Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9844
Peer-review started: July 22, 2016
First decision: August 22, 2016
Revised: August 29, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: November 28, 2016
To assess disease-specific circulating microRNAs (miRNAs) in non-alcoholic steatohepatitis (NASH) patients.
A total of 111 biopsy-proven non-alcoholic fatty liver disease (NAFLD) or chronic hepatitis B (CHB) patients and healthy controls from mainland China were enrolled to measure their serum levels of miR-122, -125b, -146b, -16, -21, -192, -27b and -34a. The correlations between serum miRNAs and histological features of NAFLD were determined. The diagnostic value of miRNA in NASH and significant fibrosis was analyzed and compared with that of cytokeratin-18 (CK-18), fibrosis-4 (FIB-4), and aspartate aminotransferase to platelet ratio index (APRI), respectively.
Circulating miR-122, -16, -192 and -34a showed differential expression levels between NAFLD and CHB patients, and miR-34a had an approximately 2-fold increase in NAFLD samples compared with that of CHB samples (P < 0.01). Serum miR-122, -192 and -34a levels were correlated with steatosis (R = 0.302, 0.323 and 0.470, respectively, P < 0.05) and inflammatory activity (R = 0.445, 0.447 and 0.517, respectively, P < 0.01); only serum miR-16 levels were associated with fibrosis (R = 0.350, P < 0.05) in patients with NAFLD. The diagnostic value of miR-34a for NASH (area under the receiver operating characteristic, 0.811, 95%CI: 0.670-0.953) was superior to that of alanine aminotransferase, CK-18, FIB-4 and APRI in NAFLD, but miR-16 showed a limited performance in the diagnosis of significant fibrosis in NASH.
Circulating miR-34a may serve as a disease-specific noninvasive biomarker for the diagnosis of NASH.
Core tip: Circulating miR-122, -192, -34a and -16 showed differential expression levels between non-alcoholic fatty liver disease and chronic hepatitis B patients, and serum miR-122, -192 and -34a could also differentiate non-alcoholic steatohepatitis (NASH) from non-alcoholic fatty liver. The latter were correlated with steatosis and inflammatory activity, and only serum miR-16 was associated with hepatic fibrosis. The diagnostic value of miR-34a for NASH was superior to that of alanine aminotransferase and cytokeratin-18, but miR-16 showed a slightly poorer performance than that of fibrosis-4 and aspartate aminotransferase to platelet ratio index in the diagnosis of significant fibrosis in NASH.
- Citation: Liu XL, Pan Q, Zhang RN, Shen F, Yan SY, Sun C, Xu ZJ, Chen YW, Fan JG. Disease-specific miR-34a as diagnostic marker of non-alcoholic steatohepatitis in a Chinese population. World J Gastroenterol 2016; 22(44): 9844-9852
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9844.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9844
With advances in the quality of life and various lifestyle transitions, non-alcoholic fatty liver disease (NAFLD) has become a growing public health concern worldwide, with a prevalence of about 15% in the general population of China[1]. NAFLD refers to a disease spectrum with progressive histological changes ranging from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH), which is characterized by hepatocyte injury with the presence of hepatic steatosis. If left untreated, NASH will result in irreversible liver damage, such as fibrosis, cirrhosis and hepatocellular carcinoma (HCC)[2]. Currently, NASH is the most common cause of chronic liver disease and the second most common indication for liver transplantation in America[3]. Thus, there is an urgent need for improved detection of NASH. Invasive liver biopsy remains the gold standard for the diagnosis of NASH, and thus there is a strong interest in the development of noninvasive biomarkers for this disease, such as serum cytokeratin-18 (CK-18) and ferritin[4,5]. However, these serum biomarkers do not have sufficient sensitivity or specificity to act as robust predictors of NASH[6].
Chronic hepatitis B (CHB), a global health burden caused by hepatitis B virus (HBV) infection, affects approximately 350 million people worldwide, and 780000 die each year due to HBV-related diseases[7]. In Asia and the western Pacific, especially China, CHB is still a leading cause of cirrhosis and HCC[8-10]. Given the high prevalence of CHB in China, it cannot be excluded in the identification of biomarkers of NAFLD and NASH.
Recently, circulating microRNAs (miRNAs) have emerged as attractive candidate biomarkers for early detection and monitoring of liver disease progression and response to treatment, as they are protected from RNases in the body fluids and are extremely stable[11]. The use of miRNAs as noninvasive biomarkers of NAFLD is of particular interest. Recent studies have identified a series of miRNAs that are dysregulated in NAFLD, among which miR-122, -125b, -146b, -16, -21, -192, -27b and -34a are the most widely reported to contribute to the development of NAFLD and NASH[12-17].
Although many studies have been conducted to identify the biomarkers and mechanisms associated with NAFLD, many issues remain unsolved. For example, there is still a lack of reliable disease-specific biomarkers for the diagnosis of NASH. Therefore, we assessed whether circulating miRNAs can distinguish NAFLD from CHB and also diagnose NASH and significant fibrosis in NAFLD. In the present study, we analyzed the serum expression profile of eight miRNAs (miR-122, -125b, -146b, -16, -21, -192, -27b and -34a) in healthy controls, CHB controls and NAFLD patients from mainland China.
This study was carried out in 111 Chinese Han individuals between May 2012 and May 2014. The inclusion criteria were as follows: (1) age of 18-60 years-old; (2) NAFLD group: evidence of hepatic steatosis by histology; and (3) CHB group: serum hepatitis B surface antigen (HBsAg) positivity for at least 6 mo, a liver biopsy showing chronic hepatitis with moderate or severe necro-inflammation, and patients not meeting the diagnostic criteria for cirrhosis or steatosis. The exclusion criteria were as follows: (1) excessive alcohol consumption (140 g per week for men, 70 g for women); (2) use of hepatotoxic medications or herbal products; (3) other viral hepatitis; (4) diabetes; and (5) pregnancy or lactation. The healthy control group consisted of healthy age- and sex-matched subjects from our institutional staff with normal body mass index (BMI; calculated as body weight/height2), liver enzymes, and abdominal ultrasonography findings. The Ethics Committees of Xinhua Hospital approved this study, and all the subjects provided informed written consent prior to enrollment.
Clinical information, including age, sex, BMI and frequency and average daily consumption of alcohol, was collected at the time of liver biopsy. Blood samples were drawn from an antecubital vein after overnight fasting; the blood analysis, including platelet (PLT) count and levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL) and low density lipoprotein (LDL), were determined with an automatic biochemical analyzer. The fibrosis-4 (FIB-4) index was calculated as follows: age (y) × AST (U/L)/[platelet count (109/L) × ALT (U/L)]1/2[18]. The AST to PLT ratio index (APRI) was calculated as AST (× upper limit of normal)/PLT (109/L) × 100[19]. HBsAg was detected by a commercially available enzyme immunoassay (Abbot Laboratories, Chicago, IL, United States). Additionally, the serum CK-18 M30 and M65 levels were measured using a human CK18-M30 enzyme linked immunosorbent assay (ELISA) Kit (U-1197; Peviva, Nacka, Sweden) and a CK18-M65 ELISA Kit (U-2098; Peviva), respectively.
The liver biopsy specimens were fixed in formalin, embedded in paraffin and stained with hematoxylin-eosin (HE) and Masson. An experienced hepato-pathologist, who was blinded to the clinical data, analyzed the liver biopsies. The steatosis score (S; 0-3) assessed the quantities of large or medium-sized lipid droplets (S0: < 5%; S1: 5%-33%; S2: 34%-66%; S3: > 67%). The activity grade (A; 0-4) was the unweighted addition of hepatocyte ballooning (0-2) and lobular inflammation (0-2). The fibrosis stage (F; 0-4) was assessed as follows: F0: none; F1: 1a or 1b perisinusoidal zone 3 or 1c portal fibrosis; F2: perisinusoidal and periportal fibrosis without bridging; F3: bridging fibrosis; and F4: cirrhosis. NASH was diagnosed based on the steatosis, activity, fibrosis (SAF) score of the fatty liver inhibition of progression (FLIP) algorithm (S ≥ 1A ≥ 2Fany)[20].
Total miRNA was isolated from the serum using a miRNeasy Mini Kit (No. 217004; Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Complementary DNA was synthesized using a TaqMan MicroRNA Reverse Transcription Kit (ABI, Foster City, CA, United States). We performed quantitative real-time polymerase chain reaction (qRT-PCR) on a 7900 HT Sequence Detection System (ABI) using a TaqMan Universal PCR Master Mix (ABI) to assess the distribution of eight miRNAs in serum. The miRNA abundance of miR-122, -125b, -146b, -16, -21, -192, -27b and -34a was normalized to that of miR-1228 and was calculated based on the comparative 2ΔΔCt method. The relative miRNA expression levels were transformed into their natural logarithm to eliminate heteroscedasticity.
All continuous variables are presented as the mean ± standard error of the mean. Statistical comparisons among groups were performed by a one-way analysis of variance followed by a Newman-Keuls post-test for continuous variables and by a χ2 test for categorical variables. Correlations between serum miRNAs and hepatic histological features were assessed using Spearman’s correlation. A P value < 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were plotted, and the area under the ROC (AUROC) was calculated with a 95% confidence interval (CI) to identify the diagnostic efficacy. Calculations were performed using SPSS version 16.0 (Chicago, IL, United States), and graphs were generated using GraphPad Prism Software version 6.0 (San Diego, CA, United States). The statistical methods used in this study were reviewed by Dr. Guang-Yu Chen from the Clinical Epidemiology Center of Shanghai Jiao Tong University School of Medicine.
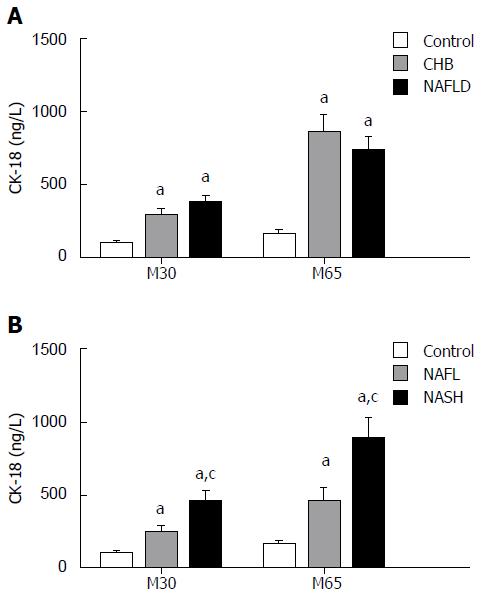
The characteristics of the study subjects are summarized in Table 1 and Table 2. Based on the SAF score, 31 patients were diagnosed with NASH. Age and sex were comparable among the groups. The NAFLD group displayed a significantly higher BMI compared with that of the control and CHB groups, and the NASH group had higher ALT and AST levels than those of the control and NAFL groups (P < 0.05). CK-18 (M30) and CK-18 (M65) levels were higher in the NASH group than in the NAFL group (P < 0.001), but there was no significant difference between the NAFLD and CHB groups (Figure 1).
| Parameters | Group | ||
| Control (n = 37) | CHB (n = 26) | NAFLD (n = 48) | |
| General parameters | |||
| Age (yr) | 43.8 ± 1.3 | 40.1 ± 2.7 | 38.1 ± 1.8 |
| Male n (%) | 18 (48.6) | 16 (61.5) | 35 (72.9) |
| BMI (kg/m2) | 21.4 ± 0.3 | 23.0 ± 0.61 | 26.9 ± 0.512 |
| Biochemical parameters | |||
| ALT (U/L) | 19.1 ± 2.4 | 138.2 ± 30.81 | 68.7 ± 7.412 |
| GGT (U/L) | 19.1 ± 1.9 | 83.8 ± 16.7 | 110.5 ± 35.9 |
| TC (mmol/L) | 4.5 ± 0.1 | 4.5 ± 0.2 | 4.9 ± 0.1 |
| TG (mmol/L) | 0.8 ± 0.1 | 1.7 ± 0.21 | 2.1 ± 0.21 |
| HDL (mmol/L) | 1.4 ± 0.0 | 1.1 ± 0.11 | 1.2 ± 0.01 |
| LDL (mmol/L) | 2.4 ± 0.1 | 2.5 ± 0.1 | 2.9 ± 0.112 |
| Parameters | Group | ||
| Control (n = 37) | NAFL (n = 17) | NASH (n = 31) | |
| General parameters | |||
| Age (yr) | 43.8 ± 1.3 | 37.1 ± 3.1 | 38.1 ± 2.3 |
| Male n (%) | 18 (48.6) | 13 (76.4) | 22 (71.0) |
| BMI (kg/m2) | 21.4 ± 0.3 | 26.3 ± 0.91 | 27.2 ± 0.61 |
| Biochemical parameters | |||
| ALT (U/L) | 19.1 ± 2.4 | 47.8 ± 6.21 | 80.2 ± 10.412 |
| AST (U/L) | 18.8 ± 1.4 | 30.2 ± 3.8 | 51.5 ± 6.012 |
| GGT (U/L) | 19.1 ± 1.9 | 78.2 ± 36.8 | 128.2 ± 51.9 |
| PLT (× 109/L) | 200.4 ± 11.3 | 237.0 ± 14.8 | 207.3 ± 9.1 |
| TC (mmol/L) | 4.5 ± 0.1 | 4.7 ± 0.2 | 5.0 ± 0.1 |
| TG (mmol/L) | 0.8 ± 0.1 | 1.9 ± 0.31 | 2.2 ± 0.31 |
| HDL (mmol/L) | 1.4 ± 0.0 | 1.2 ± 0.11 | 1.2 ± 0.11 |
| LDL (mmol/L) | 2.4 ± 0.1 | 2.9 ± 0.21 | 2.9 ± 0.11 |
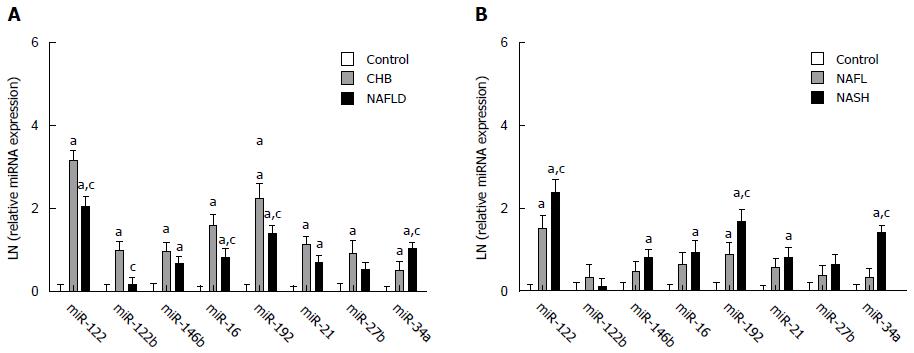
All eight miRNAs, except for miR-125 and -27b, were up-regulated in the NAFLD group compared with that of the controls (P < 0.05), with miR-34a being even higher and miR-122, -16 and -192 being lower in the NAFLD group compared with that of the CHB group (P < 0.01; Figure 2A). Among them, miR-122, -192 and -34a displayed the highest fold-change in NAFLD compared with the controls, which were 7.9, 4.0 and 2.8, respectively. When we divided NAFLD patients into NAFL and NASH subgroups, the results showed that miR-122, -192 and -34a had significant differences not only between the NAFL and the control groups but also between the NASH and the NAFL groups (P < 0.001; Figure 2B).
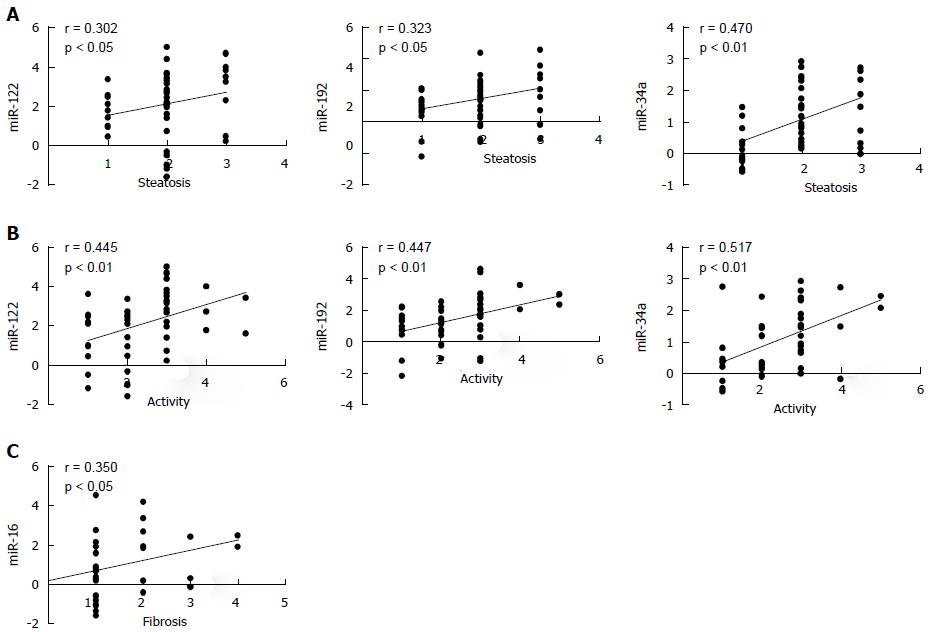
A correlation analysis was performed between serum miRNAs and hepatic histological features in NAFLD patients (Table 3). miR-122, -192 and -34a were correlated with hepatic steatosis (R = 0.302, 0.323 and 0.470, respectively, P < 0.05) and inflammatory activity (R = 0.445, 0.447 and 0.517, respectively, P < 0.01). Notably, miR-34a showed the strongest correlation with lobular inflammation (R = 0.552, P < 0.01), and miR-122 showed the strongest association with hepatocellular ballooning (R = 0.477, P < 0.01). However, only miR-16 was associated with hepatic fibrosis (R = 0.350, P < 0.05; Figure 3).
| R | miR-122 | miR-125b | miR-146b | miR-16 | miR-192 | miR-21 | miR-27b | miR-34a |
| Steatosis | 0.3021 | 0.143 | 0.230 | 0.068 | 0.3231 | 0.199 | 0.166 | 0.4702 |
| Activity | 0.4452 | 0.052 | 0.218 | 0.267 | 0.4472 | 0.3201 | 0.179 | 0.5172 |
| Lobular inflammation | 0.2851 | -0.064 | 0.137 | 0.159 | 0.3962 | 0.254 | 0.184 | 0.5522 |
| Hepatocellular ballooning | 0.4772 | 0.154 | 0.225 | 0.2901 | 0.3501 | 0.281 | 0.101 | 0.3171 |
| Fibrosis | -0.021 | -0.078 | 0.125 | 0.3501 | 0.145 | 0.199 | -0.028 | 0.134 |
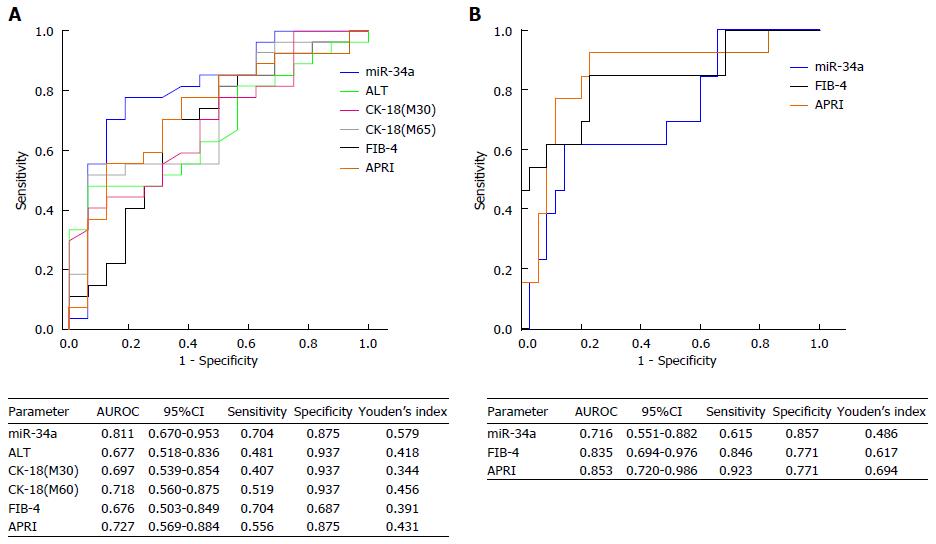
We estimated the value of the eight miRNAs in the diagnosis of NASH and significant fibrosis in patients with NAFLD using a logistic regression model. Upon adjustment for BMI, miR-34a was shown to be a significant predictor of NASH (P < 0.01) and miR-16 was found to be a significant predictor of significant fibrosis (≥ F2; P < 0.05). However, none of the eight miRNAs could predict advanced fibrosis (≥ F3) or cirrhosis. The diagnostic performance of miR-34a for NASH and miR-16 for significant fibrosis was evaluated using ROC analysis. Interestingly, the AUROC of miR-34a for NAFLD was very high (0.811, 95%CI: 0.670-0.953; Figure 4A). Although serum ALT, M30 and M65 had a specificity of 0.937 for the diagnosis of NASH, their sensitivities, which were 0.481, 0.407 and 0.519, respectively, were relatively poor. Fortunately, miR-34a significantly increased the sensitivity to 0.704, with a comparable specificity. Compared with miR-34a, FIB-4 had the same sensitivity (0.704) but lower specificity (0.687) for the diagnosis of NASH, and APRI showed the same specificity (0.875) but lower sensitivity (0.556). For the diagnosis of significant fibrosis, miR-16 displayed a relatively lower AUROC (0.716, 95%CI: 0.551-0.882) than that of FIB-4 (0.835, 95%CI: 0.694-0.976) and APRI (0.853, 95%CI: 0.720-0.986; Figure 4B).
The past decade has witnessed growing interest in miRNAs for their noninvasive diagnostic value in a variety of diseases, including NAFLD. Previous human studies have examined the miRNA expression pattern in NAFLD, and several miRNAs have been proposed to be predictors of this disease[12-17]. However, there is limited information about the disease specificity and correlation with hepatic histological features of these miRNAs. Therefore, our study explored the serum levels of miR-122, -125b, -146b, -16, -21, -192, -27b and -34a in biopsy-proven NAFLD patients, CHB patients, and healthy controls from mainland China. Among these miRNAs, miR-122, -16, -192 and -34a could differentiate NAFLD from healthy controls and CHB patients, and miR-122, -192 and -34a could distinguish NASH from NAFL. In addition, miR-34a showed a superior ability to diagnose NASH compared with that of ALT, CK-18 (M30) and CK-18 (M65).
Among the eight miRNAs selected from previous articles, miR-122, -16, -192 and -34a showed differential expression in NAFLD and CHB, which are the most common liver diseases in China. Interestingly, only miR-34a was up-regulated in NAFLD compared with CHB patients, indicating that miR-34a may be a more sensitive indicator of NAFLD than CHB. Increased miR-34a levels have been reported in the serum and liver of NAFLD patients and animal models[15,16,21], and our results suggested that miR-34a could distinguish NAFLD not only from healthy controls but also from other liver diseases, such as CHB.
miR-122, a liver-enriched and liver-specific miRNA, has received considerable attention as a biomarker of NAFLD. It is released into circulation during hepatocyte damage and has been shown to be key regulator of lipid metabolism[22]. However, increased miR-122 has also been found in many liver conditions, including hepatitis C virus (HCV) and HBV infections and alcohol- and drug-induced liver injury[23-26]. The serum miR-122 levels in CHB patients were much higher than those in NAFLD patients, limiting its value in the diagnosis of NAFLD. Consequently, miR-122 may serve as a marker of general liver damage due to its increased expression in a broad range of liver diseases. In contrast to miR-122, miR-192 has been identified as an oncogene in several cancers, such as colon cancer, breast cancer and gastric cancer[27], and is negatively correlated with the liver metastatic potential of colon cancer cells[28]. Although two independent studies reported increased serum miR-192 levels in NAFLD[12,14], little is known about its function in other liver diseases. Similarly, miR-192 had lower serum levels in NAFLD patients than those of CHB patients, which casts doubt on its diagnostic value in NAFLD.
An intriguing finding in this study is the putative role of serum miR-34a as an extra-hepatic fingerprint of NASH. In recent years, many noninvasive, diagnostic biomarkers have been extensively examined for clinical applications for NASH. CK-18 has potential value in differentiating NASH from NAFL, but consistent with the results by Cusi et al[29], it exhibited limited sensitivity in the diagnosis of NASH in our study. Fortunately, miR-34a significantly increased the sensitivity to 0.704, with a specificity of 0.875. Furthermore, our results showed that neither M30 nor M65 could differentiate etiology-related liver diseases. Compared with CK-18, miR-34a not only had a higher AUROC in the diagnosis of NASH but also had good disease specificity. To the best of our knowledge, we report here the first identification of miRNA biomarkers in NASH with regard to their disease specificity.
Our study found a good correlation between hepatic steatosis and serum miR-122, -192 and -34a, especially for miR-34a. Meanwhile, serum miR-34a levels showed the strongest correlation with the severity of hepatic inflammatory activity, including lobular inflammation and hepatocellular ballooning. miR-34a can down-regulate sirtuin 1 (SIRT1), leading to adenosine monophosphate-activated protein kinase (AMPK) dephosphorylation and ultimately cholesterol accumulation[30]. Other studies reported that miR-34a regulates lipoprotein metabolism and promotes liver steatosis and hypolipidemia in a peroxisome proliferator-activated receptor (PPAR) α-dependent manner[31].
There is also a functional link between aberrantly elevated miR-34a and impaired expression of the cytochrome P450 family and the fibroblast growth factor (FGF) family, which indicates the importance of miR-34a in metabolic disorders[32,33]. In addition, the down-regulation of SIRT1 by miR-34a also increased acetylation of p53, thereby activating apoptosis of hepatocytes[34]. CK-18 and miR-34a reflect a common pathophysiological status, a caspase-related apoptosis pathway, which may explain the good correlation between the serum levels of these markers in our study. In contrast to the findings that miR-34a promotes hepatic fibrosis through activation of hepatic stellate cells (HSCs)[15], our study found no positive relationship between serum miR-34a and hepatic fibrosis in NAFLD patients.
Nevertheless, the circulating miR-16 levels were positively correlated with hepatic fibrosis and showed a good ability to diagnose significant fibrosis in NAFLD, although its diagnostic value was slightly lower than that of FIB-4 and APRI. The FIB-4 and APRI were identified as good biomarkers for the presence and severity of hepatic fibrosis in NAFLD patients[35]. The activation of HSCs is generally considered to be the major mechanism responsible for liver fibrosis. Our previous work showed that miR-16 is related to the activation of cultured rat HSCs and likely participates in HSC apoptosis by targeting Bcl-2 and the downstream protease cascade[36].
Additionally, the up-regulation of miR-16 has been shown to contribute to the development of liver fibrosis and HCC in HCV and HBV infections, respectively[37,38]. miR-16 in visceral adipose tissue also showed an increase in obese NASH patients[17], while the data from liver tissues of NAFLD patients are limited. Therefore, it would be interesting to explore the behavior of miR-16 in livers from NASH patients to confirm the specific function of miR-16 in NAFLD in future studies.
Finally, several limitations of this study need to be noted. The subjects enrolled in our study were Han individuals from mainland China, and the results should be validated in other ethnic groups and races with larger samples. The above results did not provide direct evidence for the involvement of these differentially expressed miRNAs in the progression of NAFLD, but they could serve as the basis for functional research on this disease in the future.
In conclusion, our study demonstrated that the serum levels of miR-122, -16, -192 and miR-34a showed differential expression in NAFLD and CHB patients. Circulating miR-34a had a moderate correlation with hepatic steatosis and inflammatory activity and showed a superior ability to diagnose NASH compared to that of ALT and CK-18, which highlights its potential value as a biomarker of disease severity. In addition, circulating miR-16 exhibited a good correlation with hepatic fibrosis but had a slightly poorer performance than that of FIB-4 and APRI in distinguishing significant fibrosis in NAFLD.
Non-alcoholic fatty liver disease (NAFLD) has become a growing public health concern worldwide. Currently, non-alcoholic steatohepatitis (NASH) is the most common cause of chronic liver disease. Thus, there is an urgent need in the development of noninvasive biomarkers for this disease. In Asia, especially China, chronic hepatitis B (CHB) is still a leading cause of cirrhosis and hepatocellular carcinoma (HCC). Given the high prevalence of CHB in China, it cannot be excluded in the identification of biomarkers of NAFLD and NASH.
Recently, circulating microRNAs (miRNAs) have emerged as attractive candidate biomarkers for early detection and monitoring of disease progression and response to treatment. The use of miRNAs as noninvasive biomarkers of NAFLD is of particular interest.
Recent studies have identified miR-122, -125b, -146b, -16, -21, -192, -27b and -34a as the most common regulators contributing to the development of NAFLD. However, there is still a lack of reliable disease-specific biomarkers for the diagnosis of NASH. Therefore, we assessed whether circulating miRNAs can distinguish NAFLD from CHB and also diagnose NASH and significant fibrosis in NAFLD.
The current study identified that the serum levels of miR-122, -16, -192 and miR-34a could differentiate NAFLD from CHB patients, and that miR-122, -192 and miR-34a could differentiate NASH from NAFL. Circulating miR-34a showed a superior ability to diagnose NASH compared to that of alanine aminotransferase and cytokeratin-18, highlighting its potential value as a biomarker of NASH in clinical practice. Meanwhile, the results above could serve as the basis for functional research on this disease in the future. The subjects enrolled in our study were Han individuals from mainland China, so the results should be validated in other ethnic groups and races with larger samples in the future.
MiRNAs are short noncoding RNAs composed of 18-25 nucleotides that regulate gene expression at the post-transcriptional level. Each miRNA has numerous target genes, and hundreds of miRNAs can regulate one target gene. MiRNAs are important players in a wide spectrum of biological processes and metabolic homeostasis, including protein secretion and fatty acid metabolism. Meanwhile, circulating miRNAs are protected from RNase in circulation and are extremely stable, so they have emerged as attractive candidate biomarkers for early diagnosis of disease and to monitor disease progression.
This paper by Liu et al is an interesting study on a new diagnostic biomarker of NASH. This study examined the performance of a new diagnostic biomarker panel for NASH. They found that serum level of miR-34a correlated with histological features of NASH including steatosis, hepatocyte ballooning and lobular inflammation. This study is well conducted and the methods used are appropriate. The data is presented clearly. These findings will be of interest to [clinical] practitioners as well as researchers in the field.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Namisaki T, Rodrigues GB S- Editor: Yu J L- Editor: Filipodia E- Editor: Liu WX
| 1. | Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 424] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver (EASL), Electronic address: easloffice@easloffice. eu, European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2290] [Cited by in F6Publishing: 2686] [Article Influence: 335.8] [Reference Citation Analysis (2)] |
| 3. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1211] [Cited by in F6Publishing: 1259] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 4. | Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 5. | Goh GB, Issa D, Lopez R, Dasarathy S, Dasarathy J, Sargent R, Hawkins C, Pai RK, Yerian L, Khiyami A. The development of a non-invasive model to predict the presence of non-alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:995-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 370] [Cited by in F6Publishing: 397] [Article Influence: 39.7] [Reference Citation Analysis (2)] |
| 7. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1004] [Cited by in F6Publishing: 1041] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 8. | Kao JH. Risk stratification of HBV infection in Asia-Pacific region. Clin Mol Hepatol. 2014;20:223-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 845] [Article Influence: 84.5] [Reference Citation Analysis (1)] |
| 10. | Chang PE, Wong GW, Li JW, Lui HF, Chow WC, Tan CK. Epidemiology and Clinical Evolution of Liver Cirrhosis in Singapore. Ann Acad Med Singapore. 2015;44:218-225. [PubMed] [Cited in This Article: ] |
| 11. | Weiland M, Gao XH, Zhou L, Mi QS. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol. 2012;9:850-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, Flichman D, Mirshahi F, Sanyal AJ, Sookoian S. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2015;64:800-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 13. | Celikbilek M, Baskol M, Taheri S, Deniz K, Dogan S, Zararsiz G, Gursoy S, Guven K, Ozbakır O, Dundar M. Circulating microRNAs in patients with non-alcoholic fatty liver disease. World J Hepatol. 2014;6:613-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Becker PP, Rau M, Schmitt J, Malsch C, Hammer C, Bantel H, Müllhaupt B, Geier A. Performance of Serum microRNAs -122, -192 and -21 as Biomarkers in Patients with Non-Alcoholic Steatohepatitis. PLoS One. 2015;10:e0142661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Yan G, Li B, Xin X, Xu M, Ji G, Yu H. MicroRNA-34a Promotes Hepatic Stellate Cell Activation via Targeting ACSL1. Med Sci Monit. 2015;21:3008-3015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Braza-Boïls A, Marí-Alexandre J, Molina P, Arnau MA, Barceló-Molina M, Domingo D, Girbes J, Giner J, Martínez-Dolz L, Zorio E. Deregulated hepatic microRNAs underlie the association between non-alcoholic fatty liver disease and coronary artery disease. Liver Int. 2016;36:1221-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Sharma H, Estep M, Birerdinc A, Afendy A, Moazzez A, Elariny H, Goodman Z, Chandhoke V, Baranova A, Younossi ZM. Expression of genes for microRNA-processing enzymes is altered in advanced non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2013;28:1410-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2987] [Article Influence: 165.9] [Reference Citation Analysis (0)] |
| 19. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 2976] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 20. | Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751-1759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 526] [Cited by in F6Publishing: 555] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 21. | Tryndyak VP, Latendresse JR, Montgomery B, Ross SA, Beland FA, Rusyn I, Pogribny IP. Plasma microRNAs are sensitive indicators of inter-strain differences in the severity of liver injury induced in mice by a choline- and folate-deficient diet. Toxicol Appl Pharmacol. 2012;262:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 455] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 23. | Luna JM, Michailidis E, Rice CM. Mopping up miRNA: An integrated HBV transcript disrupts liver homeostasis by sequestering miR-122. J Hepatol. 2016;64:257-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Butt AM, Raja AJ, Siddique S, Khan JS, Shahid M, Tayyab GU, Minhas Z, Umar M, Idrees M, Tong Y. Parallel expression profiling of hepatic and serum microRNA-122 associated with clinical features and treatment responses in chronic hepatitis C patients. Sci Rep. 2016;6:21510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Ambade A, Satishchandran A, Szabo G. Alcoholic hepatitis accelerates early hepatobiliary cancer by increasing stemness and miR-122-mediated HIF-1α activation. Sci Rep. 2016;6:21340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | McGill MR, Jaeschke H. MicroRNAs as Signaling Mediators and Biomarkers of Drug- and Chemical-Induced Liver Injury. J Clin Med. 2015;4:1063-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Chiang Y, Zhou X, Wang Z, Song Y, Liu Z, Zhao F, Zhu J, Xu H. Expression levels of microRNA-192 and -215 in gastric carcinoma. Pathol Oncol Res. 2012;18:585-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Geng L, Chaudhuri A, Talmon G, Wisecarver JL, Are C, Brattain M, Wang J. MicroRNA-192 suppresses liver metastasis of colon cancer. Oncogene. 2014;33:5332-5340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, Ortiz-Lopez C, Hecht J, Feldstein AE, Webb A. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 31. | Ding J, Li M, Wan X, Jin X, Chen S, Yu C, Li Y. Effect of miR-34a in regulating steatosis by targeting PPARα expression in nonalcoholic fatty liver disease. Sci Rep. 2015;5:13729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 32. | Oda Y, Nakajima M, Tsuneyama K, Takamiya M, Aoki Y, Fukami T, Yokoi T. Retinoid X receptor α in human liver is regulated by miR-34a. Biochem Pharmacol. 2014;90:179-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Fu T, Choi SE, Kim DH, Seok S, Suino-Powell KM, Xu HE, Kemper JK. Aberrantly elevated microRNA-34a in obesity attenuates hepatic responses to FGF19 by targeting a membrane coreceptor β-Klotho. Proc Natl Acad Sci USA. 2012;109:16137-16142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, Rodrigues CM. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58:119-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 35. | Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 571] [Cited by in F6Publishing: 564] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 36. | Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J Hepatol. 2009;50:766-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 37. | Zhu B, Wei XX, Wang TB, Zhou YC, Liu AM, Zhang GW. Increased miR-16 expression induced by hepatitis C virus infection promotes liver fibrosis through downregulation of hepatocyte growth factor and Smad7. Arch Virol. 2015;160:2043-2050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Wang Y, Gao Y, Shi W, Zhai D, Rao Q, Jia X, Liu J, Jiao X, Du Z. Profiles of differential expression of circulating microRNAs in hepatitis B virus-positive small hepatocellular carcinoma. Cancer Biomark. 2015;15:171-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |