Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2757
Peer-review started: December 18, 2016
First decision: January 19, 2017
Revised: February 9, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 21, 2017
To evaluate the changes in the 8th edition American Joint Committee on Cancer (AJCC) for defining stage IB and IIA pancreatic cancer and identify their prognostic factors.
Pancreatic cancer patients were selected from the Surveillance Epidemiology and End Results database (1973-2013). The enrolled patients were divided into IB and IIA groups based on tumor size according to the 8th edition AJCC criteria. Clinical characteristics, including age, gender, race, tumor size, primary site, and grade were summarized. Univariate and multivariate analyses were performed to explore the prognostic factors of the IB and IIA stages of pancreatic cancer under new criteria.
A total of 1349 pancreatic cancer patients were included. More patients had stage IB rather than stage IIA. Stage IB tumors (54.85%) were mainly located in the head of the pancreas, while stage IIA tumors were more often located in the tail and head of the pancreas (35.21% and 31.75%, respectively). The survival time of stage IB and IIA patients had no significant difference. Univariate and multivariate analyses indicated that the prognostic factors of survival for stage IB and IIA patients were different. For stage IB patients, age and primary site were the independent prognostic factors; for stage IIA patients, age and grade were the independent prognostic factors. The risk of death was lower among patients aged ≤ 65 years than those aged > 65 years.
The prognostic factors for stage IB and IIA patients are different, but age is the independent prognostic factor for all patients. The survival time of stage IB and IIA patients has no significant difference.
Core tip: The 8th edition American Joint Committee on Cancer TNM criteria for pancreatic cancer emphasize the tumor size cutoff point of 4 cm for the first time. Thus we used the Surveillance Epidemiology and End Results database, a population-based database, to evaluate the new changes in pancreatic cancer staging and the prognostic factors of stage IB and IIA pancreatic cancer.
- Citation: Li Y, Tang CG, Zhao Y, Cao WY, Qu GF. Outcomes and prognostic factors of patients with stage IB and IIA pancreatic cancer according to the 8th edition American Joint Committee on Cancer criteria. World J Gastroenterol 2017; 23(15): 2757-2762
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2757.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2757
Pancreatic cancer is an aggressive and devastating disease, which is characterized by invasiveness, rapid progression, and profound resistance to treatment[1-3]. The incidence of pancreatic cancer in the United States and western Europe is 10/100000 per year and almost approaches mortality[4]. The overall survival rate at 5 years is less than 5%[5,6]. Surgical resection is still the only treatment providing prolonged survival; however, even after a curative resection, the 5-year survival rate remains low[7].
Tumor size is the basis of cancer staging, which is one of the strongest prognostic factors in various cancers, including pancreatic cancer[8-10]. Compared with the 6th and 7th edition of the American Joint Committee on Cancer (AJCC) system for defining stage IB pancreatic cancer (IB: tumor diameter > 2 cm, no regional lymph node metastasis, no distant metastasis)[11], the 8th edition AJCC criteria emphasize the cutoff point of 4 cm (IB: tumor diameter > 2 but ≤ 4 cm; IIA: tumor diameter > 4 cm, both with no regional lymph node metastasis and no distant metastasis)[12,13]. Clinically, the size and location of pancreatic tumor determine the type of surgical resection[14-16], which suggests the important role of tumor size and location. Therefore, the aim of this study was to evaluate the changes in the AJCC system for defining stage IB and IIA pancreatic cancer and identify their prognostic factors.
The Surveillance Epidemiology and End Results (SEER) database (1973-2013) was used for the study. The National Cancer Institute’s SEER*Stat software (Version 8.2.0) was used to identify patients. All patients underwent surgical treatment and had a pathologically confirmed diagnosis of stage IB pancreatic tumor according to the 6th and 7th edition of the AJCC criteria. Patients with unknown tumor size were excluded. Demographics, including age, gender, and race, were retrieved. Tumor variables included location of the primary tumor, tumor size, and grade. Survival data were extracted at 1 mo intervals for a follow-up period between 1 mo and 110 mo.
The enrolled patients were divided into two groups based on tumor size according to the 8th edition AJCC criteria (IB: tumor diameter > 2 but ≤ 4 cm, IIA: tumor diameter > 4 cm). The independent t-test and the χ2 test were used for the difference analysis between the two groups. Univariate analysis with the log-rank test and multivariate analysis with the Cox proportional hazards regression model were performed to explore the difference in prognostic factors between the two groups, with P values < 0.05 considered statistically significant. All analyses were performed using SPSS software, version 13.0 for Windows.
A total of 1349 pancreatic cancer patients were selected from the SEER database. The age of the patients ranged from 18 to 90 years, with a median age of 65 years. There were 626 male patients and 723 female patients. The median tumor diameter was 43.4 mm (range, 21-540 mm). The pathological stage was classified as IB in 886 patients and IIA in 463 patients, according to the AJCC 8th criteria. The total median survival of these patients was 62 mo, and their 1-, 3-, and 5-year survival rates were 83.8%, 58.9%, and 50.6%, respectively. The patients’ clinical characteristics are presented in Table 1.
| Characteristic | Entire cohort | Stage IB | Stage IIA | P value |
| Number of patients | 1349 | 886 | 463 | |
| Age (yr), median (range) | 65 (18-90) | 65 (18-90) | 63 (18-90) | 0.000 |
| Tumor size (mm), mean (range) | 43.4 (21-540) | 29.5 (21-40) | 70.0 (41-540) | 0.000 |
| Gender | ||||
| Male | 626 (46.40) | 405 (45.71) | 221 (47.73) | 0.480 |
| Female | 723 (53.60) | 481 (54.29) | 242 (52.27) | |
| Primary site | ||||
| Head | 633 (46.9) | 486 (54.85) | 147 (31.75) | 0.000 |
| Body | 165 (12.23) | 103 (11.6) | 62 (13.39) | |
| Tail | 350 (25.95) | 186 (20.99) | 164 (35.21) | |
| Other | 201 (14.90) | 111 (12.53) | 90 (19.44) | |
| Grade | ||||
| I | 348 (25.80) | 221 (24.94) | 127 (27.43) | 0.006 |
| II | 484 (35.88) | 335 (37.81) | 149 (32.18) | |
| III | 230 (17.05) | 164 (18.52) | 66 (14.25) | |
| IV | 17 (1.26) | 10 (1.13) | 7 (1.51) | |
| Unknown | 270 (20.01) | 156 (17.61) | 114 (24.62) | |
| Race | ||||
| White | 1060 (78.58) | 694 (78.33) | 366 (79.05) | 0.760 |
| Other | 289 (21.42) | 192 (21.67) | 97 (20.95) | |
Under the new criteria, the median tumor diameter of stage IB patients was 29.5 mm, while the median tumor diameter of stage IIA patients was 70.0 mm. The primary site of 54.85% of stage IB tumors was the head of the pancreas, while the primary site of stage IIA tumors was mainly the tail and head of the pancreas (35.21% and 31.75%, respectively). Among both stage IB and IIA patients, the majority (approximately 60%) were in grade I and II.
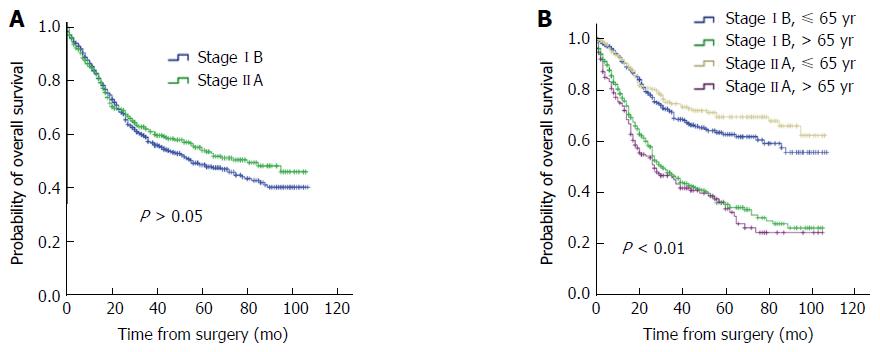
Univariate survival analysis of clinical characteristics was evaluated with a log-rank test (Table 2). Age, grade and primary site were significantly associated with the overall survival of stage IB patients (P < 0.05), while gender and race showed no significant association with survival (P > 0.05). For stage IIA patients, age, gender, grade, and primary site were significantly associated with overall survival (P < 0.05), but race showed no significant association with survival (P > 0.05). Multivariate analyses for stage IB and IIA patients were performed with the Cox regression model (Table 3). The results indicated that for stage IB patients, age and primary site were the independent prognostic factors; for stage IIA patients, age and grade were the independent prognostic factors (P < 0.05). Overall, the survival time of stage IB and IIA patients had no significant difference (Figure 1A); whereas, for both stage IB and IIA, the risk of death was lower for patients aged ≤ 65 years than those aged > 65 years (Figure 1B).
| Variable | Stage IB | Stage IIA | ||
| n | Log-rank | n | Log-rank | |
| Age (yr) | ||||
| ≤ 65 | 444 | 0.000 | 270 | 0.000 |
| > 65 | 442 | 193 | ||
| Gender | ||||
| Male | 405 | 0.162 | 221 | 0.002 |
| Female | 481 | 242 | ||
| Tumor size (mm) | ||||
| 21-30 | 584 | 0.258 | ||
| 31-40 | 302 | |||
| 41-70 | 332 | 0.013 | ||
| > 70 | 131 | |||
| Primary site | ||||
| Head | 486 | 0.000 | 147 | 0.004 |
| Body | 103 | 62 | ||
| Tail | 186 | 164 | ||
| Other | 111 | 90 | ||
| Grade | ||||
| I | 221 | 0.000 | 127 | 0.000 |
| II | 335 | 149 | ||
| III | 164 | 66 | ||
| IV | 10 | 7 | ||
| Unknown | 156 | 114 | ||
| Race | ||||
| White | 694 | 0.148 | 366 | 0.685 |
| Other | 192 | 97 | ||
| Variable | Stage IB | Stage IIA | ||||
| RR | 95%CI | P value | RR | 95%CI | P value | |
| Age | 1.037 | 1.025-1.049 | 0.000 | 1.045 | 1.029-1.062 | 0.000 |
| Gender (male vs female) | 1.128 | 0.887-1.434 | 0.327 | 1.235 | 0.852-1.790 | 0.265 |
| Tumor size | 1.018 | 0.996-1.039 | 0.000 | 1.003 | 0.999-1.008 | 0.173 |
| Primary site (head vs body-tail) | 1.734 | 1.311-2.294 | 0.000 | 1.197 | 0.825-1.736 | 0.345 |
| Grade (I + II vs III + IV) | 0.570 | 0.443-0.734 | 0.104 | 0.490 | 0.333-0.721 | 0.000 |
As one of the most lethal human cancers, pancreatic cancer staging is of important significance clinically. Regardless of how the AJCC definitions of pancreatic cancer staging change, the diameter of the tumor has been shown to be a strong predictor of prognosis. The current cutoff points of > 2 but ≤ 4 and > 4 cm have been proposed to be the sole factor governing the IB and IIA stages in pancreatic cancer. However, the results from the current study suggest that they are not statistically sound, since the patients with stage IB and IIA cancer had similar outcomes (P > 0.05). The findings reported by Burcu[17] contradicts our findings. Thus, further studies or more clinical data are required to evaluate the cutoff point of 4 cm tumor diameter.
In addition, moving to a different staging system has implications and comes with its challenges, such as hampered comparison with earlier data. In this study, all patients were pathologically diagnosed with stage IB pancreatic tumor according to the 6th and 7th edition AJCC criteria, which means the data were discrete over past decades. Therefore, further work needs to be done to evaluate the quality of the data.
Pancreatic cancer can be divided into head and body/tail cancers according to the anatomy. In this study, we found that stage IB tumors were mainly located in the head of the pancreas, while stage IIA tumors were more often located in both the tail and head of the pancreas (35.21% and 31.75%, respectively). Generally, pancreatic development begins with the formation of a ventral and a dorsal bud, which become the ventral head (lower head and uncinate process) and dorsal pancreas (upper head, body, and tail), respectively. This difference in ontogeny leads to significant differences in cell composition, blood supply, lymphatic and venous backflow, and innervations between the head and body/tail of the pancreas[18]. For instance, the number of islets of Langerhans is greater in the body and tail. There have been some reports showing the significance of tumor location in terms of the prognosis of pancreatic tumor. For example, in pancreatic serous cystic neoplasms and intraductal papillary mucinous neoplasms, tumor location in the head of the pancreas was independently associated with local invasiveness and recurrence[19,20], while in pancreatic neuroendocrine tumors, tumors located at the body/tail of the pancreas were more likely to be associated with shorter progression-free survival[21]. Our analysis indicates that tumor location has a correlation with the prognosis in stage IB pancreatic cancer patients.
Prognostic factors combining clinical and laboratory variables with physician’s estimates have been developed in recent years[22]. However, in this study, we just selected patients from the SEER database to analyze the prognostic factors. It is necessary for us to include more variables using our own patient database to verify the new TNM staging system.
In conclusion, our analysis demonstrates that more patients tend to be stage IB rather than stage IIA when they are diagnosed. Overall survival is mainly associated with age and primary site for stage IB patients, while for stage IIA patients, age and grade are the independent prognostic factors. The common independent prognostic factor for both patient groups is age. However, the survival time of stage IB and IIA patients has no significant difference. The results suggest that the new AJCC criteria need further evaluation.
Compared with the 6th and 7th edition American Joint Committee on Cancer (AJCC) system for TNM staging of pancreatic cancer, the 8th edition AJCC criteria emphasize the cutoff point of 4 cm.
AJCC TNM staging of pancreatic cancer has just been updated to the 8th edition. The aim of our study was to evaluate the changes in the AJCC system for defining stage IB and IIA pancreatic cancer - the cutoff point of 4 cm and to identify their prognostic factors.
The prognostic factors for stage IB and IIA patients are different, and age is the common factor. But the survival time of stage IB and IIA patients has no significant difference.
The new AJCC criteria need further evaluation.
This is a large retrospective study of patients undergoing resection for pancreatic cancer. The authors have chosen to look at tumor size as an absolute value for determining survival in patients having resection for pancreatic cancer. The manuscript is succinct and reasonably well written. The figures and tables are appropriate.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bramhall S, Peng SY S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 848] [Cited by in F6Publishing: 824] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 2. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2078] [Cited by in F6Publishing: 2117] [Article Influence: 151.2] [Reference Citation Analysis (2)] |
| 3. | Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1481] [Cited by in F6Publishing: 1512] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 4. | Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician. 2006;73:485-492. [PubMed] [Cited in This Article: ] |
| 6. | Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, Tang L, Klimstra D, Allen PJ. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Smeenk HG, Tran TC, Erdmann J, van Eijck CH, Jeekel J. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005;390:94-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Winter JM, Jiang W, Basturk O, Mino-Kenudson M, Fong ZV, Tan WP, Lavu H, Vollmer CM, Furth EE, Haviland D. Recurrence and Survival After Resection of Small Intraductal Papillary Mucinous Neoplasm-associated Carcinomas (≤20-mm Invasive Component): A Multi-institutional Analysis. Ann Surg. 2016;263:793-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Rohan VS, Hsu JT, Liu KH, Yeh CN, Yeh TS, Jan YY, Hwang TL. Long-term results and prognostic factors in resected pancreatic body and tail adenocarcinomas. J Gastrointest Cancer. 2013;44:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ueda M, Endo I, Nakashima M, Minami Y, Takeda K, Matsuo K, Nagano Y, Tanaka K, Ichikawa Y, Togo S. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009;33:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, Talamonti MS. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 356] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 12. | Cho JH, Ryu JK, Song SY, Hwang JH, Lee DK, Woo SM, Joo YE, Jeong S, Lee SO, Park BK. Prognostic Validity of the American Joint Committee on Cancer and the European Neuroendocrine Tumors Staging Classifications for Pancreatic Neuroendocrine Tumors: A Retrospective Nationwide Multicenter Study in South Korea. Pancreas. 2016;45:941-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Lee SM, Katz MH, Liu L, Sundar M, Wang H, Varadhachary GR, Wolff RA, Lee JE, Maitra A, Fleming JB. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am J Surg Pathol. 2016;40:1653-1660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 632] [Cited by in F6Publishing: 608] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 15. | Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas. 2010;39:458-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Saka B, Balci S, Basturk O, Bagci P, Postlewait LM, Maithel S, Knight J, El-Rayes B, Kooby D, Sarmiento J. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: ≤2, pT2: & gt; 2-≤4, pT3: & gt; 4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol. 2016;23:2010-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Ling Q, Xu X, Zheng SS, Kalthoff H. The diversity between pancreatic head and body/tail cancers: clinical parameters and in vitro models. Hepatobiliary Pancreat Dis Int. 2013;12:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Khashab MA, Shin EJ, Amateau S, Canto MI, Hruban RH, Fishman EK, Cameron JL, Edil BH, Wolfgang CL, Schulick RD. Tumor size and location correlate with behavior of pancreatic serous cystic neoplasms. Am J Gastroenterol. 2011;106:1521-1526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Park J, Lee KT, Jang TH, Seo YW, Lee KH, Lee JK, Jang KT, Heo JS, Choi SH, Choi DW. Risk factors associated with the postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2011;40:46-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Oh TG, Chung MJ, Park JY, Bang SM, Park SW, Chung JB, Song SY. Prognostic factors and characteristics of pancreatic neuroendocrine tumors: single center experience. Yonsei Med J. 2012;53:944-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14:999-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |