucosa with very small pale areas and no gross mucosal defects, a colon segment of normal diameter, and few adhesions (Table 3, Figure 20).
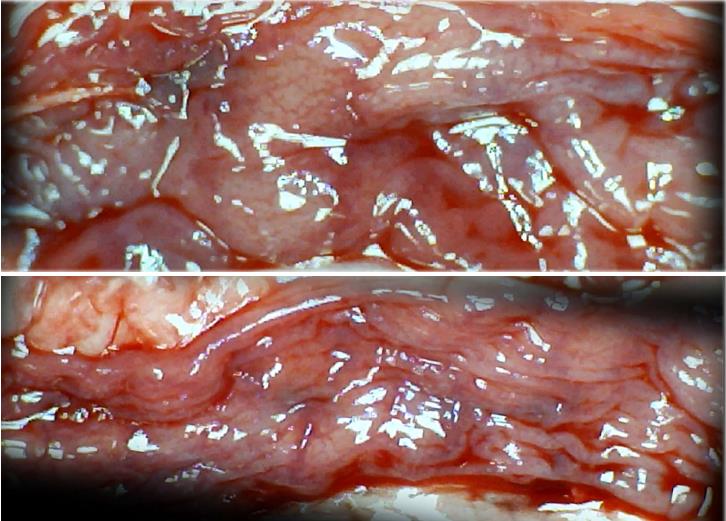
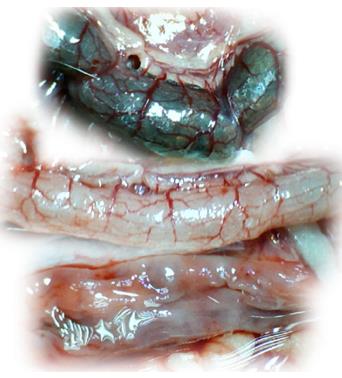
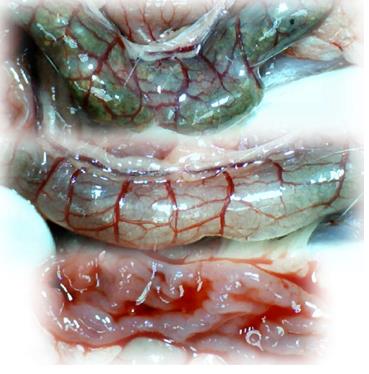
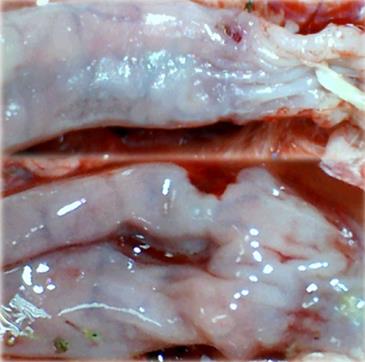
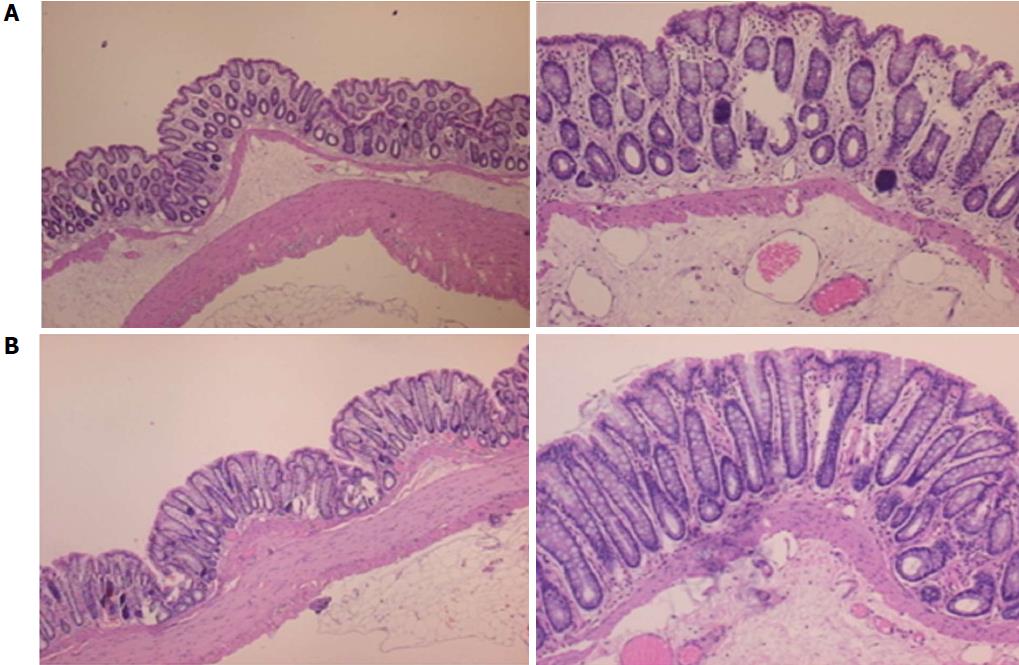
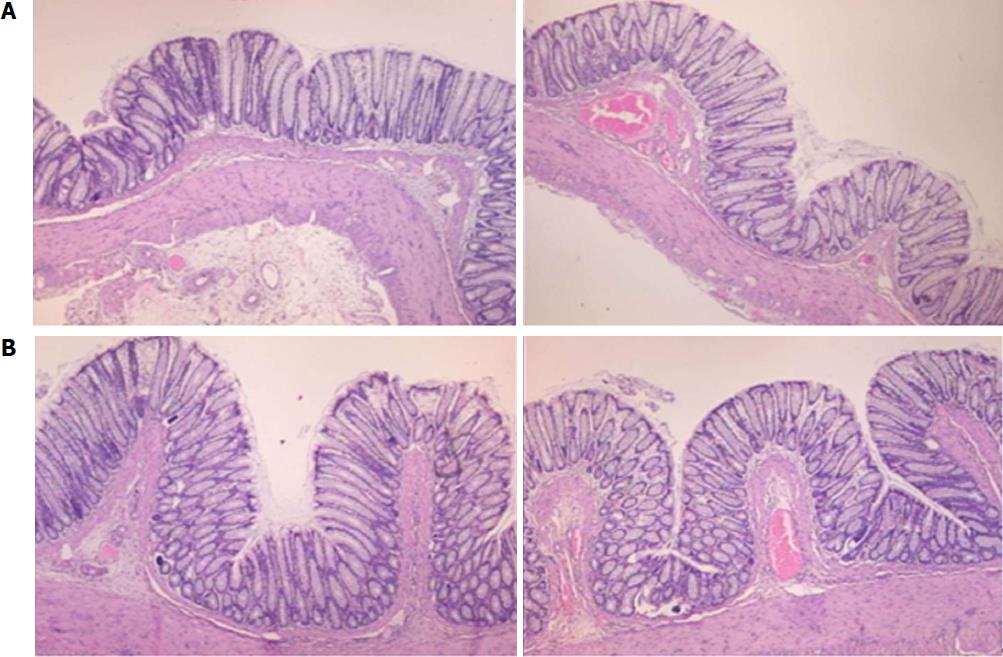
Microscopically, at 15 min after the beginning of ligation in IC rats, there was failed vascular presentation, with gross pale flat areas and the disappearance of mucosal folds (Figure 2). Severe edema of the lamina propria and continuous diffuse edema of the submucosa was also evident (Figure 23A). In addition, there was pronounced dilatation and stasis in the submucosal blood vessels (Figure 23A). By contrast, in BPC 157-treated rats, there was recovered vascular presentation, with mucosal folds present and markedly fewer pale areas (Figure 2), mild edema of the lamina propria and focally present mild-to-intermediate edema of the submucosa (Figure 23B). Moreover, stasis of the submucosal blood vessels was present, but the dilatation of veins appeared to be less pronounced (Figure 23B).
In IC + RL rats after 15 min of ligation and 15 min of full reperfusion, there was failed vascular presentation leading to an increasing number of gross pale flat areas and the disappearance of mucosal folds (Figure 12). Microscopically, even more, mucosal and submucosal edema, as well as more pronounced cyanosis, were evident. These effects were consistently counteracted by the application of BPC 157 after reperfusion initiation (Figure 24).
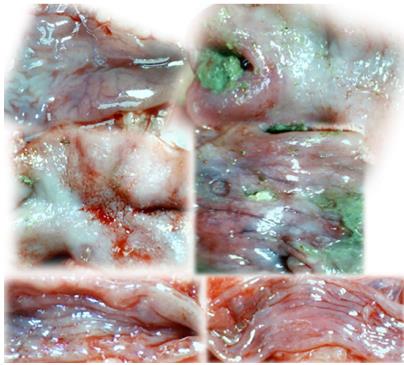
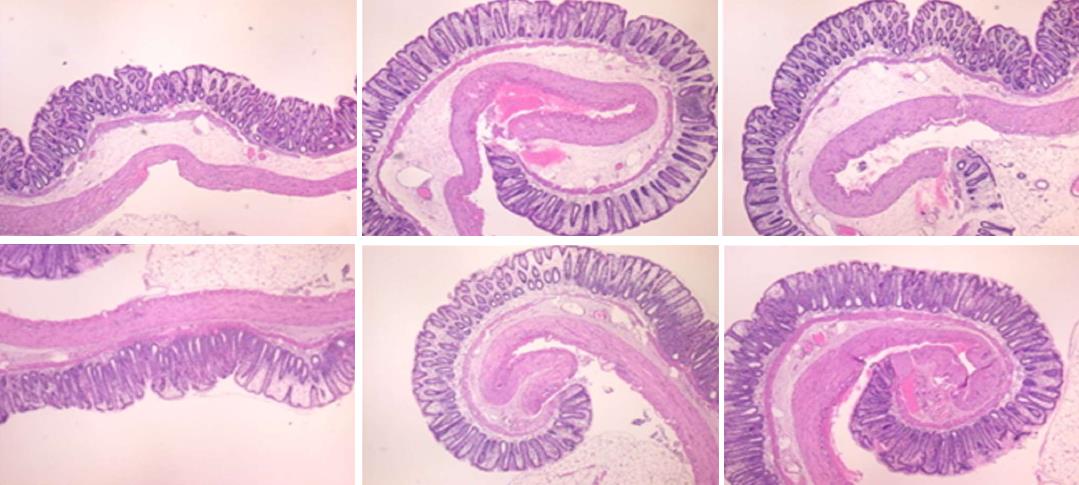
In IC + OB rats on day 3, immediately after removing the additional colon obstruction and the obstructing ring, grossly large pale flat areas without mucosal folds occupying the entire ligated segment and failed vascular presentation appeared (Table 3, Figures 17 and 20-22), with even more pronounced severe edema of the lamina propria and continuous diffuse edema of the submucosa as well as pronounced dilatation and stasis of the submucosal blood vessels. This effect was associated with mild edema of the lamina propria and diffuse mild-to-intermediate edema of the submucosa, with collagen formation, one week later on day 10 after ligation (Figure 25A). Stasis of the submucosal blood vessels was also present (Figure 25A). In these samples, the rugae are broadened and flattened, so the mucosa appears macroscopically flattened (Figure 17). By contrast, BPC 157-treated rats grossly presented almost completely spared mucosa (Figure 20), with mild edema of the lamina propria and practically no edema of the submucosa (Figure 25B). Stasis of the submucosal blood vessels was also present but to a much lesser extent than in controls. The rugae were histologically well formed, only occasionally displaying slight broadening. There was the minimal apparent formation of new collagen fibers in the submucosa (Figure 25B).
In summary, these results show that BPC 157 protects colon tissue from the endothelial damage induced by ischemia and reperfusion. The cytoprotective effect of BPC 157 against colonic ischemia/reperfusion-mediated mucosal damage is associated with the activation of the collateral circulation, which circumvents obstructed sites and results in the resolution of the obstruction, reduction of oxidative stress and normalization of NO synthesis.
We demonstrated that well-placed arcade vessels respond poorly to the increased demands that occur upon blood supply deprivation, reperfusion or additional bowel obstruction. Thus, the particular susceptibility of the colon to insufficient vascular perfusion[1] mandates that the main focus of the treatment of such conditions should be bypassing one or more of the vascular obstructions. Then, the main focus is maintaining vessel function upon the first innate reperfusion (as evidenced by an initial innate recovery while the blood supply is deprived) in IC rats [which is also applicable much later with additional bowel obstruction (IC + OB rats)], and subsequently upon massive reperfusion following the removal of vascular obstruction(s) (IC + RL rats).
Based on this reasoning, therapy for ischemic colitis was administered once at early ligation-time (IC-rats) or alternatively at early post-ligation-time (IC + RL rats). In some animals, the therapy was administered once at a later time after additional colon obstruction (IC + OB rats). BPC 157 therapy was shown to cure rat ischemic colitis in both the very early and late time points and under diverse harmful conditions (short-lasting blood deprivation (IC rats) vs reperfusion (IC + RL rats) vs long-lasting blood deprivation and additional bowel obstruction (IC + OB rats)). Obviously, such BPC 157 therapy involves analogous cytoprotection/endothelium mechanisms[2-8,12-16,20], such as the cytoprotective response that was previously highlighted in original stomach cytoprotection studies of cytoprotective agents in general and of BPC 157 specifically[12-16,20] in the immediate post-injury time period[12-16,20]. These results may underlie the aforementioned response for both ischemic and reperfusion injuries. The effects of BPC 157 may be usefully upgraded and accordingly rendered advantageous for arcade collateralization and for bypassing obstructive defects, thereby resulting in recovery throughout the course of ischemic colitis.
Thus, influencing not only the early period (which has not been validated before in studies of ischemic colitis lesions[17-19]) but also the later period should also be crucial in determining how to treat more complex ischemic colitis lesions. Interestingly, in rats with a prolonged obstruction of blood vessels that underwent additional colon obstruction due to a ring around the colon (IC + OB rats), the evidence suggests that a bath administration of BPC 157 immediately (IC rats) or shortly thereafter (IC + RL rats) produced a beneficial effect and that the effect was even greater when BPC 157 was administered later (IC + OB rats).
Notably, a post-injury course that is not reversed always presents a devastating progression. In cases in which the blood vessels disappeared with blood deprivation and did not recover with reperfusion, extremely large pale mucosal areas, flat and without folds, were found. In IC and IC + RL rats, ischemia/reperfusion injury[9,10] increased and increased dramatically, respectively, whereas the MDA oxidative stress levels progressively increased, NO tissue values decreased with ligation and increased with ligation removal.
The course of development described above is in sharp contrast to the observed course of ischemic colitis course in rats that received BPC 157 therapy. Instead of regularly failing in either experiment, the gross presentation of the blood vessels was recovered, and collateral blood vessels made interconnections that propagated toward the obstruction injury and bypassed it, rescuing blood flow. Severe edema of the lamina propria and continuous diffuse edema of the submucosa, pronounced dilatation and stasis in the submucosal blood vessels were all markedly attenuated. The animals in which BPC 157 therapy attenuated/counteracted the ischemia/reperfusion injury[9,10] showed no MDA oxidative stress and displayed normalized NO tissue values. Mucosal folds remained present (IC rats) or were recovered (IC + RL rats, IC + OB rats), and there were markedly fewer pale areas in both the short and long periods after ligation. At the end of the experiment, these animals displayed mucosa that was almost completely spared, with no ulceration, and the otherwise regular perilous outcome of extensive pale and flattened mucosal areas with severe ulcerations was avoided. In addition, microscopic examination showed only mild edema of the lamina propria and practically no edema of the submucosa; the rugae were well formed and only occasionally slightly broadened, and there was minimal formation of new collagen fibers in the submucosa. These aforementioned points, with or without therapy, would have a NO background.
Notably, for quick endothelium rescue, operating collaterals (there are rich anastomoses between individual vessels on both surfaces of the large intestine[41]) were needed for blood flow reorganization. This process occurred in rats with obstructed vessels and with vessels that used to be obstructed, after BPC 157 therapy. By contrast, in the experiments in which the course of ischemia was not reversed, decreased NO levels in the colon tissue appeared as an immediate heavy loss of endothelial cells from the vascular wall, which resulted in a lower eNOS production ability[42]. Similarly, the endothelium has general significance; failure[43] due to oxidative stress regularly appears as a result of the lysis of endothelial cells[9,10], and it may be further aggravated by additional reperfusion. Together, these reflect first a sudden decrease of colonic blood supply[1] and inadequate eNOS system function[2]. Subsequently, when the ligation is removed, the massive induced reperfusion likely shifts the tissue toward a state of noxious inducible isozyme or vascular wall and other tissue cell damage[44]. These developments can hardly be prevented spontaneously unless a specific agent such as BPC 157[2-8] is used as a therapeutic to produce a rapid and persistent recovery of vascular function.
BPC 157 was shown to restore endothelial integrity and to actually reverse most of the oxidative damage, even during reperfusion and exaggerated reperfusion. This effect was noticeable in IC rats and even more noticeable in IC + RL rats. The effect of BPC 157 is not like reperfusion injury, which must be regularly ameliorated by interventions initiated at the end of the ischemic period[45]. BPC 157 was previously shown to have a beneficial effect and to induce NO release in gastric mucosa from rat stomach tissue homogenates[46,47]. This effect occurred even under conditions in which L-arginine was not effective, a finding that could be generalized to other tissues[3,46,47]. Additionally, various free-radical-induced lesions in other organs were counteracted by BPC 157 administration[45,48,49]. The pentadecapeptide BPC 157 contains four carboxylic groups that may act as possible antioxidants; all of them could be active in the scavenger process, and if they are reactivated (by, e.g., glutathione or enzymes), the overall antioxidant activity could be very high. Additionally, BPC 157 is present in most tissues, where it can bind reactive free radicals and inactivate them at crucial positions that other antioxidants cannot reach[50].
Finally, to envisage all possibilities for the involvement of the NO system in colonic ischemic injury (opposing healing and bleeding capabilities; L-NAME opposes healing and decreases bleeding time, whereas L-arginine promotes healing and prolongs bleeding time[3,51]), we conducted a parallel investigation of the effects of NOS blockade (L-NAME) and NOS substrate (L-arginine) administration, which is an important point that should be addressed[3].
In addition to experiments in which blood flow is instantly interrupted by means of two ligations in rats that underwent interventions to produce ischemic colitis, the relationship between BPC 157 and NO has been established in various experimental models and species. The results of these studies suggest that BPC 157 might interfere with the effects of NOS blocking agent or NOS substrate application[3]. As noted in this study, the complex action of BPC 157 tends to rule out a simple effect of either the vasoconstrictor (L-NAME) or the vasodilator (L-arginine)[3] in the noted rapid presentation of blood vessels that bypass the two obstructions of major vessels and reestablishing the blood flow that was interrupted by ligation in rats which subjected to the ischemic colitis procedure. Namely, upon encountering the regular vicious course of double obstruction of vessels, as individual agents, both L-NAME and L-arginine affect the course of the lesion in a particular way, and neither appears to be completely effective. L-NAME initially caused all vessels to disappear more rapidly, thereby inducing larger pale areas, whereas L-arginine increased the number of vessels present but also induced larger pale areas (note that L-arginine, unlike BPC 157, did not produce proper collateral arcade interconnection).
This effect is likely a specific aspect of the dual role of the NO system (L-NAME vs. L-arginine vs. combination of the two) (for a review, see[3,52,53]). Each of these effects is specific (i.e., NO-related) because when they were administered together, L-NAME and L-arginine regularly attenuated or antagonized each other’s responses[3,52,53]. Likewise, the remaining serious pathology in the animals treated with L-NAME + L-arginine indicates that other system(s) (i.e., the BPC 157 system) may function along with the NO system, which was previously supposed to be inactivated by the combined action of L-NAME and L-arginine[3,52,53]. In this view, BPC 157 would be effective regardless of whether the NO system is inactivated (L-NAME + L-arginine), overstimulated (L-arginine) or blocked (L-NAME). Thus, BPC 157 may consolidate the stimulatory and inhibitory effects of the NO system to produce more effective healing (i.e., by promoting the interconnection of arcade vessels to bypass major obstructions)[3]. This rapid recruitment of existing blood vessels that would otherwise respond poorly to increased demands following ongoing harmful events may indicate that BPC 157 also affects several other molecular pathways[11,25-29], the effects which were seen at the later time point. In particular, the immediate recovery of collaterals and bypassing of the obstruction, a feature noted in the present study, and the rapid reestablishment of blood flow in both the ischemic and reperfusion conditions may be responsible for its subsequent strong angiogenic effect and its healing effects[2,22-28], which are more pronounced than those of standard anti-ulcer agents[23].
The special feature noted in the present study could be responsible, in particular, for the increased expression and internalization of VEGFR2, which is known to be essential for endothelial function, and the activation of the VEGFR2-Akt-eNOS signaling pathway[25]. Previous studies have substantiated that BPC 157 induces an acceleration of blood flow recovery and vessel number within days in rats with hind limb ischemia[23]. Other studies have demonstrated the expression of growth hormone receptor at both the mRNA and protein levels and have shown that BPC 157 regulates the phosphorylation of extracellular-signal-regulated kinases 1 and 2 (ERK1/2) as well as their downstream targets, including c-Fos, c-Jun, and Egr-1, key molecules involved in cell growth, migration, and angiogenesis[11,25-29]. BPC 157 also induced expression of the Egr-1 gene and its co-repressor gene NaB2, suggesting that it may serve as part of a feedback system[29]. Together, these findings indicate how complex the BPC 157 pathways may be, pathways that remain to be further defined.
The above considerations suggest that two pharmacologically distinct mechanisms with opposite effects on the same signaling pathway are involved in a normally unresolvable physiological failure (complete obstruction (ligation) of vessels and ischemia due to a cause that could be not removed, inadequate rescue and unable to heal). Additionally, in practice, this means that two distinct end points should both be overwhelmed to produce the effects seen in rats that received BPC 157 application[3]. Notably, BPC 157 instantly prevents and reverses L-NAME-induced hypertension as well as L-arginine-induced hypotension[46]. After amputation and/or anticoagulant application, BPC 157 counteracts prolonged bleeding and thrombocytopenia; it also counteracts the side effects of both L-NAME-(thrombocytopenia) and L-arginine (prolonged bleeding, thrombocytopenia)[51]. Additionally, in pupil dilation assays, a common point of action of L-NAME/L-arginine was noted (in miosis, both L-NAME and L-arginine antagonized the response to the other); this effect can be explained by an interaction with the cholinergic system[54]. BPC 157 counteracts the actions of both L-NAME (miosis) and L-arginine (miosis); interestingly, BPC 157 counteracts atropine-induced mydriasis as well[54].
The pentadecapeptide BPC 157 was also shown to counteract peritonitis and adhesion formation and to have a beneficial effect in diverse intestinal lesion models[30,36-40]. This particular activity is supported by the variety of beneficial effects of BPC 157 noted in colitis lesion studies[2-7]. A likely generalization follows the association of models of chemically induced inflammation (trinitrobenzenesulfonic acid and dextran sodium sulfate) with a marked reduction in blood flow[17-19]. A further generalization may be that BPC 157 effectiveness over the background of the NO-system[3,46,51,54], immobilized (L-NAME + L-arginine), (over)stimulated (L-arginine) or blocked (L-NAME), BPC 157 prevented MDA oxidative stress and normalized the NO tissue values. In addition to its use in ulcerative colitis clinical trials[2-7] and its effectiveness in various models of colitis[30-35], BPC 157 reduces high myeloperoxidase (MPO) activity in colonic tissue[33], heals fistulas[36-38], rescues failed anastomosis healing[30,34,39,40] and markedly improves intestinal adaptation following massive bowel resection[40]. It is likely that the particular molecular pathways[11,25-29] that have been suggested to be involved in the healing effects of BPC 157, as well as its recently demonstrated ability to cause the increased expression and internalization of VEGFR2 and the activation of the VEGFR2-Akt-eNOS signaling pathway[25], contribute to its action.
Finally, the aforementioned rapid and successful recruitment of the blood vessels during harmful events suggests that the application of BPC 157 may offer a fundamental treatment by providing the cytoprotection/endothelium protection that is essential[2-8,12-16,20] to quickly restore blood supply to the ischemically injured area and rapidly activate collaterals during various harmful conditions such as vascular obstruction, short-lasting blood deprivation, reperfusion, long-lasting blood deprivation and additional bowel obstruction.
Stable gastric pentadecapeptide BPC 157 would provide new insights in the treatment of colitis and ischemia and reperfusion. Likely, gastric cytoprotection and ischemic colitis (IC) lesions may have analogous therapy. Thereby, prototype cytoprotective agent gastric pentadecapeptide BPC 157, which has been used in trials for ulcerative colitis and now for multiple sclerosis, would rescue IC lesions in rats (IC rats). We studied the cytoprotective mechanism of BPC 157 because cytoprotection and cytoprotective agents restore the integrity of damaged stomach epithelium by rapidly rescuing damaged endothelium. This effect would involve the NO system. BPC 157 would be subsequently shown to be effective in IC rats underwent full reperfusion (removed ligations (RL) (IC + RL rats) and IC rats with additional colon obstruction (OB) (IC + OB rats).
The main focus of the intervention was that BPC 157 rapidly activates collaterals due to its particular direct and rapid effect on vessel presentation, the bypassing of one or more of the vascular obstructions and thereby achieving a therapeutic effect.
The next focus was on NO-system, the effect of NO system agents in IC rats, NOS blocker L-NAME and/or NOS substrate, L-arginine; in colon tissue, assessing NO levels and oxidative stress (MDA levels) (as result of the lysis of endothelial cells) and ischemia/reperfusion injury, both as a spontaneous course [when blood supply was deprived via ligation (IC rats)] and a more exaggerated course [after ligation removal (IC + RL rats)] in immediate post-ligation time. Notably, although the NO system is largely implicated in stomach cytoprotection and colitis lesions, the application of L-NAME (a vasoconstrictor) and/or L-arginine (a vasodilator) has not been investigated with respect to the immediate presentation of the blood vessels after a segment of left colic artery and vein was occluded by two ligations. By contrast, BPC 157 largely interacts with the NO system in various models and species, as shown in cytoprotection studies, in particular, studies using both L-NAME and L-arginine as individual agents or in combination.
We rescued rat ischemic colitis. The gastric pentadecapeptide BPC 157, which has been used in clinical trials for ulcerative colitis, exerted rapid cytoprotective endothelium rescue against the disabled left colic artery and vein after blood deprivation via two ligations and during reperfusion (ligations removed). By bypassing obstructions, quickly rescuing blood supply, rapidly activating collaterals, and restoring arcade interconnections, as a new integrative beneficial effect, BPC 157 prevented the occurrence of pale lesions without mucosal folds and normalized the levels of NO and MDA, two oxidative stress markers, in tissues. BPC 157 showed effectiveness over the NO-system background, immobilized (L-NAME + L-arginine), (over)stimulated (L-arginine) or blocked (L-NAME). Likewise, later application of BPC 157 in a bath treatment to rats with pertinently obstructed vessels that underwent additional colon obstruction for three days produced a similar beneficial effect.
BPC 157 is a fundamental treatment that quickly restores blood supply to the ischemically injured area and rapidly activates collaterals. This effect involves the NO system.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bourgoin SG, Fujita T S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ
| 1. | Feuerstadt P, Brandt LJ. Update on Colon Ischemia: Recent Insights and Advances. Curr Gastroenterol Rep. 2015;17:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Seiwerth S, Brcic L, Vuletic LB, Kolenc D, Aralica G, Misic M, Zenko A, Drmic D, Rucman R, Sikiric P. BPC 157 and blood vessels. Curr Pharm Des. 2014;20:1121-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20:1126-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19:126-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17:1612-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Sikiric P, Seiwerth S, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16:1224-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Sikiric P, Seiwerth S, Rucman R, Drmic D, Stupnisek M, Kokot A, Sever M, Zoricic I, Zoricic Z, Batelja L. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr Pharm Des. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Schiller HJ, Reilly PM, Bulkley GB. Tissue perfusion in critical illnesses. Antioxidant therapy. Crit Care Med. 1993;21:S92-S102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 87] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Rangan U, Bulkley GB. Prospects for treatment of free radical-mediated tissue injury. Br Med Bull. 1993;49:700-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Cesarec V, Becejac T, Misic M, Djakovic Z, Olujic D, Drmic D, Brcic L, Rokotov DS, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol. 2013;701:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Szabo S. Mechanism of mucosal protection. Gastric cytoprotection. A clinician's guide. New York, London: Plenum Medical Book Company 1989; 49-90. [DOI] [Cited in This Article: ] |
| 13. | Szabo S, Trier JS. Pathogenesis of acute gastric mucosal injury: Sulfhydrils as a protector, adrenal cortex as a modulator, and vascular endothelium as a target. Mechanism of mucosal protection in the upper gastrointestinal tract. New York: Raven 1984; 387-393. [Cited in This Article: ] |
| 14. | Trier JS, Szabo S, Allan CH. Ethanol-induced damage to mucosal capillaries of rat stomach. Ultrastructural features and effects of prostaglandin F2 beta and cysteamine. Gastroenterology. 1987;92:13-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88:228-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 323] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Sikiric P, Seiwerth S, Grabarevic Z, Petek M, Rucman R, Turkovic B, Rotkvic I, Jagic V, Duvnjak M, Mise S. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life Sci. 1994;54:PL63-PL68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Turhan A, Konerding MA, Tsuda A, Ravnic DJ, Hanidziar D, Lin M, Mentzer SJ. Bridging mucosal vessels associated with rhythmically oscillating blood flow in murine colitis. Anat Rec (Hoboken). 2008;291:74-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Konerding MA, Turhan A, Ravnic DJ, Lin M, Fuchs C, Secomb TW, Tsuda A, Mentzer SJ. Inflammation-induced intussusceptive angiogenesis in murine colitis. Anat Rec (Hoboken). 2010;293:849-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Ravnic DJ, Konerding MA, Tsuda A, Huss HT, Wolloscheck T, Pratt JP, Mentzer SJ. Structural adaptations in the murine colon microcirculation associated with hapten-induced inflammation. Gut. 2007;56:518-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761-767. [PubMed] [Cited in This Article: ] |
| 21. | Djakovic Z, Djakovic I, Cesarec V, Madzarac G, Becejac T, Zukanovic G, Drmic D, Batelja L, Zenko Sever A, Kolenc D. Esophagogastric anastomosis in rats: Improved healing by BPC 157 and L-arginine, aggravated by L-NAME. World J Gastroenterol. 2016;22:9127-9140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Sikiric P, Seiwerth S, Brcic L, Blagaic AB, Zoricic I, Sever M, Klicek R, Radic B, Keller N, Sipos K. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response. Inflammopharmacology. 2006;14:214-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Sikiric P, Separovic J, Anic T, Buljat G, Mikus D, Seiwerth S, Grabarevic Z, Stancic-Rokotov D, Pigac B, Hanzevacki M. The effect of pentadecapeptide BPC 157, H2-blockers, omeprazole and sucralfate on new vessels and new granulation tissue formation. J Physiol Paris. 1999;93:479-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Brcic L, Brcic I, Staresinic M, Novinscak T, Sikiric P, Seiwerth S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. J Physiol Pharmacol. 2009;60 Suppl 7:191-196. [PubMed] [Cited in This Article: ] |
| 25. | Hsieh MJ, Liu HT, Wang CN, Huang HY, Lin Y, Ko YS, Wang JS, Chang VH, Pang JS. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl). 2017;95:323-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, Mu N, Gu J, Zhang W, Wang Y. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485-2499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19:19066-19077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985). 2011;110:774-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Tkalcević VI, Cuzić S, Brajsa K, Mildner B, Bokulić A, Situm K, Perović D, Glojnarić I, Parnham MJ. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007;570:212-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Klicek R, Kolenc D, Suran J, Drmic D, Brcic L, Aralica G, Sever M, Holjevac J, Radic B, Turudic T. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol. 2013;64:597-612. [PubMed] [Cited in This Article: ] |
| 31. | Sikiric P, Seiwerth S, Aralica G, Perovic D, Staresinic M, Anic T, Gjurasin M, Prkacin I, Separovic J, Stancic-Rokotov D. Therapy effect of antiulcer agents on new chronic cysteamine colon lesion in rat. J Physiol Paris. 2001;95:283-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Sikiric P, Seiwerth S, Grabarevic Z, Balen I, Aralica G, Gjurasin M, Komericki L, Perovic D, Ziger T, Anic T. Cysteamine-colon and cysteamine-duodenum lesions in rats. Attenuation by gastric pentadecapeptide BPC 157, cimetidine, ranitidine, atropine, omeprazole, sulphasalazine and methylprednisolone. J Physiol Paris. 2001;95:261-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Veljaca M, Lesch CA, Pllana R, Sanchez B, Chan K, Guglietta A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther. 1995;272:417-422. [PubMed] [Cited in This Article: ] |
| 34. | Lojo N, Rasic Z, Zenko Sever A, Kolenc D, Vukusic D, Drmic D, Zoricic I, Sever M, Seiwerth S, Sikiric P. Effects of Diclofenac, L-NAME, L-Arginine, and Pentadecapeptide BPC 157 on Gastrointestinal, Liver, and Brain Lesions, Failed Anastomosis, and Intestinal Adaptation Deterioration in 24 Hour-Short-Bowel Rats. PLoS One. 2016;11:e0162590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Sandor ZS, Vincze A, Jadus MR, Dohoczky CS, Erceg D, Brajsa K, Kolega M, Szabo S. The protective effect of newly isolated peptide PL-10 in the iodoacetamide colitis model in rats. Gastroenterology. 1997;112:A400. [Cited in This Article: ] |
| 36. | Grgic T, Grgic D, Drmic D, Sever AZ, Petrovic I, Sucic M, Kokot A, Klicek R, Sever M, Seiwerth S. Stable gastric pentadecapeptide BPC 157 heals rat colovesical fistula. Eur J Pharmacol. 2016;780:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Baric M, Sever AZ, Vuletic LB, Rasic Z, Sever M, Drmic D, Pavelic-Turudic T, Sucic M, Vrcic H, Seiwerth S. Stable gastric pentadecapeptide BPC 157 heals rectovaginal fistula in rats. Life Sci. 2016;148:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Klicek R, Sever M, Radic B, Drmic D, Kocman I, Zoricic I, Vuksic T, Ivica M, Barisic I, Ilic S. Pentadecapeptide BPC 157, in clinical trials as a therapy for inflammatory bowel disease (PL14736), is effective in the healing of colocutaneous fistulas in rats: role of the nitric oxide-system. J Pharmacol Sci. 2008;108:7-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Vuksic T, Zoricic I, Brcic L, Sever M, Klicek R, Radic B, Cesarec V, Berkopic L, Keller N, Blagaic AB. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL14736, Pliva, Croatia) heals ileoileal anastomosis in the rat. Surg Today. 2007;37:768-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Sever M, Klicek R, Radic B, Brcic L, Zoricic I, Drmic D, Ivica M, Barisic I, Ilic S, Berkopic L. Gastric pentadecapeptide BPC 157 and short bowel syndrome in rats. Dig Dis Sci. 2009;54:2070-2083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Kachlik D, Baca V, Stingl J. The spatial arrangement of the human large intestinal wall blood circulation. J Anat. 2010;216:335-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Berra-Romani R, Avelino-Cruz JE, Raqeeb A, Della Corte A, Cinelli M, Montagnani S, Guerra G, Moccia F, Tanzi F. Ca²⁺-dependent nitric oxide release in the injured endothelium of excised rat aorta: a promising mechanism applying in vascular prosthetic devices in aging patients. BMC Surg. 2013;13 Suppl 2:S40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Urso C, Caimi G. [Oxidative stress and endothelial dysfunction]. Minerva Med. 2011;102:59-77. [PubMed] [Cited in This Article: ] |
| 44. | Luetic K, Sucic M, Vlainic J, Halle ZB, Strinic D, Vidovic T, Luetic F, Marusic M, Gulic S, Pavelic TT. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology. 2017;25:255-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Reilly PM, Schiller HJ, Bulkley GB. Pharmacologic approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am J Surg. 1991;161:488-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 299] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 46. | Sikirić P, Seiwerth S, Grabarević Z, Rucman R, Petek M, Jagić V, Turković B, Rotkvić I, Mise S, Zoricić I. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur J Pharmacol. 1997;332:23-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Turkovic B, Sikiric P, Seiwerth S, Mise S, Anic T, Petek M, Rucman R. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology. 2004;126:287. [Cited in This Article: ] |
| 48. | Ilic S, Drmic D, Zarkovic K, Kolenc D, Coric M, Brcic L, Klicek R, Radic B, Sever M, Djuzel V. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J Physiol Pharmacol. 2010;61:241-250. [PubMed] [Cited in This Article: ] |
| 49. | Sikiric P, Seiwerth S, Grabarevic Z, Rucman R, Petek M, Rotkvic I, Turkovic B, Jagic V, Mildner B, Duvnjak M. Hepatoprotective effect of BPC 157, a 15-amino acid peptide, on liver lesions induced by either restraint stress or bile duct and hepatic artery ligation or CCl4 administration. A comparative study with dopamine agonists and somatostatin. Life Sci. 1993;53:PL291-PL296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Sikirić P, Petek M, Rucman R, Seiwerth S, Grabarević Z, Rotkvić I, Turković B, Jagić V, Mildner B, Duvnjak M. A new gastric juice peptide, BPC. An overview of the stomach-stress-organoprotection hypothesis and beneficial effects of BPC. J Physiol Paris. 1993;87:313-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Stupnisek M, Kokot A, Drmic D, Hrelec Patrlj M, Zenko Sever A, Kolenc D, Radic B, Suran J, Bojic D, Vcev A. Pentadecapeptide BPC 157 Reduces Bleeding and Thrombocytopenia after Amputation in Rats Treated with Heparin, Warfarin, L-NAME and L-Arginine. PLoS One. 2015;10:e0123454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] [Cited in This Article: ] |
| 53. | Whittle BJ, Boughton-Smith NK, Moncada S. Biosynthesis and role of the endothelium-derived vasodilator, nitric oxide, in the gastric mucosa. Ann N Y Acad Sci. 1992;664:126-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Kokot A, Zlatar M, Stupnisek M, Drmic D, Radic R, Vcev A, Seiwerth S, Sikiric P. NO system dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis: Reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur J Pharmacol. 2016;771:211-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |