Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.4007
Peer-review started: January 14, 2017
First decision: February 23, 2017
Revised: April 5, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: June 14, 2017
To examine the influence on apoptotic mechanisms following inhibition of polo-like kinases as therapeutically approach for cholangiocellular cancer treatment.
As most cholangiocarcinomas are chemotherapy-resistant due to mechanisms preventing tumor cell death, we investigated the effect of Cisplatin on cholangiocellular carcinoma (CCA) cell lines KMCH-1 and Mz-Ch-1. Polo-like kinases (PLK) are important regulators of the cell cycle and their inhibition is discussed as a potential therapy while PLK inhibition can regulate apoptotic mediators. Here, cells were treated with PLK inhibitor BI6727 (Volasertib), Cisplatin, and in combination of both compounds. Cell viability was assessed by MTT; apoptosis was measured by DAPI staining and caspase-3/-7 assay. Western blot and qRT-PCR were used to measure expression levels of apoptosis-related molecules Bax and Bcl-2.
The cell viability in the CCA cell lines KMCH-1 and Mz-Ch-1 was reduced in all treatment conditions compared to vehicle-treated cells. Co-treatment with BI6727 and cisplatin could even enhance the cytotoxic effect of cisplatin single treatment. Thus, co-treatment of cisplatin with BI6727 could slightly enhance the cytotoxic effect of the cisplatin in both cell lines whereas there was evidence of increased apoptosis induction solely in Mz-Ch-1 as compared to KMCH-1. Moreover, PLK inhibition decreases protein levels of Bcl-2; an effect that can be reversed by the proteasomal degradation inhibitor MG-132. In contrast, protein levels of Bax were not found to be altered by PLK inhibition. These findings indicate that cytotoxic effects of Cisplatin in Mz-Ch-1 cells can be enhanced by cotreatment with BI6727.
In conclusion, BI6727 treatment can sensitize CCA cells to cisplatin-induced apoptosis with proteasomal Bcl-2 degradation as an additional pro-apoptotic effect.
Core tip: This manuscript addresses the timely and topical roles of cell cycle/apoptosis modulating enzymes for the tumor biology of human cholangiocarcinoma. These data suggest that polo-like kinases -Inhibition by BI6727 (volasertib) sensitizes some cholangiocarcinoma cell lines to cisplatin-induced apoptosis. Our findings include an enhanced cytotoxic effect of cisplatin by co-treatment with BI6727 (volasertib) and results in decreased protein expression levels of the anti-apoptotic molecule Bcl-2, which appears to be mediated via proteasomal degradation. Taken together, these data reveal another pro-apoptotic mechanism of polo-like kinase inhibition emphasizing the potential therapeutic benefit of polo-like kinase inhibitors for the treatment of cholangiocarcinoma.
- Citation: Sydor S, Jafoui S, Wingerter L, Swoboda S, Mertens JC, Gerken G, Canbay A, Paul A, Fingas CD. Bcl-2 degradation is an additional pro-apoptotic effect of polo-like kinase inhibition in cholangiocarcinoma cells. World J Gastroenterol 2017; 23(22): 4007-4015
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/4007.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.4007
Cholangiocellular carcinoma (CCA) represents the most common primary liver cancer with biliary differentiation and its incidence is increasing constantly in Western countries[1-5]. Therapeutic options are limited for CCA as tumors can be multifocal in advanced stages being surgically non-accessible. Additionally, CCA is often resistant to conventional chemotherapy and, therefore, associated with poor prognosis[6]. Development and progression of CCA are in part mediated by mechanisms that prevent tumor cell death[3,7]. For example, these cancer cells paradoxically express tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) as well as its cognate receptors[8-10] but are quite resistant to TRAIL-induced apoptosis[10-13]. The underlying mechanisms are complex and seem to be mediated by effective survival signals that prevent TRAIL-induced apoptosis (e.g., upregulation of anti-apoptotic proteins of the Bcl-2 family)[8,14,15].
Polo-like kinases (PLK) represent a highly conserved family of several members of serine/threonine kinases regulating cell cycle division and are often overexpressed in tumor tissue of many different tumors including CCA[16]. PLK 1/2 expression is known to be associated with a poor prognosis and short overall survival rates in CCA[17]. PLK2 has been shown to be upregulated by Hedgehog (Hh) signaling - another important survival mechanism in CCA[8,18].
Thus, PLK2 appears to be an important mediator of Hh survival signaling as its expression is reduced when Hh signaling is inhibited[8]. In this context, PLK inhibition is discussed as a new potential therapeutical approach for the treatment of different cancers and has been described to decrease myeloid cell leukemia-1 (Mcl-1) - an anti-apoptotic member of the Bcl2 protein family that has been identified as an important survival factor in CCA[15,19-21]. Members of the Bcl-2 family include anti- as well as pro-apoptotic proteins. The anti-apoptotic members Bcl-2 and Mcl-1 can prevent apoptosis induction by inhibition of mitochondrial cytochrome C release while the pro-apoptotic protein Bax can induce apoptosis by stimulating cytochrome C release[22-24]. We have recently shown that PLK2 inhibition can decrease Mcl-1 levels by proteasomal degradation inducing apoptosis in CCA cells, which finally results in tumor suppression in vivo[8].
Beside gemcitabine, cisplatin is a conventional chemotherapeutic drug used for CCA treatment that can induce cell death in fast replicating cells by inhibition of DNA replication causing DNA damage and apoptosis[22,25]. However, treatment with cisplatin often results in chemoresistance and therapy failure[25]. Many different underlying mechanisms have been described in this context such as defective DNA binding, premature degradation, and increased activation of specific transporter proteins[25].
In this study, we aimed to investigate the impact of PLK inhibition in cisplatin-treated CCA cell lines and its effect on several Bcl-2 family members (beside Mcl-1) that might be involved in the mechanism of apoptosis resistance in CCA.
Human CCA cell lines KMCH-1 and Mz-Ch-1 were cultured in Dulbecco’s modified eagle medium/high-glucose medium (Invitrogen, Carlsbad, CA, United States) containing 10% fetal bovine serum, 1000 U/mL Penicillin, 0.1 mg/mL streptomycin and 2 mmol/L L-glutamine (PAA, Pasching, Austria) at 5% CO2 and 37 °C. The selective PLK-inhibitor BI6727/Volasertib[26] (Selleckchem, Houston, TX, United States) and the proteasome inhibitor MG-132 (Merck, Rockland, MA, United States) were dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, United States), Cisplatin (Merck, Rockland, MA, United States) was dissolved in PBS, stock solutions of substances or vehicle as control were subsequently diluted in cell culture medium for experiments.
For assessing cell viability 5 × 104cells/well were plated in 96-multiwell plates. Twenty-four h after plating, cells were incubated with 200 nmol/L BI6727 or 1 mmol/L cisplatin for 24 h as described previously[8]. Cell viability was measured by MTT assay. The cells incubated with vehicle (DMSO) for 24 h were considered 100% viable.
Apoptosis in CCA cells was quantified by assessing characteristic nuclear changes of apoptosis after staining with 4’, 6-diamino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich, St. Louis, MO, United States) using fluorescence microscopy as described previously[27]. Caspase-3/-7 activity was assessed by Caspas-3/-7 assay (Promega, Madison, WI, United States) according to manufacturer’s recommendations.
Cells were seeded at a density of approx. 1 × 106 cells/cm2 for in vitro experiments. At the end of the stimulation period total RNA extraction and purification was performed using RNeasy Mini Kit (Qiagen, Hilden, Germany) following manufacturer instructions. Reverse transcription was performed with the QuantiTect RT kit (Qiagen; Hilden, Germany) using 1 μg of total RNA. Quantitative realtime PCR (qRT-PCR) for specific mRNA sequences was performed on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, United States) using QuantiTect SYBR Green Kit (Qiagen, Hilden, Germany) in a final volume of 15 μL including 2 μL of cDNA. Oligonucleotide sequences for Bax primers were used as follows: Bax forward: 5’-TCTGACGGCAACTTCAACTG-3’; Bax reverse: 5’-GGAGGAAGTCCAATGTCCAG-3’. Melting curves were collected to ascertain specificity of PCR products. Changes in mRNA expression were calculated by the ΔΔ-ct method and are presented as foldchanges in relation to expression of a reference gene (hypoxanthine-guanine phosphoribosyltransferase, HPRT) in vehicle-treated cells.
Cells were seeded at a density of approx. 1 × 106 cells/cm2 for in vitro experiments, at the end of the stimulation period protein lysates were prepared using lysis buffer (50 mmol/L Tris-HCl; 150 nmol/L NaCl; 0.1% NP-40; 1% desoxycholic acid) containing complete mini EDTA-free protease inhibitor cocktail and phosphostop (Roche, Mannheim, Germany). In all, 30 μg of total protein were separated using SDS-PAGE, immunoblotting was performed using standard procedures with the following primary antibodies (incubation: overnight at 4 C): Actin (1/1000; #5125; Cell Signaling, Cambridge, United Kingdom), Bax (1/1000; #2772; Cell Signaling) and Bcl-2 (1/1000; #2876; Cell Signaling), cleaved PARP (1/1000; #5625, Cell Signaling). After incubation with the appropriate horseradish peroxidase-conjugated secondary antibody, bound antibodies were visualized using chemiluminescence reagent ECLPrime reagent (GE Healthcare, Chalfont St. Giles, United Kingdom) according to the supplier’s protocol. Blotting images and densitometric quantification of protein bands was generated using Fusion detection system (PeqLab Biotechnology, Erlangen, Germany).
Statistical significance for in vitro experiments was determined by one-way ANOVA (with Tukey’s post-hoc test for individual experimental conditions) and by a two-tailed unpaired Student t test performed with Prism 5 (GraphPad Software, Inc.; San Diego, CA, United States). Data are presented as mean ± SEM. Differences were considered significant at P < 0.05.
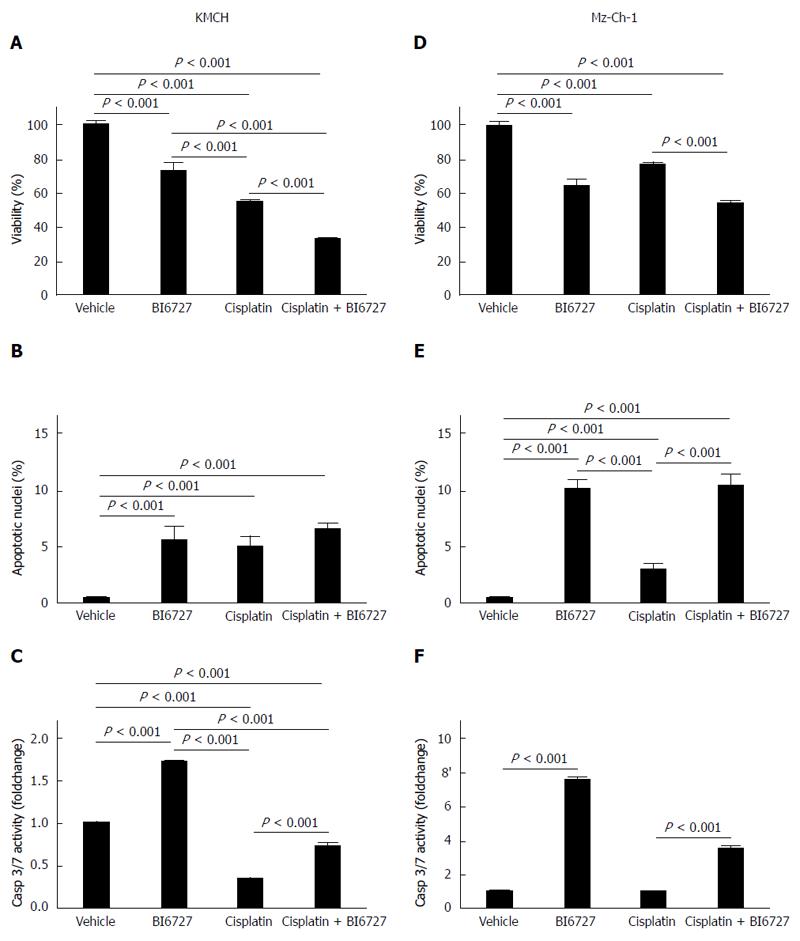
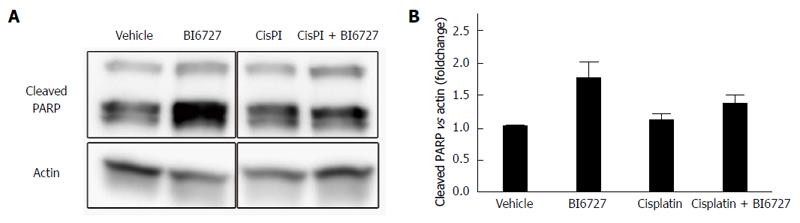
In previous studies, we demonstrated that the PLK inhibitor BI6727 had a pro-apoptotic effect in CCA cell lines and could exacerbate TRAIL-induced cell death[8]. Cisplatin is one of the conventional cytostatic drugs that are used for the chemotherapy of CCA. Here, we aimed to further investigate the effect of the PLK inhibitor BI6727 on cell death in CCA cell lines in the presence or absence of cisplatin. We measured viability in the CCA cell lines KMCH-1 and Mz-ChA-1 treated with cisplatin and BI6727 for 24 h via a MTT viability assay. Cell viability was reduced in all treatment conditions compared to vehicle-treated KMCH-1 (Figure 1A) and Mz-Ch-1 cells (Figure 1D). Co-treatment with BI6727 and cisplatin could even enhance the cytotoxic effect of cisplatin single treatment. By means of the MTT viability assay, we could demonstrate that cell viability was reduced under different treatment conditions in these two cell lines; however, with this assay the type of cell death cannot be determined. To assess apoptosis induction caused by the different treatments, we performed DAPI staining with quantitation of apoptotic nuclei by fluorescence microscopy as well as fluorescent analysis of caspase-3/-7 activity. In KMCH-1 cells treated with BI6727, cisplatin and the combination of BI6727 and cisplatin, some apoptotic nuclei were found (Figure 1B). Moreover, caspase-3/-7 activity was slightly induced in BI6727-treated KMCH-1 cells (Figure 1C). In KMCH-1 cells treated with the cytotoxic drug cisplatin, caspase-3/-7 activity was reduced compared to vehicle-treated cells whereas co-treatment with BI6727 and cisplatin induced caspase -3/-7 activitiy as compared to cisplatin only-treated cells. In KMCH-1 cells, viability was reduced by treatment with BI6727 or cisplatin and the combination of BI6727 with cisplatin could even exacerbate this effect (which does not appear to be mediated by apoptosis induction). In Mz-Ch-1 cells, we could observe a similar effect as cotreatment of BI6727 and cisplatin could slightly enhance the cytotoxic effect of both single agents (Figure 1D). Quantification of apoptotic nuclei demonstrated that BI6727 or cisplatin treatment increased the number of apoptotic nuclei. The number of apoptotic nuclei was increased in BI6727-treated cells as compared to cisplatin-treated cells while the combination of these substances enhanced the apoptotic effect of cisplatin single treatment (Figure 1E). Caspase-3/-7 activity was induced in BI6727-treated as compared to vehicle-treated cells whereas cisplatin treatment did not enhance caspase activity (Figure 1F). Under co-treatment conditions with BI6727 and cisplatin, caspase-3/-7 activity could be stronger induced as compared to cisplatin single treatment. In order to confirm apoptosis induction we also checked cleavage of the chromatin-associated enzyme PARP [poly (ADP-ribose) polymerase 1]. PARP is involved in DNA repair and replication but PARP cleavage has been described to be an early event during apoptosis[28] while cleavage may diminish DNA-repair and replication processes[29]. In KMCH-1 cells PARP cleavage was not detected (data not shown) while in Mz-Ch-1 cells, protein levels of cleaved PARP were slightly induced after BI6727 single treatment and cisplatin/BI6727 combination treatment (Figure 2A and B).
Thus, co-treatment of cisplatin with the PLK-inhibitor BI6727 could (slightly) enhance the cytotoxic effect of the cytostatic drug cisplatin in both cell lines whereas there was evidence of increased apoptosis induction solely in Mz-Ch-1 cells as compared to KMCH-1 cells.
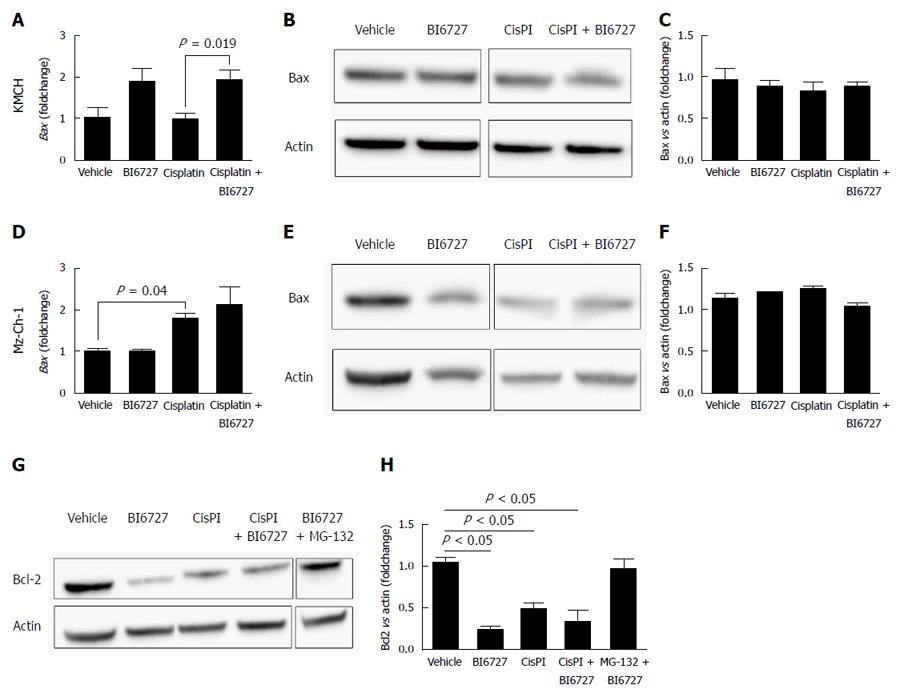
As shown previously, pro-apoptotic effects of BI6727 treatment are in part mediated by Mcl-1 down regulation[8]. As other important regulators of apoptosis, we here aimed to investigate the effect of BI6727 on the anti-apoptotic protein Bcl-2 as well as the pro-apoptotic Bcl-2 family member Bax. We determined Bax mRNA expression levels in KMCH-1 and Mz-Ch-1 cells by qRT-PCR treated with BI6727, cisplatin or both substances. In KMCH-1 cells, BI6727 treatment induced Bax expression and this effect was also present in cells co-treated with BI6727 and cisplatin. Interestingly, cisplatin as a single agent did not induce Bax expression (Figure 3A). In contrast, Bax protein levels, determined via western blot analysis and quantified by densitometric measurement, were not changed in KMCH-1 cells treated with BI6727 and/or cisplatin (Figure 3B and C). In Mz-Ch-1 cells, Bax expression was significantly induced by cisplatin treatment whereas BI6727 treatment had no effect on Bax. Following combination treatment of BI6727 with cisplatin, Bax was not further induced (Figure 3D). Western blot analyses did not show alterations regarding the Bax expression levels in Mz-Ch-1 cells after treatment with one of the components or after combination treatment (Figure 3E and F).
In Mz-Ch-1 cells, protein levels of Bcl-2 were reduced after treatment with cisplatin single treatment, cisplatin/BI6727 combination treatment, and especially BI6727 single treatment (Figure 3G and H). To assess whether the Bcl-2 decrease is a result of proteasomal degradation, cells were co-treated with the potent proteasome inhibitor MG-132. Indeed, inhibition of proteasomal degradation by co-treatment of BI6727 with MG-132 restored Bcl-2 to normal levels similar to the vehicle control.
Thus, PLK inhibition reduces Bcl-2 protein levels in a posttranslational manner by proteasomal degradation resulting in enhanced apoptosis (Figure 3G and H).
This study reveals an additional pro-apoptotic effect of polo-like kinase inhibition in CCA cell lines. The findings indicate that the cytotoxic effect of cisplatin can be enhanced by co-treatment with the PLK inhibitor BI6727 and that PLK inhibition (beside Mcl-1) decreases Bcl-2 via its proteasomal degradation[8].
Cholangiocellular carcinoma represents a deadly disease with rising prevalence in Western countries and its pathogenesis is understood insufficiently. Effective treatment options are still rare due to missing understanding of pathogenic mechanisms. TRAIL has been discussed as an effective agent to target tumor cell growth and to support existing therapy options in different types of cancers[30-32]. However CCA tumor cells already express TRAIL and its cognate receptor in vivo but are resistant to TRAIL[14]. Many other pathways and factors regulating cell growth, migration and invasion have become potential targets in cancer therapy, such as the Wnt, Notch or Hh pathway (which has already been associated to PLK)[8,33-35]. Various specific inhibitors are tested in different studies for their usability as potent anti-cancer drugs or additives to existing therapies[8,36]. The potent PLK inhibitor BI6727 (volasertib) has been identified as a promising candidate for cancer therapy as PLK are upregulated in many different cancers[16,17,37-39]. PLK depletion in different stromal cancer cells using small interfering RNA technique resulted in cell cycle arrest and increased apoptosis[38]. Using KMCH-1 and Mz-Ch-1 CCA cancer cells, we here could demonstrate increased overall cell death and enhanced apoptosis induction following treatment with the PLK-inhibitor BI6727. In combination with the conventional chemotherapeutic drug cisplatin, BI6727 could even (slightly) enhance cell death in KMCH-1 and Mz-Ch-1 cells whereas the pro-apoptotic effect was more potent in MzCh1 as compared to KMCH-1 cells. It is well known that PLK inhibition has an impact on regulation of proteins belonging to the Bcl-2 family as PLK inhibition decreases protein levels of Mcl-1 in esophageal squamous cell carcinomas and osteosarcomas as well as in KMCH-1 cells[8,38,40]. Here, we focused on the effect of BI6727 on the pro-apoptotic protein Bax and the anti-apoptotic molecule Bcl-2. Bax mRNA levels were slightly induced in KMCH-1 and Mz-Ch-1 cells treated with BI6727 and cisplatin, whereas Bax protein levels were not found to be changed in both cell lines. Moreover, Bcl-2 levels were decreased in Mz-Ch-1 cells treated with BI6727 (and cisplatin). This effect was not enhanced after combination of both components. In a previous study we could demonstrate that BI6727 treatment reduced Mcl-1 levels but not Bcl-2 levels after 8 h of incubation[8]. In the present study we observed a significant reduction of Bcl-2 protein levels due to a longer incubation period with BI6727 of 24 h.
Thus, the pro-apoptotic effect of BI6727 treatment appears to be mediated by proteasomal degradation not only of Mcl-1 but also of the anti-apoptotic protein Bcl-2 (without affecting Bax protein levels). Overexpression of Bcl-2 is common in many types of human cancer and has been correlated with decreased susceptibility to chemotherapeutical drugs[22]. However, in CCA, Mcl-1 plays a more pivotal role as tumor cell survival factor than Bcl-2[19,41].
Treatment of CCA cells with the PLK inhibitor BI6727 beside Mcl-1[8] decreases Bcl-2 protein levels thereby reducing cell viability and enhancing apoptosis. In combination with the chemotherapeutical drug cisplatin, BI6727 treatment could even enhance the cytotoxic effect of cisplatin single treatment.
In conclusion, BI6727 treatment sensitizes some CCA cell lines to cisplatin-induced apoptosis with proteasomal Bcl-2 degradation as an additional pro-apoptotic effect.
Most cholangiocarcinoma are chemotherapy-resistant and these tumors show a poor therapeutic prognosis. Development and progression of cholangiocarcinoma are in part mediated by complex mechanisms that prevent tumor cell death by stimulation of death receptors such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Polo-like kinases are important regulators of the cell cycle and their inhibition is discussed as a potential therapeutic approach for cancer treatment as polo-like kinase inhibition can decrease protein levels of anti-apoptotic mediators such as myeloid cell leukemia-1 (Mcl-1). The authors here aimed to study the chemotherapeutic effect of the conventionally used drug cisplatin in combination with polo-like kinase inhibition in cholangiocarcinoma cell lines in order to investigate mechanisms of drug resistance and potential benefits of polo-like kinase inhibition in cancer and especially in cholangiocarcinoma therapy.
The manuscript addresses the very timely and topical roles of cell cycle/apoptosis modulating enzymes for the tumor biology of human cancer and especially in cholangiocarcinoma. In particular, the data suggest that polo-like kinase inhibition can sensitize some cholangiocarcinoma cell lines to cisplatin-induced apoptosis and therefore can address a new mechanism for enhancements of cancer therapies.
It was already known that polo-like kinase inhibition could decrease expression levels of the anti-apoptotic molecule Mcl-1 in cholangiocarcinoma cells. Data of this manuscript reveal another pro-apoptotic mechanism of polo-like kinase inhibition emphasizing the potential therapeutic benefit of polo-like kinase inhibitors for the treatment of cholangiocarcinoma. Polo-like kinase inhibition by BI6727 (volasertib) could enhance cytotoxic effect of cisplatin in cholangiocarcinoma cell lines by reducing expression of the anti-apoptotic molecule Bcl-2 that seems to be mediated via proteasomal degradation.
These data reveal another pro-apoptotic mechanism of polo-like kinase inhibition emphasizing the potential therapeutic benefit of polo-like kinase inhibitors for the treatment of cholangiocarcinoma.
Polo-like kinases: Polo-like kinases are important cell cycle regulating enzymes with a conserved N-terminal kinase domain and a C-terminal polo box domain. Polo-like kinases are involved in formation of the spindle apparatus in mitosis and may activate cdk/cycline complexes of the cell cycle. In different tumors PLK1 has been described to be up regulated. Due to pro-proliferative effects these tumors are associated with enhanced tumor growth and worse outcome and therefore PLK-inhibition is tested as potential cancer treatment. TRAIL: Tumor necrosis factor related apoptosis-inducing ligand belongs to the TNF/TNFR superfamily that can induce apoptosis in target cells via binding to special death receptors. Important target cells represent tumor cells while “normal” non-tumorous cells are less susceptible to TRAIL-induced cell death and mainly remain less harmed compared to tumor cells after treatment with TRAIL. Due to these findings in different in vitro and in vivo experiments TRAIL has been discussed as a potential cancer treatment agent. Interestingly in cholangiocellular carcinoma TRAIL seems to contribute to therapy resistance of tumors.
The manuscript is interesting, but needs more improvements. For example, the confirmation of apoptosis at the molecular level by the analysis of PARP cleave, or caspase-3 using western blot analysis. Also, the analysis of Bax expression at the protein level.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hassan M S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 520] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 3. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1072] [Cited by in F6Publishing: 1199] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 4. | von Hahn T, Ciesek S, Wegener G, Plentz RR, Weismüller TJ, Wedemeyer H, Manns MP, Greten TF, Malek NP. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand J Gastroenterol. 2011;46:1092-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806-813. [PubMed] [Cited in This Article: ] |
| 7. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 856] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 8. | Fingas CD, Mertens JC, Razumilava N, Sydor S, Bronk SF, Christensen JD, Rizvi SH, Canbay A, Treckmann JW, Paul A. Polo-like kinase 2 is a mediator of hedgehog survival signaling in cholangiocarcinoma. Hepatology. 2013;58:1362-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 10. | Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 645] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 11. | Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129-G136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1924] [Cited by in F6Publishing: 1897] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 13. | Guicciardi ME, Mott JL, Bronk SF, Kurita S, Fingas CD, Gores GJ. Cellular inhibitor of apoptosis 1 (cIAP-1) degradation by caspase 8 during TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Exp Cell Res. 2011;317:107-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Fingas CD, Blechacz BR, Smoot RL, Guicciardi ME, Mott J, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. A smac mimetic reduces TNF related apoptosis inducing ligand (TRAIL)-induced invasion and metastasis of cholangiocarcinoma cells. Hepatology. 2010;52:550-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, Kaufmann SH, Gores GJ. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517-3524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Juntermanns B, Sydor S, Kaiser GM, Jaradat D, Mertens JC, Sotiropoulos GC, Swoboda S, Neuhaus JP, Meng W, Mathé Z. Polo-like kinase 3 is associated with improved overall survival in cholangiocarcinoma. Liver Int. 2015;35:2448-2457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Schöffski P. Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist. 2009;14:559-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Kurita S, Mott JL, Cazanave SC, Fingas CD, Guicciardi ME, Bronk SF, Roberts LR, Fernandez-Zapico ME, Gores GJ. Hedgehog inhibition promotes a switch from Type II to Type I cell death receptor signaling in cancer cells. PLoS One. 2011;6:e18330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, Gores GJ. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42:1329-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Okaro AC, Deery AR, Hutchins RR, Davidson BR. The expression of antiapoptotic proteins Bcl-2, Bcl-X(L), and Mcl-1 in benign, dysplastic, and malignant biliary epithelium. J Clin Pathol. 2001;54:927-932. [PubMed] [Cited in This Article: ] |
| 21. | Fingas CD, Katsounas A, Kahraman A, Siffert W, Jochum C, Gerken G, Nückel H, Canbay A. Prognostic assessment of three single-nucleotide polymorphisms (GNB3 825C& gt; T, BCL2-938C& gt; A, MCL1-386C& gt; G) in extrahepatic cholangiocarcinoma. Cancer Invest. 2010;28:472-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1400] [Cited by in F6Publishing: 1413] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 24. | Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1249] [Cited by in F6Publishing: 1258] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 25. | Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869-1883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1561] [Cited by in F6Publishing: 1820] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 26. | Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J, Haslinger C, Garin-Chesa P, Adolf GR. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15:3094-3102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 27. | Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares H, Smulson ME. Involvement of PARP and poly(ADP-ribosyl)ation in the early stages of apoptosis and DNA replication. Mol Cell Biochem. 1999;193:137-148. [PubMed] [Cited in This Article: ] |
| 29. | Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976-3985. [PubMed] [Cited in This Article: ] |
| 30. | White-Gilbertson SJ, Kasman L, McKillop J, Tirodkar T, Lu P, Voelkel-Johnson C. Oxidative stress sensitizes bladder cancer cells to TRAIL mediated apoptosis by down-regulating anti-apoptotic proteins. J Urol. 2009;182:1178-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Zheng SJ, Wang P, Tsabary G, Chen YH. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest. 2004;113:58-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000;97:1754-1759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 377] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 33. | Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, Ridgway RA, Samuel K, Van Rooijen N, Barry ST. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 34. | Zender S, Nickeleit I, Wuestefeld T, Sörensen I, Dauch D, Bozko P, El-Khatib M, Geffers R, Bektas H, Manns MP. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23:784-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | Pinter M, Sieghart W, Schmid M, Dauser B, Prager G, Dienes HP, Trauner M, Peck-Radosavljevic M. Hedgehog inhibition reduces angiogenesis by downregulation of tumoral VEGF-A expression in hepatocellular carcinoma. United European Gastroenterol J. 2013;1:265-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, Gürtler U, Garin-Chesa P, Lieb S, Quant J. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 650] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 37. | Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 38. | Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci USA. 2003;100:5789-5794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 391] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 39. | Weichert W, Ullrich A, Schmidt M, Gekeler V, Noske A, Niesporek S, Buckendahl AC, Dietel M, Denkert C. Expression patterns of polo-like kinase 1 in human gastric cancer. Cancer Sci. 2006;97:271-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Feng YB, Lin DC, Shi ZZ, Wang XC, Shen XM, Zhang Y, Du XL, Luo ML, Xu X, Han YL. Overexpression of PLK1 is associated with poor survival by inhibiting apoptosis via enhancement of survivin level in esophageal squamous cell carcinoma. Int J Cancer. 2009;124:578-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Bertram S, Padden J, Kälsch J, Ahrens M, Pott L, Canbay A, Weber F, Fingas C, Hoffmann AC, Vietor A. Novel immunohistochemical markers differentiate intrahepatic cholangiocarcinoma from benign bile duct lesions. J Clin Pathol. 2016;69:619-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |