Published online Apr 14, 2017. doi: 10.3748/wjg.v23.i14.2511
Peer-review started: November 17, 2016
First decision: December 19, 2016
Revised: January 6, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: April 14, 2017
To investigate the effects of berberine on esophageal cancer (EC) cells and its molecular mechanisms.
Human esophageal squamous cell carcinoma cell line KYSE-70 and esophageal adenocarcinoma cell line SKGT4 were used. The effects of berberine on cell proliferation were evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For cell cycle progression, KYSE-70 cells were stained with propidium iodide (PI) staining buffer (10 mg/mL PI and 100 mg/mL RNase A) for 30 min and cell cycle was analyzed using a BD FACSCalibur flow cytometer. For apoptosis assay, cells were stained with an Annexin V-FITC/PI apoptosis detection kit. The rate of apoptotic cells was analyzed using a dual laser flow cytometer and estimated using BD ModFit software. Levels of proteins related to cell cycle and apoptosis were examined by western blotting.
Berberine treatment resulted in growth inhibition of KYSE-70 and SKGT4 cells in a dose-dependent and time-dependent manner. KYSE-70 cells were more susceptible to the inhibitory activities of berberine than SKGT4 cells were. In KYSE-70 cells treated with 50 μmol/L berberine for 48 h, the number of cells in G2/M phase (25.94% ± 5.01%) was significantly higher than that in the control group (9.77% ± 1.28%, P < 0.01), and berberine treatment resulted in p21 up-regulation in KYSE-70 cells. Flow cytometric analyses showed that berberine significantly augmented the KYSE-70 apoptotic population at 12 and 24 h post-treatment, when compared with control cells (0.83% vs 43.78% at 12 h, P < 0.05; 0.15% vs 81.86% at 24 h, P < 0.01), and berberine-induced apoptotic effect was stronger at 24 h compared with 12 h. Western blotting showed that berberine inhibited the phosphorylation of Akt, mammalian target of rapamycin and p70S6K, and enhanced AMP-activated protein kinase phosphorylation in a sustained manner.
Berberine is an inhibitor of human EC cell growth and could be considered as a potential drug for the treatment of EC patients.
Core tip: Initial diagnosis of many esophageal cancer (EC) patients is made at an advanced stage of the disease, making surgery an undesirable option. Although advances in chemotherapy have been achieved, serious adverse effects usually limit clinical application. Exploring non-invasive strategies to prevent the growth of EC is urgently needed. The current research showed that berberine is an inhibitor of human EC cell growth and could be considered as a potential source of drugs for the treatment of EC patients.
- Citation: Jiang SX, Qi B, Yao WJ, Gu CW, Wei XF, Zhao Y, Liu YZ, Zhao BS. Berberine displays antitumor activity in esophageal cancer cells in vitro. World J Gastroenterol 2017; 23(14): 2511-2518
- URL: https://www.wjgnet.com/1007-9327/full/v23/i14/2511.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i14.2511
Esophageal cancer (EC) is the sixth most common malignant gastrointestinal carcinoma worldwide. More than 50% of the global incidence of EC is in China[1]. A report published in 2016 shows that there are an 477900 and 375000 estimated new EC cases and deaths, respectively, in China[2]. Histologically, EC is divided into two major types: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). More than 90% of EC in China is ESCC. Although advances have been achieved in surgery and chemotherapy, the 5-year survival rate of EC in China is only 19.9%[3]. Esophagostomy is so far the only potentially curative approach for EC, but many patients are at an advanced stage of disease during initial diagnosis, thus ruling them out from surgery. Therefore, there is a critical need to develop alternative and novel approaches in EC therapy.
Berberine is a quaternary ammonium salt derived from Ranunculaceae and Papaveraceae families of plants. Apart from a broad range of bioactivities, such as anti-inflammatory, antibacterial and antidiabetic actions, accumulating studies have revealed that berberine exhibits antitumor properties by interfering with the multiple features of tumorigenesis and tumor development[4]. The antitumor activity of berberine is mainly mediated through the inhibition of cancer cell proliferation by inducing cell cycle arrest at the G1 or G2/M phases and initiation of apoptosis[5,6]. Previous studies have reported that berberine inhibits the phosphatidylinositol 3-kinase (PI3K)/Akt /mammalian target of rapamycin (mTOR)[7,8] signaling cascades to inhibit cell proliferation in various cell lines derived from breast, lung, colon and liver cancer[9-12]. Berberine also activates AMP-activated protein kinase (AMPK), a major regulator of metabolic pathways, subsequently inhibiting mTOR, a downstream target of AMPK[12,13].
Although berberine possesses numerous anticancer activities in various cells, the effect of berberine on EC growth and its mechanism of action have not yet been fully elucidated. In this study, we reported that berberine inhibited EC cell growth by promoting cell cycle arrest at G2/M phase as well as apoptosis. The Akt, mTOR/p70S6K and AMPK signaling pathways were involved in the antitumor activity of berberine on EC.
Berberine hydrochloride was obtained from Ye-Yuan (Shanghai, China). 3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), propidium iodide (PI) cell cycle assay kit, Annexin V-FITC/PI apoptosis detection kit and western blot analysis ECL were purchased from Beyotime (Jiangsu, China). RPMI 1640 and fetal bovine serum (FBS) were obtained from Thermo Fisher Scientific (Waltham, MA, United States). All primary antibodies, including against p21, Akt, p-Akt (Ser473), mTOR, p-mTOR (Ser2448), p70S6K, p-p70S6K (Thr389), AMPK, p-AMPK (Thr172) and β-actin, were from Cell Signaling Technology (Danvers, MA, United States). All other common chemicals and buffers were from Boster (Wuhan, China).
Human ESCC cell line KYSE-70 and EAC cell line SKGT4 were purchased from Kebai Technology (Nanjing, China). The culture medium for both cell lines was RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were incubated in a humidified atmosphere with 5% CO2 at 37 °C.
Cell viability was measured by MTT assay. KYSE-70 (104/well) and SKGT4 (5000/well) were seeded in 96-well culture plates and incubated overnight at 37 °C in a humidified 5% CO2 incubator. On the following day, cells were treated with berberine hydrochloride at indicated concentrations for indicated durations. Then, 10 μL MTT dye was added to each well at a final concentration of 5 mg/mL. For an additional 4 h after incubation, blue MTT formazan crystals were dissolved in 100 μL/well of DMSO. The absorbance at 562 nm was measured on a Multiskan Spectrum microplate reader (Thermo Fisher Scientific). Cell viability was calculated by dividing the OD of samples by the OD of the control group. All experiments were repeated three times.
KYSE-70 cells (8 × 104/well) were seeded in six-well plates in complete culture medium. After incubating for 12 h, cells were treated with berberine hydrochloride (50 μmol/L). Cells were harvested separately at 12 and 24 h later, and immediately fixed with 75% ethanol. For the cell cycle progression analysis, cells were stained with PI staining buffer (10 mg/mL PI and 100 mg/mL RNase A) for 30 min, and fluorescence intensity was measured by BD FACSCalibur (BD Biosciences, San Jose, CA, United States). For apoptosis analysis, cells were stained with the Annexin V-FITC/PI apoptosis detection kit. The rate of apoptotic cells was analyzed using a dual laser flow cytometer and estimated using the ModFit software (BD Biosciences).
Cell lysates were prepared with RIPA lysis buffer (50 mM Tris-HCl, 150 mmol/L NaCl, 0.1% SDS, 1% NP40, 0.5% sodium deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride, 100 μmol/L leupeptin, and 2 μg/mL aprotinin, pH 8.0). Protein extract (20 μg) was subjected to SDS-PAGE and transferred onto nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, United States). After blocking with 5% nonfat dry milk, membranes were incubated at 4 °C overnight with each of the following primary antibodies: p21, pAKT (Ser473), AKT, p-mTOR (Ser2448), mTOR, pp70S6K (Thr389), p70S6K, p-AMPK (Thr172), AMPK (all 1:1000 dilution) and β-actin. Membranes were washed with phosphate buffered saline plus Tween (PBST) buffer and incubated with horseradish peroxidase-conjugated secondary antibodies. After incubation, the membranes were washed three times with PBST and immersed in a SuperSignal West Pico Chemiluminescent Substrate from the detection kit (Thermo Fisher Scientific). Chemiluminescent detection of western blots was performed using an Amersham Imager 600 System (GE Healthcare Bio-Sciences, Pittsburgh, PA, United States).
Data were analyzed using Student’s t-test, and all data were expressed as mean ± SE of the mean. P < 0.05 was considered statistically significant.
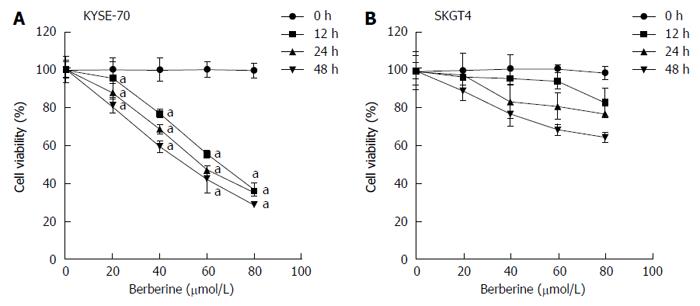
To examine the biological consequences of berberine, we first examined its effect on the proliferation of ESCC and EAC cells. We observed that berberine significantly suppressed KYSE-70 proliferation after treatment with different concentrations (20, 40, 60 and 80 μmol/L) at all tested time points (12, 24 and 48 h) (Figure 1A). Berberine had significantly suppressive effects on SKGT4 cell proliferation when tested at 24 and 48 h after treatment with berberine at 20, 40, 60 or 80 μmol/L. At the 12-h time point, berberine did not significantly inhibit SKGT4 cell proliferation until the concentration reached 80 μmol/L (Figure 1B). Upon comparison of the proliferation inhibitory effects of berberine against the two cell lines, KYSE-70 was more sensitive than SKGT4 to the dose-dependent and time-dependent suppressive effects of berberine. Therefore, we focused further on KYSE-70 cells in the following experiments.
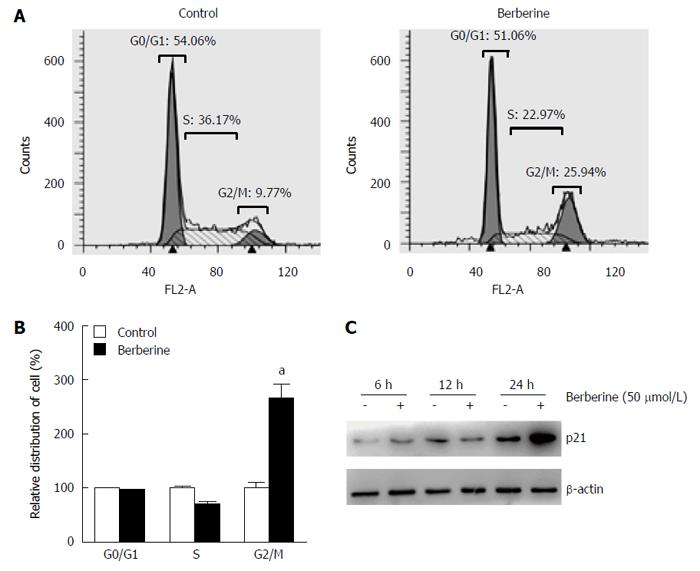
To clarify whether impairment of cell cycle involved in the reduction of KYSE-70 growth was induced by berberine, KYSE-70 cells were treated with 50 μmol/L berberine for 48 h, stained with PI, and subjected to cell cycle progression analysis using flow cytometry. As shown in Figure 2A and B, when compared with the controls, it is evident that the fraction of G2/M cells was increased after berberine treatment (9.77% vs 25.94%, P < 0.01), whereas in parallel, we did not observe significant changes in cell numbers in G0/G1 phase (54.06% vs 51.06%). To explore further the molecular signals involved in berberine-induced G2/M phase arrest, Western blot analysis was used to determine the expression of p21; a key cell cycle negatively regulated protein. As shown in Figure 2C, after application of berberine at 50 μmol/L for 24 h, p21 level was increased. This indicates that berberine-induced cell cycle arrest at G2/M phase in KYSE-70 cells is mediated through p21 down-regulation.
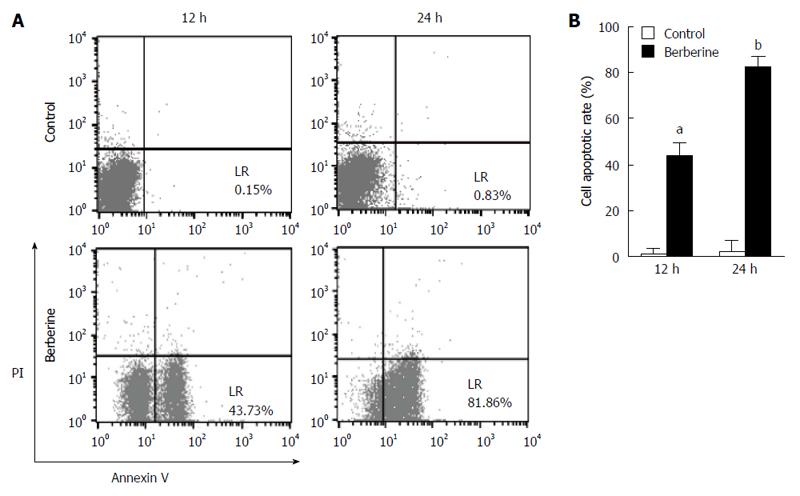
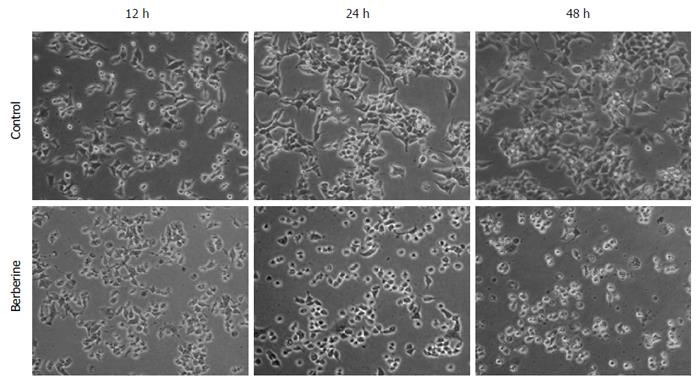
To evaluate whether the antiproliferative activity of berberine was related to its apoptotic effect, KYSE-70 cells were treated with 50 μmol/L berberine, and flow cytometric analyses were performed by double staining with Annexin-V FITC/PI. As shown in Figure 3, berberine significantly increased KYSE-70 cell apoptosis (0.15% vs 43.73% at 12 h, P < 0.05; 0.83% vs 81.86% at 24 h, P < 0.05). We next evaluated the effect of berberine on KYSE-70 cell morphology. Phase contrast imaging (Figure 4) showed that untreated control KYSE-70 cells were epithelial-like adherent cells, with a flat and polygonal shape, that grew homogeneously and showed strong refraction. When treated with berberine, the cells showed reduced refraction and shrunk to a round shape. The treated cells grew in a scattered way, resulting in loss of intercellular conjunction. Consistent with the data in Figure 1, phase contrast imaging showed that berberine suppressed proliferation and promoted apoptosis.
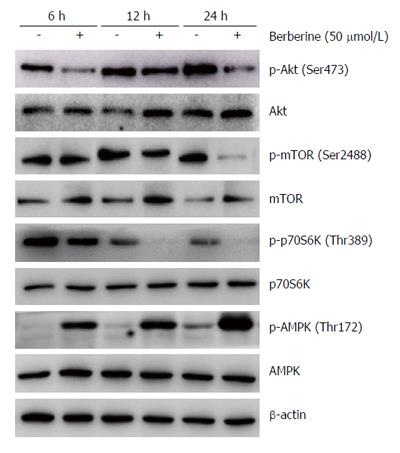
Previous studies have indicated that inhibiting Akt/mTOR/p70S6K signaling and activating AMPK contribute to berberine-induced loss of cell viability[9,14]. To address whether these signaling molecules are related to the biological consequences of berberine in KYSE-70 cells, western blot analyses were performed to examine the phosphorylation levels of these signaling molecules. Cells were treated with 50 μmol/L berberine for 6, 12 or 24 h, in comparison with control cells at each time point. Berberine markedly reduced phosphorylation of Akt at Ser473, mTOR at Ser2448 and p70S6K at Thr389, starting as early as 6 h after treatment and sustaining a reduced level for 24 h. Berberine clearly enhanced AMPK phosphorylation at Thr172 after 6 h treatment, and maintained increasing levels for 24 h after treatment. These data suggest that inhibition of Akt-mTOR/p70S6K and activation of AMPK are important targets of berberine activity (Figure 5).
The low survival rate of EC patients is associated with poor prognosis of the disease and advanced stage at initial diagnosis, thus making surgery an undesirable option. Although advances have been achieved in chemotherapy, the serious adverse effects usually limit clinical application[14]. Therefore, there is a critical need to develop non-invasive strategies to confine the growth or prevent the occurrence of EC.
Compounds derived from plants have been identified as an important source of anticancer therapies and have played a vital role in the prevention and treatment of cancer because of their availability and low toxicity when compared with chemotherapy[15,16]. A compound derived from an alkaloid-containing plant, berberine, has been shown to possess numerous anticancer activities in various cells by interfering with the multiple aspects of tumorigenesis and tumor progression[9-12]. Despite this, it has remained unconfirmed whether berberine exerts an antitumor effect against EC. In the present study, we found that berberine induced strong growth inhibition of the human ESCC cell line KYSE-70 and EAC cell line SKGT4 in a dose-dependent and time-dependent manner. KYSE-70 cells were more susceptible than SKGT4 cells to the inhibitory effects of berberine. Our findings indicate that berberine is a potent inhibitor of human EC cell growth and could be considered as a potential source of drugs for the treatment of EC patients.
Cell cycle arrest and apoptosis are closely linked to cell proliferation in mammalian cells[17]. The major regulatory mechanism of cell growth, the cell cycle dictates the timing of DNA synthesis, and is divided into four distinct phases: M phase (chromosome segregation and mitosis), G1 phase (before DNA replication), S phase (DNA replication) and G2 phase (before mitosis). The cell cycle process includes mechanisms to warrant error amendment, and if not, the cells commit apoptosis, which is one of the most important contributors to the suppression of malignant transformation and elimination of tumors. Control of cell numbers is determined by a complicated balance of cell proliferation and death.
Previous studies have shown that berberine induces cell cycle arrest in various human cancer cells[5,6]. To determine whether berberine prompts cell cycle arrest of KYSE-70 cells, the cell cycle distribution was analyzed by flow cytometry after application of berberine. Our results demonstrated that berberine significantly blocked KYSE-70 cells at the G2/M phase of the cell cycle, suggesting that berberine inhibits KYSE-70 cell proliferation by inducing G2/M cell cycle arrest. These data are in agreement with previous studies in human breast cancer cells and liver cancer cells[6,11]. Appropriate control over cell cycle progression depends on many factors, such as cyclin-dependent kinase inhibitor p21 facilitating cell cycle arrest in response to a variety of stimuli. Our results showed that berberine augmented p21 level in KYSE-70 cells, indicating that berberine-induced cell cycle arrest in G2/M phase may be through regulation of cell cycle protein p21.
The PI3K/Akt/mTOR signaling pathway plays a crucial role in controlling cell proliferation and apoptosis[18]. Constitutive activation of this pathway is considered to be important in cell growth and homeostasis[19]. Specifically, activated mTOR directly phosphorylates many downstream targets including p70S6K to promote protein synthesis[20]. As a major regulator of cellular energy metabolism, AMPK is a negative regulator of the mTOR pathway[20,21]. Berberine regulation of cell proliferation and survival has been shown to involve Akt, mTOR/p70S6K and AMPK signaling pathways[10-12]. Our results showed that berberine treatment inhibited the phosphorylation of Akt and mTOR, as well as mTOR downstream target p70S6K, but enhanced the phosphorylation of AMPK. A previous study reported that, in breast cells, berberine transiently activated AMPK and inhibited AKT, but did not inhibit mTOR activity[22]. Our results showed that treatment with berberine induced sustained alterations (6-24 h) of increased levels of AKT and mTOR phosphorylation in KYSE-70 cells or increased level of AMPK phosphorylation in KYSE-70 cells. These results suggest that berberine alters Akt, mTOR and AMPK activity in an individual cell-dependent manner.
In conclusion, it is suggested that berberine inhibits EC cell growth by promoting cell cycle arrest at G2 phase and the apoptotic process. The Akt, mTOR/p70S6K and AMPK signaling pathways are involved in the antitumor activity of berberine on EC. We have shown that berberine is an inhibitor of human EC cell growth and could be considered as a potential source of drugs for the treatment of EC patients.
The initial diagnosis of many esophageal cancer (EC) patients is at an advanced stage, making surgery an undesirable option. Although advances have been achieved in chemotherapy, serious adverse effects usually limit its clinical application. Therefore, there is an urgent need to find non-invasive strategies to confine the growth or prevent the occurrence of EC. Berberine, a compound derived from an alkaloid-containing plant, has been shown to possess numerous anticancer activities in various cells by interfering with the multiple aspects of tumorigenesis and tumor progression. Despite this, it has remained unconfirmed whether berberine exerts antitumor effects against EC.
Accumulating studies have revealed that berberine exhibits antitumor activity by interfering with the multiple features of tumorigenesis and tumor development.
This study revealed that berberine inhibited EC cell growth by promoting cell cycle arrest at G2/M phase and the apoptosis process. Human esophageal squamous cell carcinoma cells were more susceptible to the inhibitory activity of berberine than human esophageal adenocarcinoma cells. Inhibition of Akt, mTOR/p70S6K and activated AMPK signaling pathways was involved in the antitumor activity of berberine on EC.
Berberine is a quaternary ammonium salt derived from Ranunculaceae and Papaveraceae families of plants. Apart from a broad range of bioactivities that includes anti-inflammatory, antibacterial and antidiabetic activity, berberine has been shown to have antitumor activity, which it exerts by interfering with the multiple features of tumorigenesis and tumor development.
EC is still a cancer with poor prognosis. The testing of chemosensitivity of cancer cells in the esophagus to berberine is important. This enables classification of the condition for selected patients with esophageal cancer to be treated. Berberine can provide treatment options for adjuvant or preoperative treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Zielinski J S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH
| 1. | Sun X, Chen W, Chen Z, Wen D, Zhao D, He Y. Population-based case-control study on risk factors for esophageal cancer in five high-risk areas in China. Asian Pac J Cancer Prev. 2010;11:1631-1636. [PubMed] [Cited in This Article: ] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11444] [Cited by in F6Publishing: 12496] [Article Influence: 1562.0] [Reference Citation Analysis (0)] |
| 3. | Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598-5606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 657] [Cited by in F6Publishing: 695] [Article Influence: 63.2] [Reference Citation Analysis (5)] |
| 4. | Tillhon M, Guamán Ortiz LM, Lombardi P, Scovassi AI. Berberine: new perspectives for old remedies. Biochem Pharmacol. 2012;84:1260-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 5. | Wen C, Wu L, Fu L, Zhang X, Zhou H. Berberine enhances the antitumor activity of tamoxifen in drugsensitive MCF7 and drugresistant MCF7/TAM cells. Mol Med Rep. 2016;14:2250-2256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Yu R, Zhang ZQ, Wang B, Jiang HX, Cheng L, Shen LM. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 2014;14:49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39:935-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Ye B, Jiang LL, Xu HT, Zhou DW, Li ZS. Expression of PI3K/AKT pathway in gastric cancer and its blockade suppresses tumor growth and metastasis. Int J Immunopathol Pharmacol. 2012;25:627-636. [PubMed] [Cited in This Article: ] |
| 9. | Su K, Hu P, Wang X, Kuang C, Xiang Q, Yang F, Xiang J, Zhu S, Wei L, Zhang J. Tumor suppressor berberine binds VASP to inhibit cell migration in basal-like breast cancer. Oncotarget. 2016;7:45849-45862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Xi S, Chuang K, Fang K, Lee Y, Chung J, Chuang Y. Effect of berberine on activity and mRNA expression of N-acetyltransferase in human lung cancer cell line A549. J Tradit Chin Med. 2014;34:302-308. [PubMed] [Cited in This Article: ] |
| 11. | Guamán Ortiz LM, Croce AL, Aredia F, Sapienza S, Fiorillo G, Syeda TM, Buzzetti F, Lombardi P, Scovassi AI. Effect of new berberine derivatives on colon cancer cells. Acta Biochim Biophys Sin (Shanghai). 2015;47:824-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Yang X, Huang N. Berberine induces selective apoptosis through the AMPKmediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol Med Rep. 2013;8:505-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Yi T, Zhuang L, Song G, Zhang B, Li G, Hu T. Akt signaling is associated with the berberine-induced apoptosis of human gastric cancer cells. Nutr Cancer. 2015;67:523-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 14. | Satake H, Tahara M, Mochizuki S, Kato K, Hara H, Yokota T, Kiyota N, Kii T, Chin K, Zenda S. A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol. 2016;78:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Scarpa ES, Emanuelli M, Frati A, Pozzi V, Antonini E, Diamantini G, Di Ruscio G, Sartini D, Armeni T, Palma F. Betacyanins enhance vitexin-2-O-xyloside mediated inhibition of proliferation of T24 bladder cancer cells. Food Funct. 2016;7:4772-4780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Mohan A, Nair SV, Lakshmanan VK. Leucas aspera Nanomedicine Shows Superior Toxicity and Cell Migration Retarded in Prostate Cancer Cells. Appl Biochem Biotechnol. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Chen X, Wu QS, Meng FC, Tang ZH, Chen X, Lin LG, Chen P, Qiang WA, Wang YT, Zhang QW. Chikusetsusaponin IVa methyl ester induces G1 cell cycle arrest, triggers apoptosis and inhibits migration and invasion in ovarian cancer cells. Phytomedicine. 2016;23:1555-1565. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573-578. [PubMed] [Cited in This Article: ] |
| 19. | Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577-590. [PubMed] [Cited in This Article: ] |
| 20. | Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1118] [Cited by in F6Publishing: 1176] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 21. | Meijer AJ, Dubbelhuis PF. Amino acid signalling and the integration of metabolism. Biochem Biophys Res Commun. 2004;313:397-403. [PubMed] [Cited in This Article: ] |
| 22. | Lee KH, Lo HL, Tang WC, Hsiao HH, Yang PM. A gene expression signature-based approach reveals the mechanisms of action of the Chinese herbal medicine berberine. Sci Rep. 2014;4:6394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |