Published online Nov 14, 2017. doi: 10.3748/wjg.v23.i42.7551
Peer-review started: June 26, 2017
First decision: July 13, 2017
Revised: July 31, 2017
Accepted: September 9, 2017
Article in press: September 9, 2017
Published online: November 14, 2017
To investigate the therapeutic effect of combined integrin α6β4-targeted radioimmunotherapy (RIT) and PI3K/mTOR inhibitor BEZ235 in a pancreatic cancer model.
Phosphorylation of Akt, mTOR, the downstream effectors eukaryotic initiation factor 4E binding protein 1 (4EBP1) and S6 ribosomal protein (S6) were evaluated in BxPC-3 human pancreatic cancer cells treated with Yttrium-90 (90Y) labeled anti-integrin α6β4 antibody (ITGA6B4) and BEZ235 by western blotting. The cytotoxic effect of BEZ235 was investigated using a colony formation assay. Therapeutic efficacy enhancement by oral BEZ235 administration was assessed using mice bearing BxPC-3 xenograft tumors. Tumor volume measurements and immunohistochemical analyses (cell proliferation marker Ki-67, DNA damage marker p-H2AX and p-4EBP1 staining) of tumors were performed for evaluation of combined treatment with 90Y-ITGA6B4 plus BEZ235, or each arm alone.
We found that phosphorylation of Akt (p-Akt), 4EBP1 (p-4EBP1) and S6 (p-S6) was inhibited by BEZ235. Colony formation in BxPC-3 cells was additively suppressed by the combination of 90Y-ITGA6B4 and BEZ235. Pretreatment with BEZ235 before 90Y-ITGA6B4 exposure resulted in significant reduction of cells plating efficiency (PE) (0.54 ± 0.11 vs 2.81 ± 0.14 with 185 kBq/mL 90Y-ITGA6B4 exposure, P < 0.01; 0.39 ± 0.08 vs 1.88 ± 0.09 with 370 kBq/mL 90Y-ITGA6B4 exposure, P < 0.01) when 5 × 103 cells per dish were plated. In vivo, the combined treatment with 90Y-ITGA6B4 plus BEZ235 enhanced the inhibition of tumor growth and statistically significant differences of relative tumor volume were observed for 27 d after the treatment start date when compared with the 90Y-ITGA6B4 single injection treatment (1.03 ± 0.38 vs 1.5 ± 0.15 at Day 27, P < 0.05), and for 41 d when compared with the BEZ235 treatment alone (1.8 ± 0.7 vs 3.14 ± 1.19 at Day 41, P < 0.05). Tumors from treatment groups showed reduction in volumes, decreased Ki-67-positive cells, increased p-H2AX-positive cells and decreased p-4EBP1 expression.
The therapeutic efficacy of 90Y-ITGA6B4-RIT can be improved by combining with dual PI3K and mTOR inhibitor, BEZ235, in a pancreatic cancer model suggesting potential clinical application.
Core tip: We examined whether the therapeutic effect of 90Y-labeled anti-α6β4 integrin antibody (ITGA6B4)-mediated radioimmunotherapy (RIT) is improved by dual PI3K/mTOR inhibitor BEZ235 in the treatment of pancreatic cancer xenograft. There is no report about the combined therapeutic effects of RIT and BEZ235 in cancer treatment, though BEZ235 has been tested for its anticancer and potential radiosensitizing effect. Our studies (in vitro/in vivo) and results suggest for the first time that it is possible to improve the therapeutic efficacy by combining 90Y-ITGA6B4-RIT and BEZ235 and this combination can be a potential encouraging treatment modality in the future.
- Citation: Aung W, Tsuji AB, Sudo H, Sugyo A, Ukai Y, Kouda K, Kurosawa Y, Furukawa T, Saga T, Higashi T. Combined treatment of pancreatic cancer xenograft with 90Y-ITGA6B4-mediated radioimmunotherapy and PI3K/mTOR inhibitor. World J Gastroenterol 2017; 23(42): 7551-7562
- URL: https://www.wjgnet.com/1007-9327/full/v23/i42/7551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i42.7551
Pancreatic cancer is one of the most difficult malignant diseases to cure[1]. Its treatment options are limited including surgery, adjuvant chemotherapy, and radiation therapy and have not given encouraging outcomes so far. A possible solution might emerge from the use of targeted therapy such as radioimmunotherapy (RIT)[2]. RIT involves a selective internal radiation therapy using cytotoxic radionuclides conjugated to tumor-directed antibodies[3]. We recently reported the results obtained from a preclinical study of RIT using a novel monoclonal anti-integrin α6β4 antibody (ITGA6B4) labeled with beta-emitter Yttrium-90 (90Y) (90Y-ITGA6B4). According to our study, although 90Y-ITGA6B4 showed significant anti-tumor effects, the myelotoxicity caused by an overdose was a major challenge to overcome[4]. To counteract this problem, the feasibility of reducing the radiation dose by combining other therapeutic modalities and retaining the same or better therapeutic effect is important. Thus, combining RIT with other chemotherapeutic candidates is one of the options to reduce the radiation dose to normal organs especially bone marrow.
The phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is a critical intracellular signaling pathway involved in regulating cell metabolism and survival, cell cycle progression, proliferation, adhesion, and migration, particularly during cancer progression, metastasis, and radioresistance[5-10]. Moreover, this pathway is frequently aberrant and activated in cancer cells. Several downstream targets including the serine/threonine kinase Akt that activates mTOR are activated by PI3K. Furthermore, activation of this pathway is known to decrease sensitivity to chemotherapeutics as well as to irradiation (IR)[11,12], resulting in a limited treatment outcome. The PI3K/Akt and mTOR signaling pathways are also frequently dysregulated in pancreatic ductal adenocarcinoma (PDAC)[13]. Meanwhile, BEZ235 (also known as NVP-BEZ235, Dactolisib) is a potent dual pan-class I PI3K and mTOR inhibitor that suppresses PI3K and mTOR kinase activity and has been tested in preclinical studies for many cancers to demonstrate remarkable anticancer effects[14]. This orally administrable inhibitor is the first PI3K/mTOR dual inhibitor to undergo clinical trials[15,16], and already shown promising cytostatic results in breast cancer treatment[17].
Cao et al[18] reported that acute oral dosing with BEZ235 strongly suppressed the phosphorylation of protein kinase B (PKB)/Akt in primary human pancreatic cancers grown as orthotopic xenografts. They also showed the inhibition of downstream Thr37/46 eukaryotic initiation factor 4E binding protein 1 (4EBP1) and Ser235/236 S6 ribosomal protein (S6), consistent with the effects of BEZ235 as a dual PI3K/mTOR inhibitor[18]. Matsushima et al[19] reported that the phosphorylation of Akt (p-Akt), 4EBP1 (p-4EBP1), and S6 (p-S6) was inhibited in BEZ235-treated MBT-2 murine bladder cancer cells. Similarly, Kuger et al[20] reported that inhibiting the PI3K/Akt/mTOR pathway with BEZ235 caused dephosphorylation of the transcription and translation regulators 4EBP1 and S6. Although several studies have demonstrated that BEZ235 could be a potential radiosensitizer to external radiation therapy[21-29], there are currently no reports evaluating BEZ235 as a suitable drug to improve the therapeutic effect of RIT. The present study aimed to investigate whether the combination of RIT with molecular targeting BEZ235 therapy could enhance the therapeutic efficacy.
Human pancreatic cancer cell line BxPC-3 was purchased from American Type Culture Collection (Manassas, VA, United States) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich, St. Louis, MO, United States) supplemented with 10% fetal bovine serum (FBS, Nichirei Biosciences, Tokyo, Japan), 100 U/mL penicillin G sodium, and 100 mg/mL streptomycin sulfate (Invitrogen, Carlsbad, CA, United States) at 37 °C in a humidified atmosphere containing 5% CO2. NVP-BEZ235 was purchased from Selleck Chemicals (Houston, TX, United States), dissolved in dimethyl sulfoxide (Sigma-Aldrich) and the 2.5 mmol/L stock was stored at -20 °C. For in vivo treatment, it was mixed with the vehicle NMP/polyethylene glycol 300 (10/90, v/v).
Human anti-α6β4 monoclonal antibody (IgG1) was labeled with beta-emitter 90Y, as previously reported[30]. Briefly, the antibody solution and a chelating agent, N-[(R)-2-amino-3-(p-isothiocyanato-phenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine-N,N,N′,N″,N″-pentaacetic acid (CHX-A”-DTPA) (Macrocyclics, Dallas, TX, United States) were mixed at a molar ratio of 1:2.5 and incubated overnight at 37 °C. The conjugation ratio of DTPA and the antibody was estimated to be 1.3 calculated from the ratio (of 111In-DTPA-antibody to 111In-DTPA) determined by isoelectric focusing. Unconjugated DTPA was removed using a Sephadex G-50 column eluted with 0.1 mol/L sodium acetate buffer (GE Healthcare, Little Chalfont, United Kingdom). Afterward, the DTPA-conjugated antibody (71.2 μg in 0.1 mol/L sodium acetate buffer, pH 6.0) was incubated with a mixture of 90Y-chloride (74 MBq, Eckert & Ziegler Radiopharma GmbH, Berlin, Germany) and 1 mol/L sodium acetate buffer (pH 6.0) for 30 min at room temperature (RT). The radiolabeled antibody was purified using a Sephadex G-50 column (730 × g for 2 min). The radiochemical purity as determined by TLC was > 95%. The radiochemical yield was approximately 80%, and the specific activity was approximately 1500 kBq/μg.
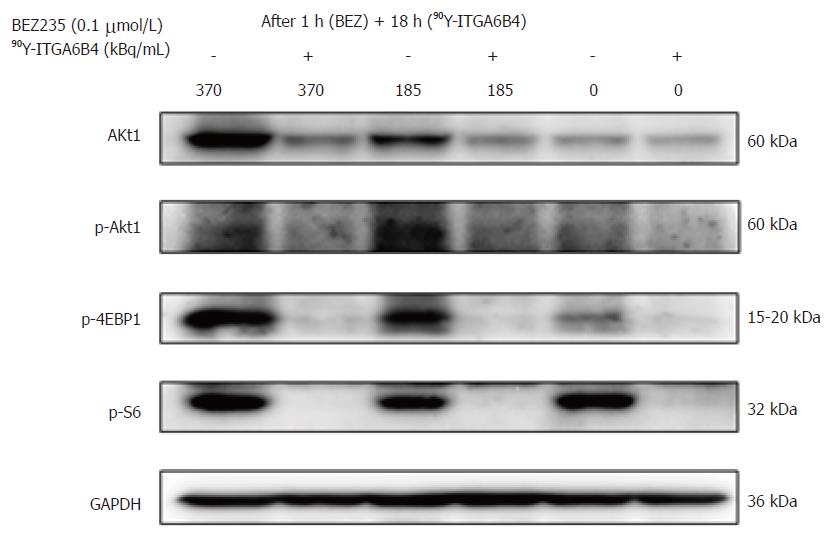
Western blotting was performed to analyze the proteins of interest from cultured cells. Cancer cells were cultured and treated with medium containing 0.1 μmol/L BEZ235 or DMSO (vehicle) for 1 h. The medium was then discarded and cells were exposed to medium containing 90Y-ITGA6B4 (indicated doses 185 and 370 kBq/mL) in the presence and absence of BEZ235 treatment. At 18 h after incubation, whole-cell lysates were prepared using radioimmunoprecipitation assay buffer (Wako Pure Chemical Industries, Osaka, Japan) with protease inhibitor cocktail. Total protein concentration was measured using the NanoDrop One Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, United States). Protein samples (45 μg) were separated on a 4%-20% polyacrylamide gel (ATTO Corporation, Tokyo, Japan) and transferred to an Immobilon-P membrane (Millipore, Billerica, MA, United States). The following antibodies: anti-human phospho-Akt (Ser473) (D9E) monoclonal antibody, anti-human phospho-4EBP1 (Thr37/46) (236B4) monoclonal antibody, anti-human phospho-mTOR (Ser2448) (D9C2) monoclonal antibody, anti-human phospho-S6 Ribosomal protein (Ser235/236) polyclonal antibody, and anti-human GAPDH monoclonal antibody were purchased from Cell Signaling technology (Danvers, MA, United States). Anti-human Akt1 (C-20) polyclonal antibody was purchased from Santa Cruz Biotechnology (Dallas, TX, United States). These were used as primary antibodies. Horseradish peroxidase (HRP)-linked anti-rabbit IgG antibody purchased from GE Healthcare (Little Chalfont, United Kingdom) was used as the secondary antibody. Immunoreactive bands were visualized using the Enhanced Chemiluminescence Plus western blotting detection system (GE Healthcare).
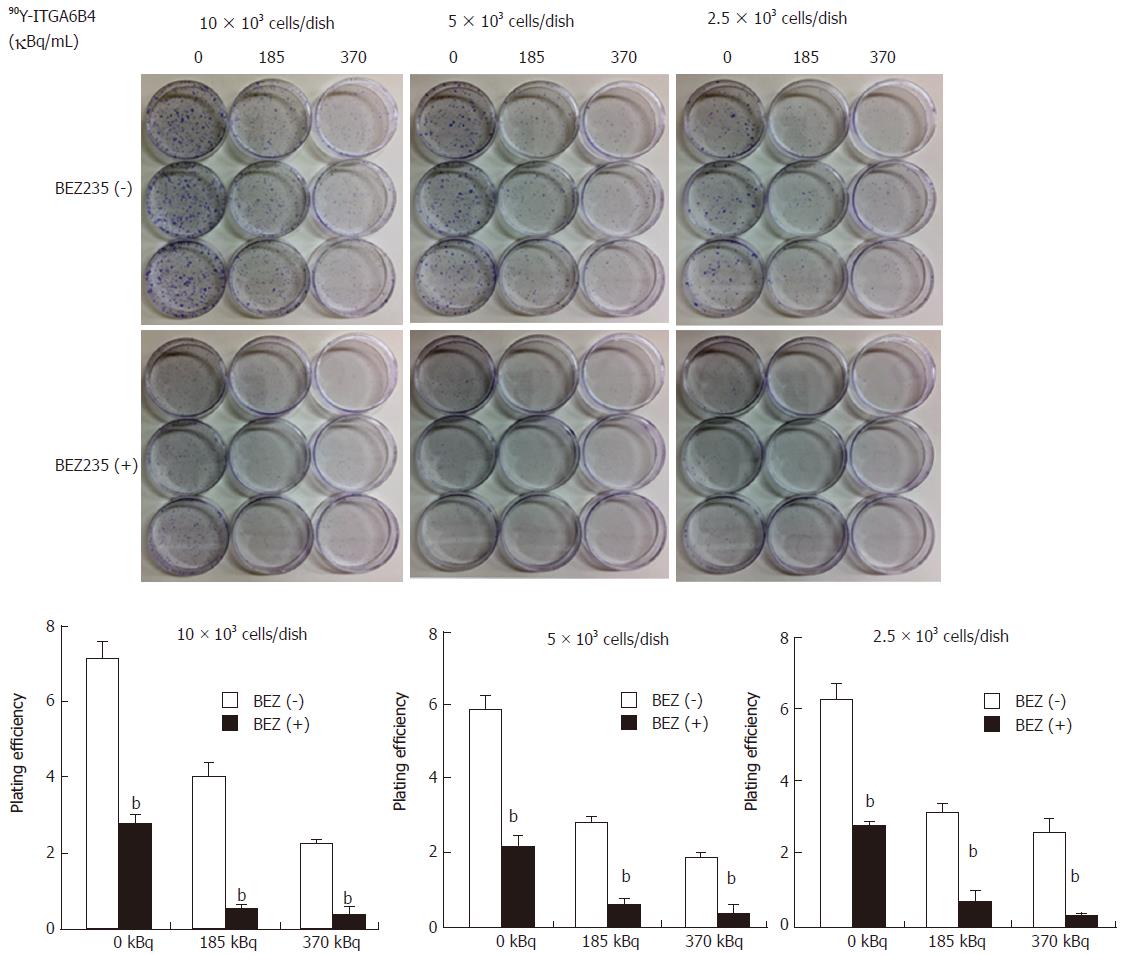
Cells (10, 5, 2.5 × 103 cells/dish) were plated in triplicate onto 60-mm dishes. After overnight incubation, exponentially growing cells were treated with the medium containing 0.1 μmol/L μmol BEZ235 or DMSO (vehicle) for 1 h. The medium was then discarded and adherent cells were exposed to medium containing 90Y-ITGA6B4 (indicated doses 185 and 370 kBq/mL) in the presence and absence of BEZ235 treatment for 24 h. The medium was then replaced with drug-free medium and the cells were cultured for 7 d for colony formation. At the indicated time point, cells were fixed and stained with Gentian violet and the grown colonies (clusters of > 50 cells) were counted. Plating efficiencies (PE) were determined as (number of colonies counted/number of cell inoculated) × 100.
All animal experiments were performed in accordance with the animal experimentation protocol approved by the Animal Care and Use Committee of National Institute of Radiological Sciences. Nude mice (7-wk-old female BALB/cA Jcl-nu/nu mice) were obtained commercially from CLEA, Shizuoka, Japan. They were housed in a restricted access room and acclimatized to standard laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, free access to food and water). Subcutaneous tumors were generated by injecting a suspension of 5 × 106 BxPC-3 cells in 100 µL RPMI medium mixed with BD Matrigel matrix (BD Biosciences, Bedford, MA, United States) into the right thigh of nude mice.
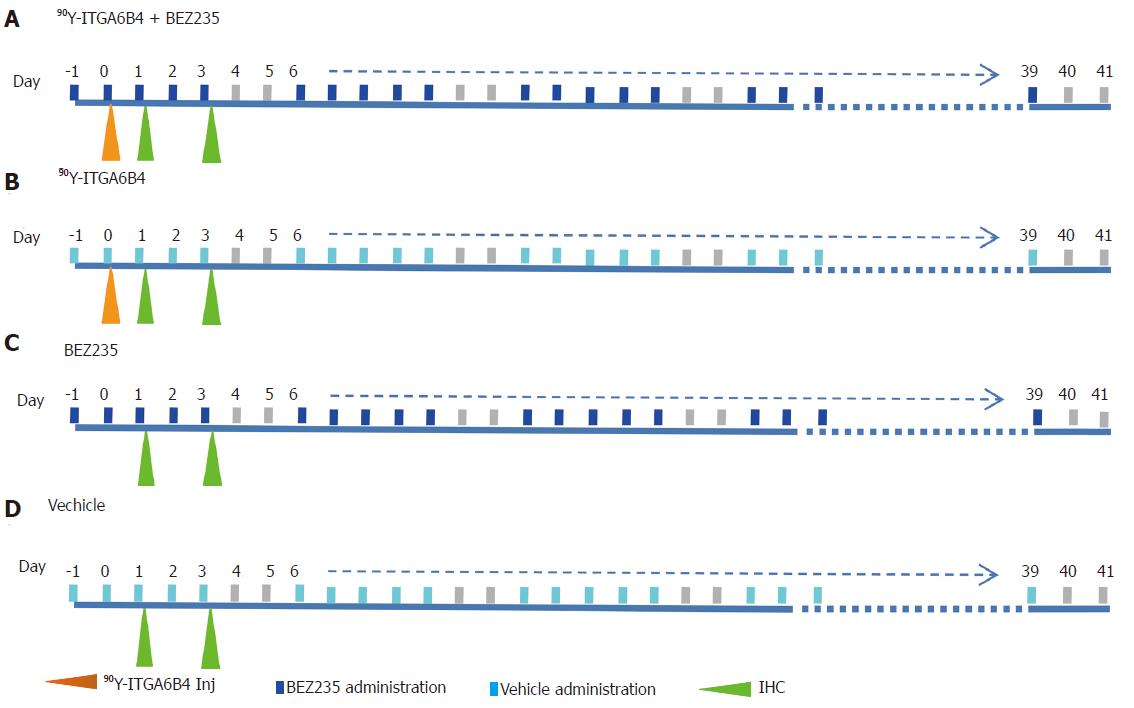
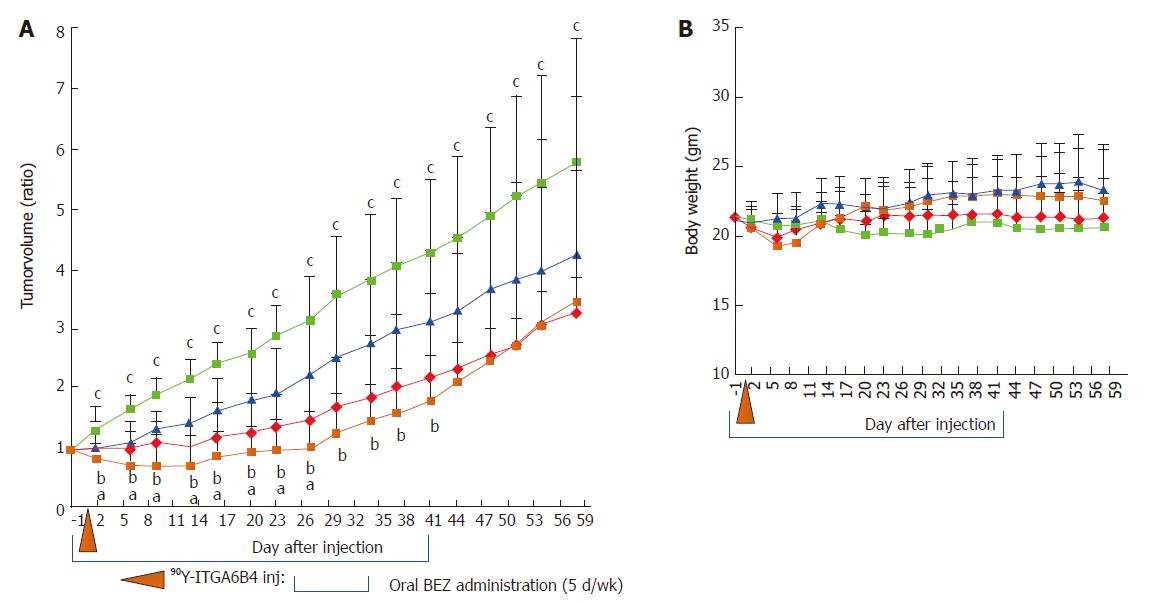
When the subcutaneous tumors in mice reached approximately 10 mm at the longest diameter, the xenograft tumor-bearing mice were randomly assigned to 4 groups (n = 10 for each group) for the treatment study. Experimental treatment was performed according to the shown scheme (Figure 1). Group A received daily oral administration of BEZ235 35 mg/kg, 5 d/wk for a 6-wk schedule with a single administration of 90Y-ITGA6B4 (2.8 MBq) following 1 h of the second BEZ235 dose, group B received a single administration of 90Y-ITGA6B4 (2.8 MBq), group C received daily oral administration of BEZ235 35 mg/kg, 5 d/wk for a 6-wk schedule, and group D received no treatments except oral vehicle administration. Intragastric gavage administration of BEZ235 was carried out with conscious mice, using disposable flexible gavage needles (1 inch length, 1.25 ball diameter). Each quantity of the injected antibody was adjusted to 20 μg by the addition of intact unlabeled antibody. According to previous pharmacokinetic studies, mice were injected with 90Y-ITGA6B4 at 1 h following BEZ235 administration because within this time frame, BEZ235 achieves effective intra-tumoral concentrations[15]. To observe the tumor response, tumor volumes of mice (n = 6 for each group) were measured twice a week throughout the experiment using calipers, and were approximated using the equation: volume (mm3) = [length (mm)] × [width (mm)] 2/2. Relative tumor volume was calculated as the volume on the indicated day divided by the volume on the day treatment began.
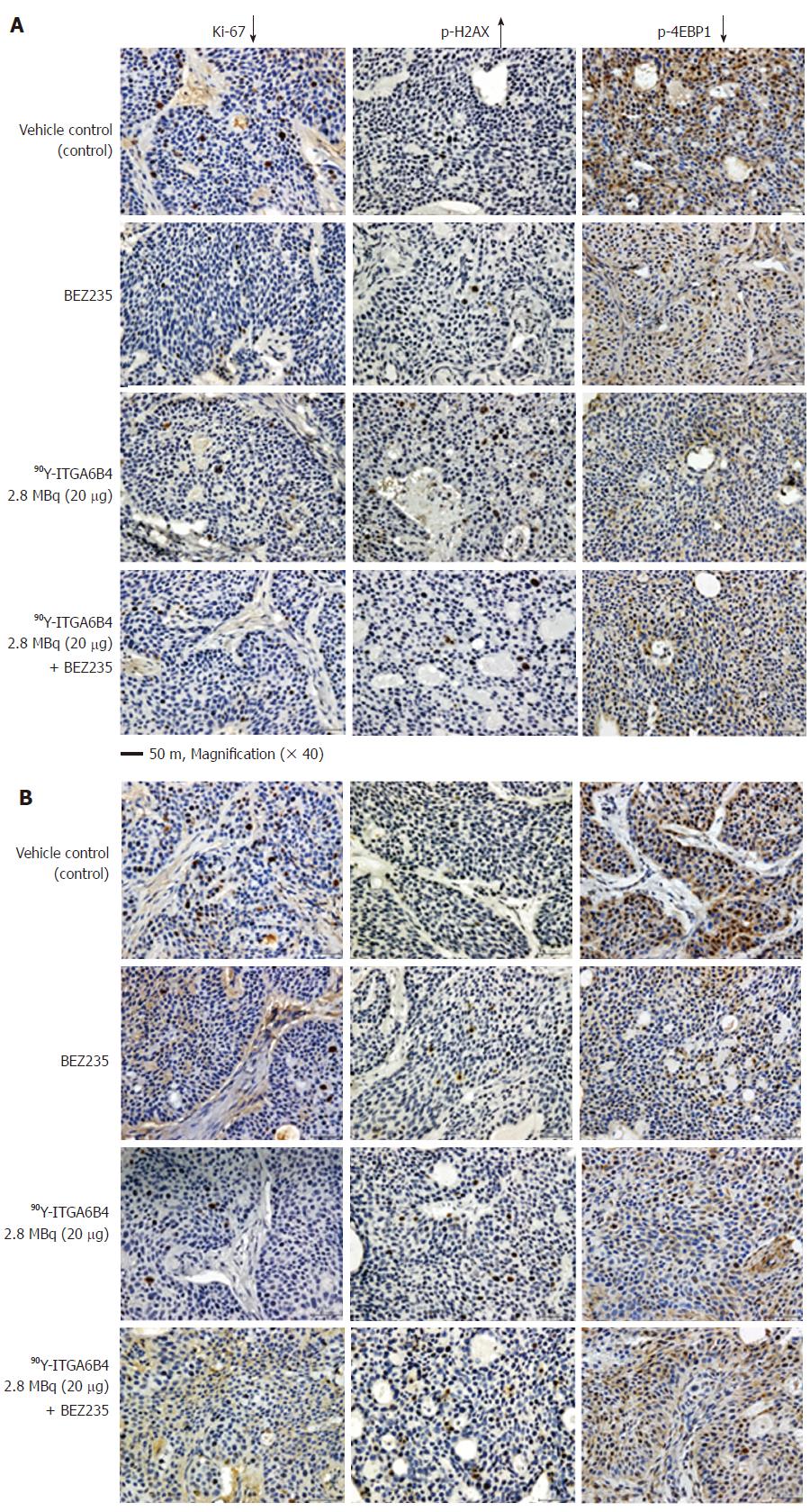
On Day 1 and Day 3 after 90Y-ITGA6B4 administration, 2 mice of each group (n = 2) were euthanized by cervical dislocation under anaesthesia (Isoflurane). Tumor tissue specimens were extirpated, fixed in 4% paraformaldehyde, and embedded in paraffin. Tumor specimens obtained from untreated mice were used as control. Paraffin-embedded tissue sections were cut (5 μm), rehydrated, and subjected to antigen retrieval. Ki-67 staining of sections was performed using an anti-human Ki-67 polyclonal antibody (Dako Denmark, Glostrup, Denmark), as previously described[31]. Phospho-Histone H2AX (p-H2AX) and Phospho-4EBP1 (p-4EBP1) staining were also detected using anti-human p-H2AX (Ser139) (20E3) monoclonal antibody and anti-human p-4EBP1 (Thr37/46) (236B4) monoclonal antibody (Cell Signaling technology), respectively. To detect the apoptotic tumor cells, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining was performed with an ApopTag Peroxidase In situ Apoptosis Detection Kit (Millipore Corporation, Temecula, CA, United States). Each slide was observed using the Olympus BX43 microscope system (Olympus, Tokyo, Japan). For quantitative and statistical analysis, we counted the number of Ki-67-positive cells observed in three random fields of view at 200× magnification in 2 different tumors sections from each group (n = 6). Similarly, p-H2AX-positive cells were also counted.
All results were expressed as mean ± SD. Significant differences between groups were determined by Student’s t-test (Excel, Microsoft, Redmond, WA, United States). Two-tailed unpaired t-test was used for comparisons of relative tumor volume, Ki-67 positive cells and p-H2AX-positive cells. Two-tailed paired t-test was used for comparisons of PE. P-values < 0.05 were considered significant.
The effects of BEZ235 on the PI3K/Akt signaling pathway were determined by western blotting using BxPC-3 cells. Cells were treated with or without 0.1 μmol/L BEZ235 for 1 h before exposure to 90Y-ITGA6B4. Total cell lysates were prepared after 18 h of 90Y-ITGA6B4 treatment. Akt1 expression and phosphorylation of Akt (p-Akt) were increased at 18 h after exposure to 185 or 370 kBq/mL 90Y-ITGA6B4. These increased expression levels were attenuated by pretreatment with 0.1 μmol/L BEZ235. Similarly, phosphorylation of 4EBP1 (p-4EBP1) and S6 (p-S6), which are Akt and mTOR downstream effectors, was inhibited by BEZ235 (Figure 2). No obvious changes in mTOR expression and phosphorylation were noted in our experiments (data not shown).
In the colony formation assay, 24 h exposure of BxPC-3 cells to 90Y-ITGA6B4 (185 or 370 kBq/mL) apparently resulted in radiation dose-dependent inhibition of cell survival and proliferation, which was evidenced by the reduced size and number of colonies and by the decreased PE. Moreover, pretreatment with 0.1 μmol/L BEZ235 for 1 h before 90Y-ITGA6B4 exposure resulted in significant reduction of colony formation and PE to a greater degree than in the single-drug treatment. For example, combined treatment with BEZ235 and 90Y-ITGA6B4 (185 kBq/mL) to cells (5 × 103 cells/dish) showed 90.9% reduction of PE from that of control while 90Y-ITGA6B4 treatment alone showed 52.5% reduction of PE from that of control. All relevant PE were compared in Figure 3 (P < 0.01). These results indicate that combination of BEZ235 and RIT markedly exaggerates the cytotoxic activity.
The anti-tumor effect of 90Y-ITGA6B4 with or without BEZ235 was evaluated using a BxPC-3 xenograft tumor model. Tumor growth was significantly delayed by 90Y-ITGA6B4 (2.8 MBq) treatment alone for 58 days following the treatment start date compared with that in vehicle treatment (control). Tumor growth was also significantly delayed by BEZ235 (35 mg/kg, 5 d/wk for 6 wk) treatment alone for 23 d compared with that in control. Combined treatment with 90Y-ITGA6B4 plus BEZ235 enhanced the inhibition of tumor growth and statistically significant differences were observed for 27 d after the treatment start date when compared with the 90Y-ITGA6B4 single injection treatment, and for 41 d when compared with the BEZ235 treatment alone (P < 0.05, Figure 4A). At 27 d after starting the treatment, the combination treatment showed 30.9% or 53.9% further reduction of the tumor volume ratio compared to that attained with RIT alone or BEZ235 alone, respectively. Neither significant average body weight loss (Figure 4B) nor differences in general conditions of mice were observed between the groups throughout the experiment.
Proportions of cell positive for the cell proliferation marker protein[32] Ki-67 were decreased in tumor sections of mice treated with the combination 90Y-ITGA6B4 plus BEZ235, or each arm alone, when compared with those of the control group. It is well-known that phosphorylation of the histone variant H2AX (p-H2AX) is a highly specific and sensitive molecular marker to monitor both DNA double-strand break (DSB) initiation and resolution, especially during radiation treatment[33]. P-H2AX-positive cells were increased in tumor sections of mice receiving the combined treatment or 90Y-ITGA6B4 treatment alone, when compared with those in the control group. The quantitative results and statistical analysis were summarized in Table 1. Immunohistochemical staining with the p-4EBP1 antibody depicted decreased expression of p-4EBP1 in the combined treatment or each arm alone, when compared with that in the control (Figure 5). On the other hand, TUNEL-positive cells were rare and there was no clear difference in the TUNEL staining patterns among all the groups (Data not shown).
Several previous in vitro and in vivo studies have revealed that BEZ235 could be a potential radiosensitizer in the treatment of various cancers such as fibrosarcoma as well as hypopharyngeal carcinoma[21], prostate carcinoma[22,23], non-small cell lung cancer (NSCLC)[24], glioblastoma[25], colorectal cancer (CRC)[26], and breast cancer[34]. Chen et al[26] reported that inactivation of the PI3K/Akt pathway by BEZ235 could enhance radiosensitivity by hindering the PI3K/Akt/mTOR pathway and DNA repair mechanisms. To prevent the radiation induced activation of PI3K/Akt/mTOR signaling, they treated the colorectal cancer cells with BEZ235 one hour before the radiation and found that BEZ235 synergistically inhibited cell viability. Their findings are in accordance with those of Kuger et al[27] demonstrating that radiosensitivity of glioblastoma cells was enhanced by BEZ235 exposure one hour before irradiation. Some recent studies described that BEZ235 could potently inhibit major DNA damage response kinases, attenuate the repair of irradiation-induced DNA damage, and confer striking tumor radiosensitization in glioblastoma[25,29] .
In contrast to external beam radiation therapy, one of the convincing benefits of RIT is its capacity to strike the primary tumor as well as the systemically metastasizing and residual lesions. However, systemic administration of large amounts of radiolabeled antibodies may cause bone marrow suppression. Small but adequate quantities of radiolabeled antibody that may bind to targets and produce efficient cytotoxic effects are therefore desirable, and new strategies are consequently needed to improve the effectiveness of RIT. We speculated that the combination of RIT and BEZ235 may help reduce the dose of RIT and generate a greater therapeutic response than each arm used alone, with less frequent treatment-related toxicity and increased radiosensitivity. The radiophysical properties of 90Y (β-particles with a maximum emitted energy of 2.28 MeV and emission range of 11 mm, half-life 2.7 d) are a relatively preferable and practical choice for RIT. In our previous study, we conducted RIT with 90Y labeled anti-integrin α6β4 antibody (90Y-ITGA6B4) in a pre-clinical mouse pancreatic cancer model[4]. As the treatment protocol, we used a single administration of 90Y-ITGA6B4 (3.7 MBq), and double administrations of 90Y-ITGA6B4 once-weekly (3.7 MBq × 2) in our previous study[4]. We found significantly reduced tumor growth rates in both groups compared with those in the untreated control. However, one mouse from the group receiving the double administrations showed pale skin and few petechiae during the study, and died on day 22 following the first dose. We considered myelotoxicity due to an overdose as the cause of death because analysis of hematological parameters at day 27 after starting the RIT indicated decreased RBC, WBC, and platelet counts in mice treated with double administrations, whereas only a decreased RBC count was observed in mice receiving a single administration. Based on these previous results[4], we chose a single administration dose of 90Y-ITGA6B4 (2.8 MBq) for RIT in this study design. Moreover, we decided to include daily oral administration of BEZ235 35 mg/kg, 5 d/wk over a 6-wk schedule. To the best of our knowledge, this is the first report about the combined therapeutic effects of RIT and BEZ235 in a preclinical pancreatic cancer model.
In the present study, inhibition of the PI3K/Akt/mTOR pathway with BEZ235 was proven by western blotting in BxPC-3 tumor cells upon observing the downregulation of p-Akt, as well as the downstream targets, p-4EBP1 and p-S6. The reduction of p-Akt, p-4EBP1 and p-S6 suggested that a plausible mechanism of the cytotoxic effect of BEZ235 derived from inhibition of the PI3K/Akt/mTOR pathway. The 4EBP1 protein integrates its function at the level of translation regulation[35]. Recently, it has been shown that some kinases phosphorylate 4EBP1 dependent or independent of mTOR, indicating that mTOR may not be the only kinase that phosphorylates 4EBP1[36], and that 4EBP1 is regarded as a point of convergence of various signaling pathways[35]. Phosphorylation of S6 ribosomal protein correlates with an increase in the translation of mRNA transcripts encoding proteins involved in cell cycle progression as well as ribosomal proteins and elongation factors necessary for translation[37]. Upregulation of mRNA translation is effectively involved in sustained cell growth and proliferation[37]. The opposite effect was observed with BEZ235 treatment.
The cytotoxic effect of BEZ235 on BxPC-3 cells was evaluated using the colony formation assay. Exposure to 90Y-ITGA6B4 alone resulted in decreased clonogenicity in cells and BEZ235 pretreatment caused more potent inhibition of colony formation indicated by reduced counts and smaller colonies. Taken together with the results of western blot analysis, BEZ235 might prevent PI3K pathway reactivation and further enhance radiation-induced cell killing. Next, we determined the anti-tumor effect of 90Y-ITGA6B4 with or without BEZ235 treatment in BxPC-3 xenograft tumors through longitudinal measurement of tumor volume and immunohistochemical analyses. We found that a single injection of 90Y-ITGA6B4 (2.8 MBq) alone significantly delayed tumor growth compared with that upon vehicle administration. Furthermore, combined treatment with 90Y-ITGA6B4 plus BEZ235 more strongly and significantly inhibited the tumor growth for 27 d when compared with that in 90Y-ITGA6B4 treatment alone, and for 41 d when compared with that in BEZ235 treatment alone (P < 0.05, Figure 4A). To further retain the superior therapeutic efficacy of the combined treatment and to impede tumor regrowth, further fractionated 90Y-ITGA6B4 administration with appropriate timings will be required and will be interesting to address in next study. Concerning toxicity, we can judge that there was no enhancement of toxicity during the combined treatment because body weight loss, obvious abnormal changes in general conditions and death were not observed in the mice receiving treatment (Figure 4B).
Previously, Fokas et al[28] has demonstrated that BEZ235 itself causes DNA damage even in nonirradiated cells, as evidenced by moderately increased phosphorylation of the histone variant H2AX, and the enhanced persistence of p-H2AX foci after irradiation, thus attributing radiosensitivity to head and neck and bladder cancer cell lines. In our immunohistochemical analyses, significantly increased DNA damage marker p-H2AX-positive cells were noted in the tumor sections of mice that received 90Y-ITGA6B4 plus BEZ235, or 90Y-ITGA6B4 treatment alone, when compared with those of the control group (Figure 4). In contrast, the proliferation marker nuclear protein Ki-67-positive cells were significantly reduced in treatment groups. Besides, immunohistochemical examination showed decreased phosphorylation of 4EBP1 in the treatment groups. These results suggest that BEZ235 treatment could potentiate the radioimmunotherapeutic effect of 90Y-ITGA6B4 in BxPC-3 tumors. We detected no appreciable induction of apoptotic cells in TUNEL-stained sections, and the staining patterns among groups were not very different at 1 and 3 d after 90Y-ITGA6B4 administration (data not shown). This result is in line with the results of our previous RIT study in which TUNEL assay was conducted at 2 d post-administration[4].
Chen et al[26] have reported that irradiation upregulates the Akt/mTOR signaling pathway including the activation of Akt and mTOR, which were attenuated by BEZ235 pretreatment. Likewise, RIT may also upregulate Akt/mTOR signaling pathway to some extent after administration, but it seems that this activated Akt/mTOR signaling pathway is attenuated by BEZ235 pretreatment. The mechanisms underlying the treatment with BEZ235 in combination with RIT for pancreatic cancer still need to be clarified with a more detailed study covering evidences at multiple times-points during treatment with various dose regimens of both drugs. In the current study, we obtained an insight in to the presumptive mechanism of a combination treatment with PI3K/mTOR inhibitors and RIT as well as its therapeutic effects.
In conclusion, our findings imply that it is possible to improve the therapeutic efficacy by combining 90Y-ITGA6B4-mediated radioimmunotherapy with the dual PI3K and mTOR inhibitor, BEZ235, and this combination is a promising treatment option for future pancreatic cancer therapy, though many hurdles remain to be overcome to reach clinical use.
Pancreatic cancer is one of the most difficult malignant diseases to cure. Its treatment options are limited and have not given encouraging outcomes so far. A possible solution might emerge from the use of radioimmunotherapy (RIT) and other molecular targeting chemotherapeutic candidates. The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is frequently dysregulated and activated in human cancers including pancreatic cancer. Several studies have revealed that BEZ235 (NVP-BEZ235) can inhibit the PI3K and mTOR kinase activity, could be a potential radiosensitizer, and has been used in preclinical studies in many cancers with excellent results of anticancer effects.
The authors have studied the radioimmunotherapeutic effect and toxicity of Yttrium-90 labeled anti-integrin α6β4 antibody (90Y-ITGA6B4) in a mouse pancreatic cancer model. In the previous study, 90Y-ITGA6B4 showed anti-tumor effects but myelotoxicity caused by an overdose was a major handicap. To overcome this obstacle, combining RIT with other chemotherapeutic candidates is one of the options for reducing the radiation dose and retaining the therapeutic effect.
To the best of our knowledge, there was no report about the therapeutic effects of combined 90Y-ITGA6B4-RIT and BEZ235 in cancer treatment. Thus, The authors examined this effect for the first time in a pre-clinical pancreatic cancer xenograft model.
These in vitro and in vivo studies and results suggest that it is possible to improve the therapeutic efficacy by combing 90Y-ITGA6B4-RIT and BEZ235 and this combination can be a potential encouraging treatment option for future pancreatic cancer therapy.
The 90Y-ITGA6B4-RIT is a specific treatment option for cancer by cytotoxic radionuclide (beta-emitter Yttrium-90) conjugated to anti-integrin α6β4 monoclonocal antibody (ITGA6B4). The PI3K/Akt/mTOR pathway is a critical intracellular signaling pathway involved in regulating cell metabolism and survival, cell cycle progression, proliferation, adhesion, and migration, particularly during cancer progression, metastasis, and radioresistance.
It’s a well-designed study and answers a good scientific question.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Roy PK, Wei DY S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19772] [Article Influence: 2196.9] [Reference Citation Analysis (17)] |
| 2. | Sabbah EN, Kadouche J, Ellison D, Finucane C, Decaudin D, Mather SJ. In vitro and in vivo comparison of DTPA- and DOTA-conjugated antiferritin monoclonal antibody for imaging and therapy of pancreatic cancer. Nucl Med Biol. 2007;34:293-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Kawashima H. Radioimmunotherapy: a specific treatment protocol for cancer by cytotoxic radioisotopes conjugated to antibodies. ScientificWorldJournal. 2014;2014:492061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Aung W, Tsuji AB, Sudo H, Sugyo A, Ukai Y, Kouda K, Kurosawa Y, Furukawa T, Saga T. Radioimmunotherapy of pancreatic cancer xenografts in nude mice using 90Y-labeled anti-α6β4 integrin antibody. Oncotarget. 2016;7:38835-38844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Vo BT, Morton D Jr, Komaragiri S, Millena AC, Leath C, Khan SA. TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology. 2013;154:1768-1779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Ni J, Cozzi P, Hao J, Beretov J, Chang L, Duan W, Shigdar S, Delprado W, Graham P, Bucci J. Epithelial cell adhesion molecule (EpCAM) is associated with prostate cancer metastasis and chemo/radioresistance via the PI3K/Akt/mTOR signaling pathway. Int J Biochem Cell Biol. 2013;45:2736-2748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 8. | Chang L, Graham PH, Hao J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. Emerging roles of radioresistance in prostate cancer metastasis and radiation therapy. Cancer Metastasis Rev. 2014;33:469-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Bartholomeusz C, Gonzalez-Angulo AM. Targeting the PI3K signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Ebrahimi S, Hosseini M, Shahidsales S, Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM, Avan A. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Curr Med Chem. 2017;24:1321-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 11. | Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Castaneda CA, Cortes-Funes H, Gomez HL, Ciruelos EM. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metastasis Rev. 2010;29:751-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Awasthi N, Yen PL, Schwarz MA, Schwarz RE. The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to enhance chemotherapy and antiangiogenic response in pancreatic cancer. J Cell Biochem. 2012;113:784-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 968] [Cited by in F6Publishing: 950] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 15. | Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851-1863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 903] [Cited by in F6Publishing: 924] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 16. | Search of: BEZ235—List Results—ClinicalTrials. gov. [Accessed May 1, 2017]. Available from: http://clinicaltrials.gov/ct2/results?term=bez235Search=Search. [Cited in This Article: ] |
| 17. | Leung E, Kim JE, Rewcastle GW, Finlay GJ, Baguley BC. Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol Ther. 2011;11:938-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Cao P, Maira SM, García-Echeverría C, Hedley DW. Activity of a novel, dual PI3-kinase/mTor inhibitor NVP-BEZ235 against primary human pancreatic cancers grown as orthotopic xenografts. Br J Cancer. 2009;100:1267-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Matsushima M, Kikuchi E, Matsumoto K, Hattori S, Takeda T, Kosaka T, Miyajima A, Oya M. Intravesical dual PI3K/mTOR complex 1/2 inhibitor NVP-BEZ235 therapy in an orthotopic bladder cancer model. Int J Oncol. 2015;47:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Kuger S, Flentje M, Djuzenova CS. Simultaneous perturbation of the MAPK and the PI3K/mTOR pathways does not lead to increased radiosensitization. Radiat Oncol. 2015;10:214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Fokas E, Im JH, Hill S, Yameen S, Stratford M, Beech J, Hackl W, Maira SM, Bernhard EJ, McKenna WG. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Cancer Res. 2012;72:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Potiron VA, Abderrahmani R, Giang E, Chiavassa S, Di Tomaso E, Maira SM, Paris F, Supiot S. Radiosensitization of prostate cancer cells by the dual PI3K/mTOR inhibitor BEZ235 under normoxic and hypoxic conditions. Radiother Oncol. 2013;106:138-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Zhu W, Fu W, Hu L. NVP-BEZ235, dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, prominently enhances radiosensitivity of prostate cancer cell line PC-3. Cancer Biother Radiopharm. 2013;28:665-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Konstantinidou G, Bey EA, Rabellino A, Schuster K, Maira MS, Gazdar AF, Amici A, Boothman DA, Scaglioni PP. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644-7652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Gil del Alcazar CR, Hardebeck MC, Mukherjee B, Tomimatsu N, Gao X, Yan J, Xie XJ, Bachoo R, Li L, Habib AA. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin Cancer Res. 2014;20:1235-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Chen YH, Wei MF, Wang CW, Lee HW, Pan SL, Gao M, Kuo SH, Cheng AL, Teng CM. Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor is an effective radiosensitizer for colorectal cancer. Cancer Lett. 2015;357:582-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Kuger S, Graus D, Brendtke R, Günther N, Katzer A, Lutyj P, Polat B, Chatterjee M, Sukhorukov VL, Flentje M. Radiosensitization of Glioblastoma Cell Lines by the Dual PI3K and mTOR Inhibitor NVP-BEZ235 Depends on Drug-Irradiation Schedule. Transl Oncol. 2013;6:169-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Fokas E, Yoshimura M, Prevo R, Higgins G, Hackl W, Maira SM, Bernhard EJ, McKenna WG, Muschel RJ. NVP-BEZ235 and NVP-BGT226, dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitors, enhance tumor and endothelial cell radiosensitivity. Radiat Oncol. 2012;7:48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Mukherjee B, Tomimatsu N, Amancherla K, Camacho CV, Pichamoorthy N, Burma S. The dual PI3K/mTOR inhibitor NVP-BEZ235 is a potent inhibitor of ATM- and DNA-PKCs-mediated DNA damage responses. Neoplasia. 2012;14:34-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 30. | Sogawa C, Tsuji AB, Sudo H, Sugyo A, Yoshida C, Odaka K, Uehara T, Arano Y, Koizumi M, Saga T. C-kit-targeted imaging of gastrointestinal stromal tumor using radiolabeled anti-c-kit monoclonal antibody in a mouse tumor model. Nucl Med Biol. 2010;37:179-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Sudo H, Tsuji AB, Sugyo A, Ogawa Y, Sagara M, Saga T. ZDHHC8 knockdown enhances radiosensitivity and suppresses tumor growth in a mesothelioma mouse model. Cancer Sci. 2012;103:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 33. | Sak A, Stuschke M. Use of γH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin Radiat Oncol. 2010;20:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Kuger S, Cörek E, Polat B, Kämmerer U, Flentje M, Djuzenova CS. Novel PI3K and mTOR Inhibitor NVP-BEZ235 Radiosensitizes Breast Cancer Cell Lines under Normoxic and Hypoxic Conditions. Breast Cancer (Auckl). 2014;8:39-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, Solit DB, Rosen N. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 36. | Qin X, Jiang B, Zhang Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle. 2016;15:781-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 37. | Peterson RT, Schreiber SL. Translation control: connecting mitogens and the ribosome. Curr Biol. 1998;8:R248-R250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |