Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2326
Peer-review started: June 6, 2015
First decision: September 29, 2015
Revised: October 18, 2015
Accepted: December 12, 2015
Article in press: December 12, 2015
Published online: February 21, 2016
AIM: To investigate the optimal magnetic pressure and provide a theoretical basis for choledochojejunostomy magnetic compressive anastomosis (magnamosis).
METHODS: Four groups of neodymium-iron-boron magnets with different magnetic pressures of 0.1, 0.2, 0.3 and 0.4 MPa were used to complete the choledochojejunostomy magnamosis. Twenty-six young mongrel dogs were randomly divided into five groups: four groups with different magnetic pressures and 1 group with a hand-suture anastomosis. Serum bilirubin levels were measured in all groups before and 1 wk, 2 wk, 3 wk, 1 mo and 3 mo after surgery. Daily abdominal X-ray fluoroscopy was carried out postoperatively to detect the path and the excretion of the magnet. The animals were euthanized at 1 or 3 mo after the operation, the burst pressure was detected in each anastomosis, and the gross appearance and histology were compared according to the observation.
RESULTS: The surgical procedures were all successfully performed in animals. However, animals of group D (magnetic pressure of 0.4 MPa) all experienced complications with bile leakage (4/4), whereas half of animals in group A (magnetic pressure of 0.1 MPa) experienced complications (3/6), 1 animal in the manual group E developed anastomotic stenosis, and animals in group B and group C (magnetic pressure of 0.2 MPa and 0.3 MPa, respectively) all healed well without complications. These results also suggested that the time required to form the stoma was inversely proportional to the magnetic pressure; however, the burst pressure of group A was smaller than those of the other groups at 1 mo (187.5 ± 17.7 vs 290 ± 10/296.7 ± 5.7/287.5 ± 3.5, P < 0.05); the remaining groups did not differ significantly. A histologic examination demonstrated obvious differences between the magnamosis groups and the hand-sewn group.
CONCLUSION: We proved that the optimal range for choledochojejunostomy magnamosis is 0.2 MPa to 0.3 MPa, which will help to improve the clinical application of this technique in the future.
Core tip: This study introduced a magnetic anastomosis device and verified the feasibility of magnetic compression anastomosis (magnamosis) in choledochojejunostomy; moreover, 3D printing technology was used to design and produce magnetic shells of different sizes to explore the optimum magnetic pressure range in choledochojejunostomy. The result of this study provided a more efficient and accurate theoretical basis for clinical application of choledochojejunostomy magnamosis in the future.
- Citation: Xue F, Guo HC, Li JP, Lu JW, Wang HH, Ma F, Liu YX, Lv Y. Choledochojejunostomy with an innovative magnetic compressive anastomosis: How to determine optimal pressure? World J Gastroenterol 2016; 22(7): 2326-2335
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2326.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2326
Roux-en-Y choledochojejunostomy is known as a standard operation for the treatment of benign biliary stricture or malignant biliary obstruction and a well-developed approach[1]. However, the currently used manual anastomosis technique is time-consuming and associated with a high risk of complications, such as stricture recurrence and anastomotic leak[2]. Staple anastomosis has been introduced to solve this problem, but many limitations remain, such as foreign residues, low histocompatibility, and anastomosis diameter mismatch[3].
The compressive anastomosis technique was invented two centuries ago, and Denans proposed the concept of compressive anastomosis as early as 1826[4]. A new device was invented by Murphy in 1892, which has been referred to as “Murphy’s button” and extensively used in intestinal anastomosis[5,6]. In 1978, Obora[7] first used magnets instead of mechanical devices; this technique cleverly avoids physical contact by using magnetic force, which is field-mediated. With advantages of simpleness, saving time, and low cost, the magnetic compressive anastomosis (magnamosis) has attracted many surgeons to solve a variety of surgical problems. Jansen et al[8] first successfully used magnetic rings for colorectal anastomosis in 1981. Mimuro et al[9] and Akita et al[10] reported many cases of the successful application of magnamosis for biliary strictures and biliary anastomoses in liver transplantation. In 2003, the Ventrica company launched magnetic devices used for vascular side-to-side anastomosis, and these devices were clinically successful[11,12]. Magnamosis has been proven to be a safe surgical technique that is equivalent or superior to anastomosis created by the hand-sewn or stapling technique[13].
Although magnetic approaches have shown promise in choledochojejunostomy, the compressive pressure and magnet specification are based on experience, and significant differences have been reported in these parameters. Furthermore, these parameters often lack systematic research, and a weaker magnetic force may create local ulceration or abscesses due to slow and contained perforation. Stronger attraction can cause severe ischemia and/or lacerating/shearing trauma, which leads to free perforation. We hypothesized that an appropriate range of magnetic pressure can create a viable and durable choledochojejunostomy, and we further proposed that the magnetic pressure affects the quality of anastomosis.
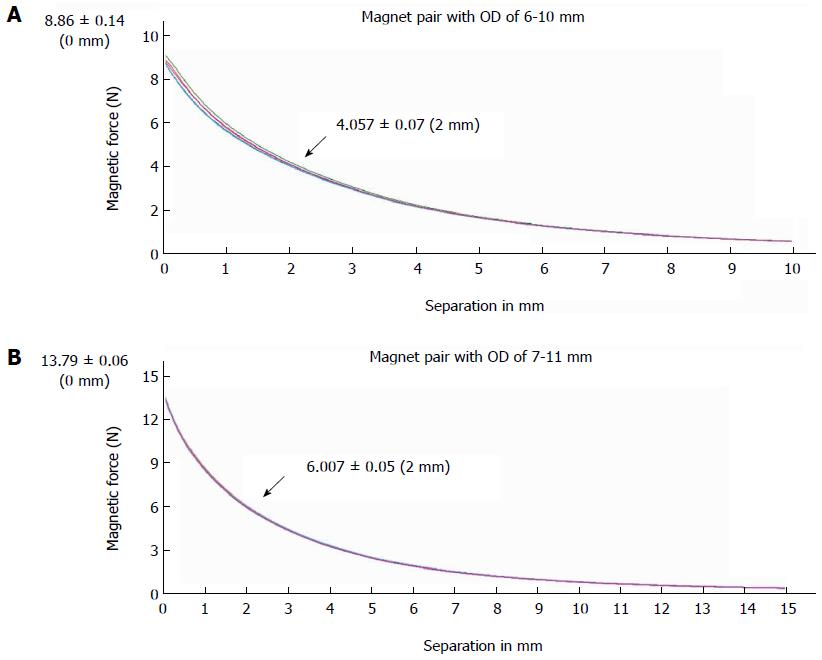
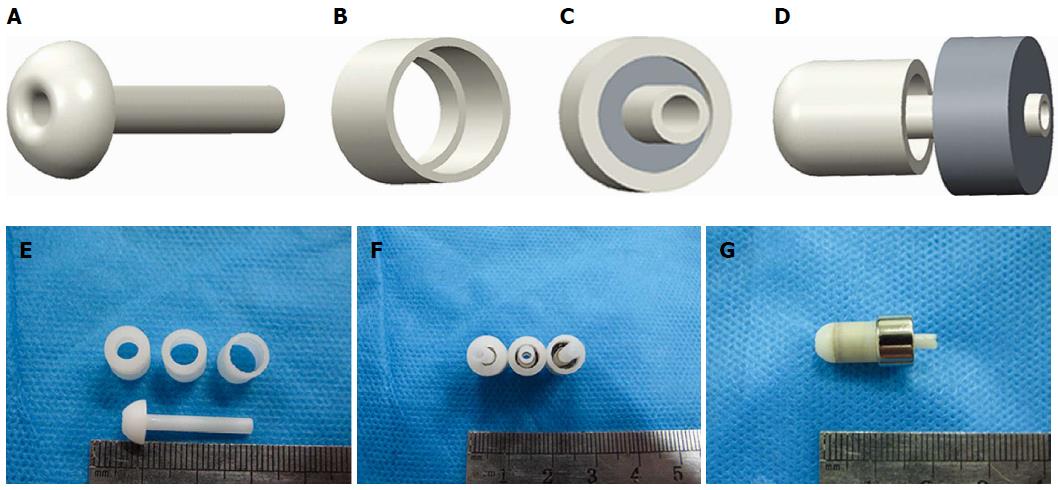
The device used for the end-to-side choledochojejunostomy consisted of two parts: the biliary part and the enteric part. Both parts featured magnetic rings constructed of sintered-type neodymium-iron-boron (NdFeB, N45); the surface field was approximately 2500 GS. These magnetic materials were all plated with titanium film on the external surfaces to maintain material stability and biocompatibility. According to a previous design[14], two magnets for the biliary part were constructed with the following respective outer diameter, inner diameter and height: 6 mm × 2.5 mm × 6 mm and 7 mm × 3 mm × 6 mm. The two magnets for the enteric part were designed with the following respective outer diameter, inner diameter and height: 10 mm × 3 mm × 6 mm and 11 mm × 3 mm × 6 mm. The force-displacement curve of the biliary-enteric magnet pair with outside diameters of 6 mm-10 mm and 7 mm-11 mm were measured using a universal tensile testing machine (UTM6202, Suns Technology Stock Co., Ltd., Shenzhen, China) (Figure 1). The magnetic forces of different magnet pairs (6-10 mm, 6-11 mm, 7-10 mm, and 7-11 mm) at separation distances of 0 mm, 2 mm and 4 mm are given in Table 1.
| Separation (mm) | Outside diameter of biliary - enteric magnet pairs (mm) | |||
| 6-10 | 6-11 | 7-10 | 6-11 | |
| 0 | 8.86 ± 0.14 | 8.24 ± 0.46 | 14.63 ± 0.24 | 13.79 ± 0.06 |
| 2 | 4.06 ± 0.07 | 4.04 ± 0.16 | 5.82 ± 0.09 | 6.01 ± 0.05 |
| 4 | 2.15 ± 0.03 | 2.30 ± 0.06 | 2.99 ± 0.05 | 3.27 ± 0.04 |
To optimize the magnetic pressure for choledochojejunostomy, four different pressures were tested: 0.1 MPa, 0.2 MPa, 0.3 MPa, and 0.4 MPa (1 MPa = 1 N/mm2). According to P = F/S, the pressure can be changed by adjusting the crimping area at a constant force. Based on these calculations, we designed a series of magnetic outer shells with different crimping areas to meet the required predetermined pressure values. The shells were made of photosensitive resin and produced using a three dimensional (3-D) printer (Figure 2). The shells feature an internal drainage duct with an inside diameter of 1.5 mm at the middle, which allowed bile to flow through and the enteric part to precisely couple with the biliary part.
Twenty-six mongrel male dogs older than 1 year and weighing more than 15 kg were provided by the Experimental Animal Center (SYXK-SHAN 2014-003) of the School of Medicine of Xi’an Jiaotong University. Male animals were selected because they cannot menstruate or become pregnant. The animals were randomly assigned to five groups: A (n = 6) - choledochojejunostomy with 0.1 MPa magnetic pressure, B (n = 6) - choledochojejunostomy with 0.2 MPa magnetic pressure, C (n = 6) - choledochojejunostomy with 0.3 MPa magnetic pressure, D (n = 4) - choledochojejunostomy with 0.4 MPa magnetic pressure, and E (n = 4) - choledochojejunostomy with traditional suture. The end-to-side enteroenteric anastomosis for the Roux-en-Y choledochojejunostomy of each group was completed using 3-0 non-absorbable sutures. One and 3 mo after surgery, postoperative complications, the bursting pressure of anastomoses, gross appearance, and pathology were evaluated.
The experimental protocol was approved by the Animal Experimentation Committee of Xi’an Jiaotong University (No. XJTULAC201-398), and the animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. They were euthanized using a barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection, and all dogs received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
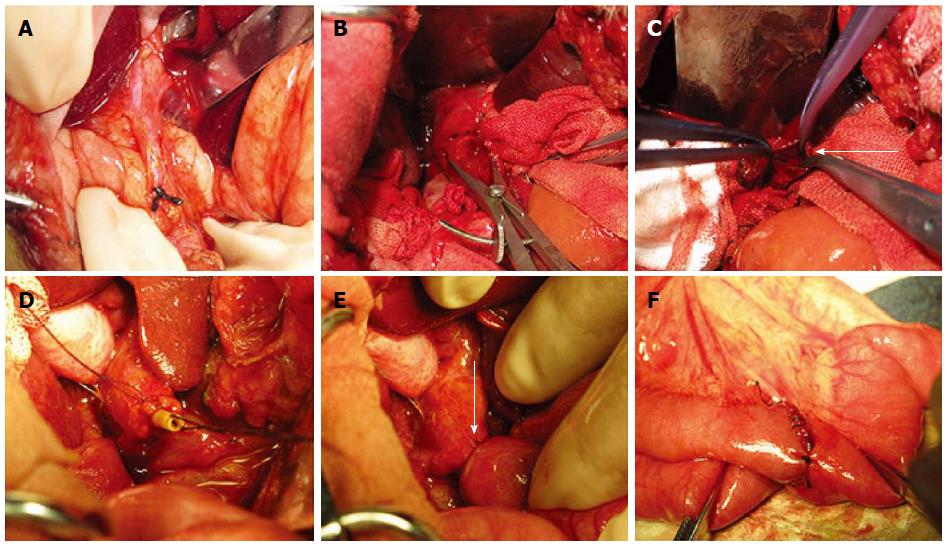
First, a common bile duct dilation animal model was established in all dogs. After being fasted for 12 h and water deprived for 4 h, the dogs were anesthetized by an intraperitoneal injection of 30 g/L pentobarbital (1 mL/kg). Penicillin (2400000 U) was injected intramuscularly 30 min before surgery to avoid postoperative infection. After disinfection with povidone iodine, sterile towels were placed and an abdominal midline incision was made. By fully exposing the hepatoduodenal ligament, the common bile duct could be easily identified. 3-0 Mersilk (Ethicon; Johnson & Johnson Medical Ltd., Shanghai, China) sutures were used to ligate the distal end of the common bile duct near the duodenum (Figure 3A). Water was freely available after recovery from anesthesia, but food was not provided until 2 d later. Penicillin (2400000 U) was injected twice daily after surgery for 3 consecutive days.
Ten days after ligation, the dogs showed obvious symptoms of biliary obstruction such as dark-yellow urine and clay-colored stools. Serum bilirubin also significantly increased, confirming that the animal model was successfully established (Figure 3B).
The repair and reconstruction process was then performed. After the same presurgical procedures described in the common bile duct ligation operation, a second laparotomy was performed. The jejunum was dissociated and cut off approximately 15 cm away from the ligament of Treitz, and the distal end was closed with a suture. An end-to-side anastomosis was created between the proximal end and jejunum 50 cm away from the distal end with a double-layer suture of 3-0 Mersilk (Ethicon; Johnson & Johnson Medical Ltd., Shanghai, China).
In groups A, B, C and D, duct parts of the devices of different magnetic pressures were inserted into the proximal end of the common bile duct, and the stump of the bile duct was purse-string fixed with 5-0 Vicryl (Ethicon; Johnson & Johnson, Somerville, New Jersey, United States) and ligated onto the internal drainage tube. The jejunum was then punched 5 cm distal to the raised loop, and the enteric part magnet was inserted into the jejunum and coupled with the duct part magnet under the guidance of a drainage tube through the punch hole. After confirming that the common bile duct wall and the intestinal wall were clamped between the two magnets, the stump of the jejunum was then closed with 3-0 non-absorbable sutures.
Group E was subjected to a hand-sewn biliary-enteric bypass using 5-0 Vicryl, and full-thickness puncture and the mucosa-to-mucosa contact of the duct and jejunum were confirmed. The abdominal wall was then closed layer by layer (Figure 3C-E).
No food was allowed until the third day after the operation, and water was freely available. Penicillin sodium (2400000 U) was postoperatively injected intravenously twice daily for 3 d.
Blood samples for total bilirubin tests were collected at each time point, including before the ligation of the common bile duct and 10 d after ligation; 1, 2 and 3 wk after choledochojejunostomy and 1 and 3 mo after surgery.
Postoperative complications mainly included biliary stenosis and bile leakage. Biliary stenosis is reflected by recurrent jaundice and a rebound in the total bilirubin. Bile leakage can be judged by the animal state and postoperative cholangiography results. In case of death, dogs were carefully autopsied to determine the exact cause.
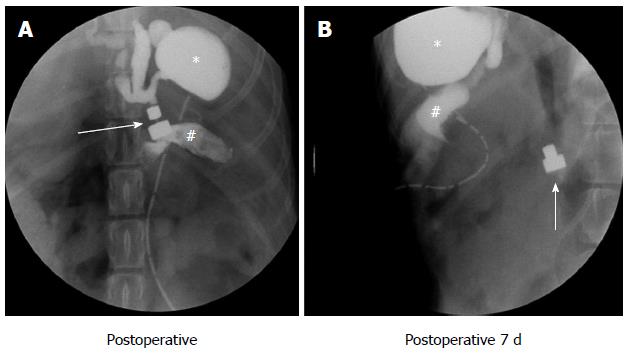
After choledochojejunostomy, a plain abdominal X-ray was immediately taken to confirm the accurate coupling of the two magnets. The X-ray test and cholangiography were carried out every day after surgery to verify the passage of the magnets until they disappeared in the photograph. The time required to shed the magnets from the anastomosis was accurately recorded for different magnet pressures (Figure 4).
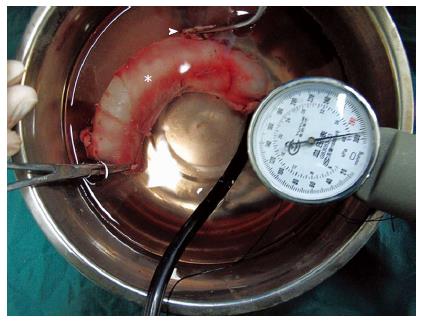
The burst pressure was measured in each anastomosis. The two ends of the anastomotic specimen were ligated using hemostatic forceps or silk sutures, and the third end was attached to the sphygmomanometer and submerged in water. The intraluminal pressure was then gradually increased, and the readings were recorded as air first rose in bubbles to the surface of the water (Figure 5). The maximum hydraulic pressure when the specimen ruptured was recorded.
Dogs from each group were sacrificed at 1 and 3 mo (half of animals were sacrificed at 1 mo, and the other half were sacrificed at 3 mo), and the anastomotic specimens were harvested. After a gross observation, the specimens were cut into sections, fixed in 10% neutral formalin for subsequent mounting and stained on slides.
The statistical methods of this study were reviewed by Qian Li from the First Affiliated Hospital at Xi’an Jiaotong University.
For descriptive statistics, the data were evaluated by analysis of variance (ANOVA) and student’s t-test. In all of the tests, the significant level was set at P < 0.05. The data were analyzed using the SPSS 17.0 software.
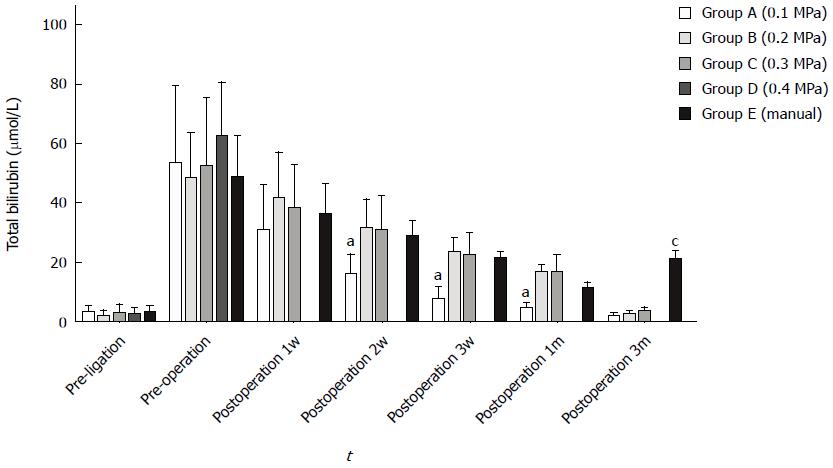
To accurately assess the patency of anastomosis, the initial bilirubin levels were ensured to be normal in each group. These levels significantly increased within 10 d after ligation, decreased during postoperative 1 wk and returned to normal within 1 mo. Bile leakage occurred in group A, which resulted in a faster bilirubin decrease than in the other groups (ANOVA, P < 0.05); in the manual group with stenosis formation, the bilirubin levels slightly increased 3 mo after a decline to normal levels (ANOVA, P < 0.01) (Figure 6).
Four dogs in group A experienced postoperative bile leakage, and 3 animals died of anastomosis leakage because of a failure of coupling. In addition, all dogs in group D died within one week because of severe bile leakage. The total mortality rate of 26 dogs was 27% (7/26), and the incidences of postoperative complications was 34.6% (9/26) (Table 2).
| Group | n | Complications, n | Death, n | Anastomotic molding time (d), mean ± SD |
| A | 6 | 4 | 3 | 9.3 ± 0.6 |
| B | 6 | 0 | 0 | 6.3 ± 0.82 |
| C | 6 | 0 | 0 | 4.5 ± 0.84 |
| D | 4 | 4 | 4 | / |
| E | 4 | 1 | 0 | / |
Daily abdominal radiography was performed strictly to monitor the exact time of anastomosis formation. We found that the magnet shedding time decreased as the pressure gradually increased; the mean times were 9.3 ± 0.6 d for group A, 6.3 ± 0.82 d for group B and 4.5 ± 0.8 d for group D, and these times significantly differed between groups (ANOVA P < 0.05) (Table 2).
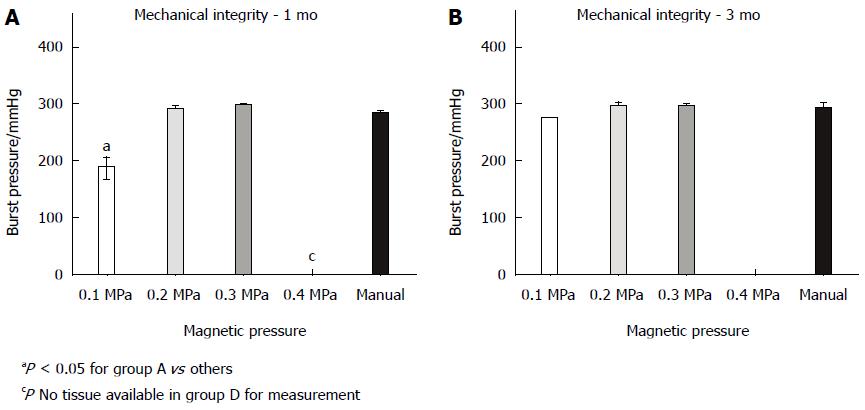
The burst pressures at 1 and 3 mo are displayed in Figure 7. At 1 mo, the burst pressure of group A was 187.5 ± 17.7 mmHg, and this pressure significantly differed from those of groups B (290 ± 10 mmHg), C (296.7 ± 5.7 mmHg) and E (287.5 ± 3.5 mmHg) (P < 0.05). All dogs in group D died of complications; therefore, the exact burst pressure could not be measured. Within 3 mo, significant differences could not be observed between groups A (275 mmHg), B (296.7 ± 5.7 mmHg), C (295 ± 5 mmHg) and E (297.5 ± 3.5 mmHg) (P = 0.052).
One month after the anastomosis, significant differences were observed between the traditional hand-sewn group and groups with magnamosis (Figure 8A1-E1). The suture knots remained evident in the manual group, and the mucosal surfaces appeared uneven and rough due to the interference of suture (Figure 8E1). By contrast, the mucosa of the magnamosis groups was smoother and flatter, but differences were evident between groups: an obvious anastomotic line in the mucosa can be observed in group A (Figure 8A1), whereas the mucosa was smoother in groups B and C, and the anastomosis line was not easily identified (Figure 8B1-C1). Animals in group D all experienced perforation (Figure 8D1, D2). Within 3 mo, all animals exhibited improved gross appearances, and the inner surfaces of anastomoses healed very well (Figure 8A2-C2). Even in the manual group, almost all the suture knots disappeared from the mucosa (Figure 8E2).
Histological observations revealed that all groups healed very well: the submucosal layer, muscular layer and collagen fibers were appropriately organized, and a continuous epithelium migrating from the bile duct wall to the jejunum wall could be observed. Within one month, the mucous of group E sloughed and exhibited higher levels of lymphocyte infiltration compared with other groups due to foreign body stimulation (Figure 9E1). Little difference could be observed between groups A-C, and all showed mild inflammation in the submucosal region (Figure 9A1-C1). Within 3 mo, healing was completed at the anastomosis site in all groups, and the serosal, submucosal, and mucosal layers were continuous without ischemia or necrosis (Figure 9A2-E2). However, the level of lymphocytes in group E remained higher than those in the magnamosis groups (Figure 9E2).
Since the introduction of the concept of magnetic compressive anastomosis (magnamosis), it has been used to resolve certain clinical dilemmas in some case reports[15-17], including a wide range of applications in diseases of the biliary system, mainly in the treatment of biliary strictures and obstructions. Although success is not uncommon, these approaches have lacked a theoretical basis to regulate their use. Because magnamosis relies on transluminal compression, the magnetic pressure must be sufficient to effect ischemia with central necrosis such that a new channel is formed rather than an ulcer or fistula. However, the pressure must allow the surrounding non-compressed tissue to have sufficient time to remodel.
Existing research has reported that an optimized range for the bilioenteric compression force is 0.18 to 0.3 N (18-31 g) and the associated pressure can vary between 1 and 3.5 N/mm2 (1 MPa = 1 N/mm2)[18]. However, these data are based on the author’s retrospective study of previously published studies with the help of the MAGDA online tool (http://magda.ucc.ie). Because the MAGDA tool is too idealistic to simulate the actual magnetic force and the pressure, the accuracy of this conclusion remains to be demonstrated. Thus, we adjusted the magnitude of compression and topology of the mated surfaces in this study to explore the relationship between ischemic processes and pressure and identified the most appropriate range of pressure.
Our research demonstrated the feasibility of magnamosis in performing choledochojejunostomies. The most prominent advantages of this technique are a low incidence of stenosis and the absence of postoperative residual foreign bodies. However, due to the low pressure intensity in group A, segments of tissue sandwiched between two magnets appeared viable, and the remaining ischemic and necrotic tissue eventually formed partially free perforations to cause leakage and death. Furthermore, a very long molding time was required in only three cases. In group D, excessive pressure led to larger cutting forces, which exerted the greatest effect toward the center but did not allow sufficient time for the surrounding tissue to remodel; therefore, all dogs died. In groups B and C, the moderate pressure optimized the effect of anastomosis without any postoperative complications.
To accurately monitor the anastomotic molding time under different pressures, abdominal X-ray examinations should routinely be conducted after surgery. The results showed that the formation time was negatively correlated with the pressure - group C showed the fastest molding time; in one case, the magnets pressed on ischemic necrotic tissue and fell off on the third day. Fortunately, bile leakage was not observed under careful monitoring, the device was found in the feces two days later, and the progression of the dog was uneventful in the following days.
Although the burst pressures at 1 mo and 3 mo after surgery were similar in each group, the suture produced significant inflammation in the hand-sewn group. This inflammation easily led to fibrin deposition around the stoma and severely limited the expansion of anastomosis due to fluctuations in pressure, which increases the risk of stenosis. The burst pressure of group A was slightly lower than those of the other groups 1 mo after surgery; therefore, a lower pressure is the key factor to influence tissue anastomosis. Histopathology showed that layers of tissue did not fully heal, and obvious breakage was observed, which led to the formation of leakage. The anastomoses healed well in the other two groups with sufficient burst pressure, indicating that the magnetic pressures in these groups were ideal to complete the choledochojejunostomy magnamosis.
In conclusion, the inappropriate selection of compression characteristics may incur difficulties. This study proved that the magnetic pressure for choledochojejunostomy anastomosis can vary between 0.1 MPa to 0.3 MPa, and the optimized pressure range is 0.2 MPa to 0.3 MPa. Designing and selecting the appropriate magnet specifications will help both physicians and engineers. Further investigation remains necessary, including finite-element modeling and an analysis of optimized pressure ranges in additional, different tissues, such as gastro-enteric, entero-enteric and vascular tissues. Only such studies can provide a more rational theoretical basis for magnamosis and improve and accelerate its clinical application.
The magnetic compression technique (MCT), which is a simple and effective way of anastomosis, has been applied in gastroenterostomy and bilioenterostomy since it was first proposed in 1978. The authors have designed and successfully applied magnetic devices for choledochojejunostomy anastomosis. However, the blind use of these devices will result in complications. Therefore, the authors examined the effect of magnetic pressure on the effectiveness of anastomosis devices in this study. These devices were tested in animal models to determine the optimal pressure intensity in choledochojejunostomy magnamosis. The result provided a more reliable theoretical basis for the scientific and clinical application of these devices in the future.
MCT utilizes a magnetic field force to achieve organ compression anastomosis, which has been widely researched and applied in bilioenterostomy and hollow organ anastomosis. However, few studies have examined the optimal magnetic pressure, and a theoretical basis is consequently lacking for these devices.
3D printing technology features advantage of high precision and easy operation. Thus, the authors combined MCT with 3D printing technology to design and produce devices used for choledochojejunostomy magnamosis with different magnetic pressures and verified the relationship between pressure gradient and tissue healing to lay a foundation for the further clinical application of these devices.
The scientific and theoretical basis provided in this study has greatly improved the safety and reliability of choledochojejunostomy magnamosis, which can be used for the treatment of obstructive jaundice as a minimally invasive approach that provides stable stomas and allows the patients to be implant free in the long term.
MCT is a novel procedure utilizing magnetic forces for suture-less anastomoses in hollow organs. Combined with endoscopic or interventional techniques, some conventional laparotomies may turn to be solved in a simplified minimal invasive procedure.
The research group performed animal experiments to examine choledochojejunostomy using MCT. Combined with 3D printing technology, the magnetic devices were cleverly designed. The animal study was well designed and executed with great innovativeness.
P- Reviewer: Harrison MR S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Ahrendt SA, Pitt HA. A history of the bilioenteric anastomosis. Arch Surg. 1990;125:1493-1500. [PubMed] [Cited in This Article: ] |
| 2. | Nealon WH, Urrutia F. Long-term follow-up after bilioenteric anastomosis for benign bile duct stricture. Ann Surg. 1996;223:639-645; discussion 645-648. [PubMed] [Cited in This Article: ] |
| 3. | Cirocchi R, Covarelli P, Mazieri M, Fabbri B, Bisacci C, Fabbri C, Bisacci R. [Choledochojejunostomy using a mechanical stapler]. Chir Ital. 1999;51:177-179. [PubMed] [Cited in This Article: ] |
| 4. | Hardy KJ. Non-suture anastomosis: the historical development. Aust N Z J Surg. 1990;60:625-633. [PubMed] [Cited in This Article: ] |
| 5. | Classical articles in colonic and rectal surgery. Cholecysto-intestinal, entero-intestinal anastomosis, and approximation without sutures. Dis Colon Rectum. 1981;24:51-59. [PubMed] [Cited in This Article: ] |
| 6. | Booth CC. What has technology done to gastroenterology? Gut. 1985;26:1088-1094. [PubMed] [Cited in This Article: ] |
| 7. | Obora Y, Tamaki N, Matsumoto S. Nonsuture microvascular anastomosis using magnet rings: preliminary report. Surg Neurol. 1978;9:117-120. [PubMed] [Cited in This Article: ] |
| 8. | Jansen A, Brummelkamp WH, Davies GA, Klopper PJ, Keeman JN. Clinical applications of magnetic rings in colorectal anastomosis. Surg Gynecol Obstet. 1981;153:537-545. [PubMed] [Cited in This Article: ] |
| 9. | Mimuro A, Tsuchida A, Yamanouchi E, Itoi T, Ozawa T, Ikeda T, Nakamura R, Koyanagi Y, Nakamura K. A novel technique of magnetic compression anastomosis for severe biliary stenosis. Gastrointest Endosc. 2003;58:283-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Akita H, Hikita H, Yamanouchi E, Marubashi S, Nagano H, Umeshita K, Dono K, Tsutsui S, Hayashi N, Monden M. Use of a metallic-wall stent in the magnet compression anastomosis technique for bile duct obstruction after liver transplantation. Liver Transpl. 2008;14:118-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Klima U, Falk V, Maringka M, Bargenda S, Badack S, Moritz A, Mohr F, Haverich A, Wimmer-Greinecker G. Magnetic vascular coupling for distal anastomosis in coronary artery bypass grafting: a multicenter trial. J Thorac Cardiovasc Surg. 2003;126:1568-1574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 12. | Klima , Macvaugh Iii, Maringka , Kirschner , Haverich . Clinical experience with the Ventrica distal anastomotic device in coronary surgery. Minim Invasive Ther Allied Technol. 2004;13:26-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Jamshidi R, Stephenson JT, Clay JG, Pichakron KO, Harrison MR. Magnamosis: magnetic compression anastomosis with comparison to suture and staple techniques. J Pediatr Surg. 2009;44:222-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Li J, Lü Y, Qu B, Zhang Z, Liu C, Shi Y, Wang B. Application of a new type of sutureless magnetic biliary-enteric anastomosis stent for one-stage reconstruction of the biliary-enteric continuity after acute bile duct injury: an experimental study. J Surg Res. 2008;148:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Zaritzky M, Ben R, Zylberg GI, Yampolsky B. Magnetic compression anastomosis as a nonsurgical treatment for esophageal atresia. Pediatr Radiol. 2009;39:945-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Marubashi S, Nagano H, Yamanouchi E, Kobayashi S, Eguchi H, Takeda Y, Tanemura M, Maeda N, Tomoda K, Hikita H. Salvage cystic duct anastomosis using a magnetic compression technique for incomplete bile duct reconstruction in living donor liver transplantation. Liver Transpl. 2010;16:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ashikawa K, Komoriyama H, Kumano R, Yamanouchi E. Magnetic compression anastomosis for rectal anastomotic stenosis: report of a case. Brit J Surg. 2010;97:S79-S80. [Cited in This Article: ] |
| 18. | Lambe T, Ríordáin MG, Cahill RA, Cantillon-Murphy P. Magnetic compression in gastrointestinal and bilioenteric anastomosis: how much force? Surg Innov. 2014;21:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |