Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9794
Peer-review started: July 25, 2016
First decision: August 29, 2016
Revised: September 24, 2016
Accepted: October 27, 2016
Article in press: October 27, 2016
Published online: November 28, 2016
To investigate the effect of Helicobacter pylori (H. pylori) status test and H. pylori eradication on the occurrence of metachronous gastric cancer (MGC) after endoscopic submucosal dissection (ESD) of early gastric cancer (EGC) and risk factors of MGC.
The authors retrospectively reviewed the medical records of 433 patients (441 lesions) who underwent ESD for EGC from January 2005 to January 2015 in Yeungnam University Hospital. Patients were categorized into two groups; the H. pylori tested group (n = 257) and the H. pylori non-tested group (n = 176) based on performance of H. pylori status test after ESD of EGC. The H. pylori tested group was further categorized into three subgroups based on H. pylori status; the H. pylori-eradicated subgroup (n = 120), the H. pylori-persistent subgroup (n = 42), and the H. pylori-negative subgroup (n = 95). Incidences of MGC and risk factors of MGC were identified.
Median follow-up duration after ESD was 30.00 mo (range, 6-107 mo). Total 15 patients developed MGC during follow-up. MGC developed in 11 patients of the H. pylori tested group (7 in the H. pylori-negative subgroup, 3 in the H. pylori-eradicated subgroup, and 1 in the H. pylori-persistent subgroup) and 4 patients of the H. pylori non-tested group (P > 0.05). The risk factors of MGC were endoscopic mucosal atrophy in the H. pylori tested group and intestinal metaplasia in all patients.
H. pylori eradication and H. pylori status test seems to have no preventive effect on the development of MGC after ESD for EGC. The risk factors of MGC development were endoscopic mucosal atrophy in the H. pylori tested group alone and intestinal metaplasia in all patients.
Core tip: This is a retrospective study to evaluate the effect of Helicobacter pylori (H. pylori) status test and H. pylori eradication on the occurrence of metachronous gastric cancer (MGC) after endoscopic submucosal dissection (ESD) of early gastric cancer (EGC) and risk factors of MGC. H. pylori status test and H. pylori eradication seems to have no preventive effect on the occurrence of MGC after ESD for EGC. The risk factors of MGC were endoscopic gastric mucosal atrophy in H. pylori tested group alone and intestinal metaplasia in all patients.
- Citation: Kim SB, Lee SH, Bae SI, Jeong YH, Sohn SH, Kim KO, Jang BI, Kim TN. Association between Helicobacter pylori status and metachronous gastric cancer after endoscopic resection. World J Gastroenterol 2016; 22(44): 9794-9802
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9794.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9794
Endoscopic resection (ER) including endoscopic mucosal resection and endoscopic submucosal dissection (ESD) is a recognized as one of treatment options for curative resection of early gastric cancer (EGC) without simultaneous concomitant lymph node metastasis[1-4]. Unlike surgery of EGC, ER preserves most part of the stomach and this leads to increased risk of metachronous gastric cancer (MGC) development in residual gastric mucosa[5]. As more EGCs are treated with ER recently, identifying risk factors of MGC development after ER of EGC is important.
Helicobacter pylori (H. pylori) infection is related to the development of gastritis, atrophy, intestinal metaplasia, dysplasia, and gastric cancer[6-9]. Among dietary, environmental, and genetic risk factors of gastric cancer, H. pylori is classified as a Group 1 or definite carcinogen for gastric cancer by the World Health Organization[10]. In previous reports, the odds for development of gastric cancer reported to increase by 2-4 folds in the patients with H. pylori infection[11,12].
The effect of H. pylori eradication on development of MGC after ER of EGC is still on debate. A study of 132 patients who underwent ER for EGC and showed positive H. pylori serologic test demonstrated that H. pylori eradication inhibited the growth of new gastric cancer[13] and a retrospective study of 283 patients with H. pylori infection at time of ESD for EGC showed that failure of H. pylori eradication was a risk factor of MGC development[5]. However, a study of 1258 patients who underwent ESD for EGC reported that the incidence rate of MGC was not significantly different between patients with or without H. pylori eradication[14] and a retrospective study of 268 patients with a 5-year follow-up reported that H. pylori eradication after ER for EGC did not significantly reduced the incidence of MGC[15]. Studies about the effect of H. pylori status test on development of MGC after ER of EGC has been scarce.
The aims of this study were to investigate the effect of H. pylori status test and H. pylori eradication on the occurrence of MGC after ESD of EGC and risk factors of MGC.
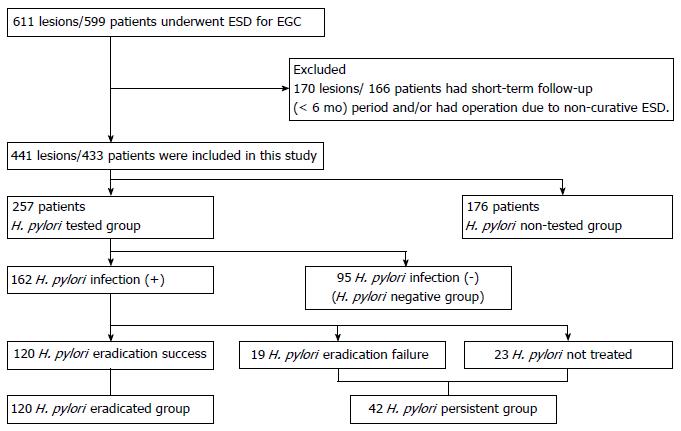
The medical records of 599 patients with 611 lesions who underwent ESD for EGC from January 2005 to January 2015 at Yeungnam university hospital were retrospectively reviewed. Exclusion criteria of the present study were as follows: additional gastrectomy due to a non-curative ESD of EGC and short-term follow-up duration (< 6 mo) and a total of 166 patients with 170 lesions were excluded from the present study. Finally, 433 patients with 441 lesions were included for analysis. Baseline clinical characteristics of the patients, characteristics and histology findings of EGC, performance of H. pylori status test and H. pylori eradication and occurrence of MGC were analyzed. Institutional review board approval was obtained for this study (2016-06-035).
Patients were divided into two groups; the H. pylori tested group (n = 257) and the H. pylori non-tested group (n = 176) based on performance of H. pylori status test after ESD of EGC. Patients in the H. pylori tested group were further divided into three subgroups; the H. pylori negative subgroup, the H. pylori eradicated subgroup, and the H. pylori persistent subgroup (Figure 1).
Among patients with positive H. pylori test results, patients who agreed to treat H. pylori infection received H. pylori eradication. The regimen for first-line H. pylori treatment was triple therapy with amoxicillin 1000 mg, clarithromycin 500 mg, and a proton-pump inhibitor (pantoprazole 40 mg, eomeprazole 40 mg, lansoprazole 30 mg or rabeprazole 20 mg) all twice daily for a week. The regimen for second-line H. pylori treatment was a quadruple therapy with metronidazole 500 mg (3 times daily), tetracycline 500 mg (4 times daily), tripotassium dicitrato bismuthate 300 mg (4 times daily), and a proton-pump inhibitor (twice daily) for 10-14 d. Eradication was confirmed by histology or rapid urase test at scheduled esophagogastroduodenoscopy (EGD) follow-up after ESD or urea breathing test. After ESD, scheduled EGD was performed at 2 or 3, 6, and 12 mo, and annually thereafter.
The presence of gastric mucosal atrophy was assessed through EGD and presence of intestinal metaplasia through histology. MGC was defined as the development of new gastric cancer at a previously uninvolved site in the stomach after the 6 mo following ESD. MGC was confirmed by histology of biopsy specimens. Incidences of MGC was compared according to performance of H. pylori status test and among the H. pylori eradicated, persistent and negative group and risk factors of MGC were analyzed.
Results are presented as means and standard deviations or as medians and ranges. The χ2 or Fisher’s exact test and one-way analysis of variance test or the Student’s t-test were used to compare categorical and continuous variables, respectively. The log-rank test was used for to compare group incidence rates. Univariate and multivariate Cox proportional hazard regression analyses were used to identify independent risk factors associated with MGC development. Covariates with P values of < 0.05 by univariate analyses were entered into multivariate analysis. Statistical analyses of the data were performed using SPSS 20 (IBM SPSS, Chicago, IL, United States). Statistical significance was accepted for P values < 0.05.
Mean age of the 433 patients included in the present study was 67.02 years and 325 (75.1%) patients were male and 108 (24.9%), female. Median follow-up duration after ESD of EGC was 30.00 mo (range, 6-107 mo).
Among 257 patients of the H. pylori tested group, 162 (63.0%) patients showed positive result for H. pylori test and 95 (37.0%) patients, negative. Of these 162 patients with positive result of H. pylori test, H. pylori eradication was done in 139 patients and eradication was successful in 120 (86.3%) patients. Ninety-five patients without H. pylori infection were classified as the H. pylori-negative subgroup, 120 patients with successful H. pylori eradication as the H. pylori-eradicated subgroup, and 42 patients (19 patients in whom H. pylori eradication failed and 23 patients not treated for H. pylori infection) as the H. pylori-persistent subgroup (Figure 1). The mean age of H. pylori tested group was 66.61 years and 189 (73.5%) patients were male. Patients in the H. pylori-eradicated subgroup were significantly younger than patients in the H. pylori-negative and H. pylori-persistent subgroups (P < 0.05). The mean follow-up duration was not significantly different between three subgroups (P > 0.05). Endoscopic mucosal atrophy and intestinal metaplasia were significantly more prevalent in the H. pylori-negative subgroup than the other two subgroups (P < 0.05). The location and macroscopic type of primary gastric cancer were not significantly different between three subgroups (P > 0.05). The H. pylori-persistent subgroup had significantly less differentiated cancers than other two subgroups (P = 0.032) (Table 1).
| H. pylori | H. pylori | H. pylori | P value | |
| negative group | persistent group | eradicated group | ||
| (n = 95) | (n = 42) | (n = 120) | ||
| Sex | ||||
| Male | 74 (77.9) | 29 (69.0) | 86 (71.7) | 0.454 |
| Female | 21 (22.1) | 13 (31.0) | 34 (28.3) | |
| Age, mean (SD) | 68.65 (8.86) | 67.31 (9.12) | 64.76 (10.10) | 0.011 |
| Follow-up period (mo), mean (SD) | 36.18 (± 26.74) | 33.29 (± 25.93) | 32.78 (± 23.72) | 0.149 |
| Endoscopic mucosal atrophy | 50 (52.6) | 8 (19.0) | 25 (20.8) | < 0.001 |
| Intestinal metaplasia | 63 (66.3) | 18 (42.9) | 32 (26.7) | < 0.001 |
| Location of primary cancer | ||||
| Upper | 10 (10.5) | 5 (11.9) | 10 (8.3) | 0.577 |
| Middle | 36 (37.9) | 14 (33.3) | 35 (29.2) | |
| Lower | 49 (51.6) | 23 (54.8) | 75 (62.5) | |
| Macroscopic type of primary cancer | ||||
| Elevated | 40 (42.1) | 122 (8.6) | 57 (47.5) | 0.177 |
| Flat | 13 (13.7) | 5 (11.9) | 9 (7.5) | |
| Depressed | 42 (37.0) | 25 (59.5) | 54 (45.0) | |
| Diameter of primary cancer (cm), mean (SD) | 14.24 (7.31) | 13.83 (6.22) | 13.67 (6.90) | 0.978 |
| Histology of primary cancer | ||||
| Differentiated | 93 (97.9) | 38 (90.5) | 118 (98.3) | 0.032 |
| Undifferentiated | 2 (2.1) | 4 (9.5) | 2 (1.7) | |
| ESD criteria | ||||
| Absolute | 73 (76.8) | 33 (78.6) | 95 (79.2) | 0.636 |
| Expended | 18 (18.9) | 5 (11.9) | 19 (15.8) | |
| Beyond expanded | 4 (4.2) | 4 (9.5) | 6 (5.0) | |
| Depth of primary cancer | ||||
| Mucosa | 89 (93.7) | 40 (95.2) | 111 (92.5) | 0.819 |
| Submucosa | 6 (6.3) | 2 (4.8) | 9 (7.5) | |
| Metachronous cancer recurrence | 7 (7.4) | 1 (2.4) | 3 (2.5) | 0.173 |
The mean age and follow-up duration were not significantly different between the H. pylori tested and the H. pylori non-tested groups (P > 0.05). Endoscopic mucosal atrophy and intestinal metaplasia was significantly more frequent in the H. pylori tested group than in the H. pylori non-tested group, and location of primary gastric cancer location was significantly lower in the H. pylori tested group than the H. pylori non-tested group (P < 0.05). In addition, the H. pylori non-tested group had more elevated lesions than the H. pylori tested group and the H. pylori tested group had more depressed lesions than the H. pylori non-tested group (P < 0.05) (Table 2).
| H. pylori tested group | H. pylori non-tested group | P value | |
| (n = 257) | (n = 176) | ||
| Sex | |||
| Male | 189 (73.5) | 136 (77.3) | 0.378 |
| Female | 68 (26.5) | 40 (22.7) | |
| Age, mean (SD) | 66.61 (9.63) | 67.60 (10.03) | 0.303 |
| Follow-up period (mo), mean (SD) | 34.12 (25.19) | 33.31 (18.11) | 0.699 |
| Endoscopic mucosal atrophy | 83 (32.3) | 12 (6.8) | < 0.001 |
| Intestinal metaplasia | 113 (44.0) | 14 (8.0) | < 0.001 |
| Location of primary cancer | |||
| Upper | 25 (9.7) | 12 (6.8) | |
| Middle | 85 (33.1) | 37 (21.0) | 0.006 |
| Lower | 147 (57.2) | 127 (72.2) | |
| Macroscopic type of primary cancer | |||
| Elevated | 109 (42.4) | 95 (54.0) | |
| Flat | 27 (10.5) | 24 (13.6) | 0.009 |
| Depressed | 121 (47.1) | 57 (32.4) | |
| Diameter of primary cancer (cm), mean (SD) | 13.91 (6.93) | 14.30 (5.74) | 0.540 |
| Histology of primary cancer | |||
| Differentiated | 249 (96.9) | 171 (97.2) | |
| Undifferentiated | 8 (3.1) | 5 (2.8) | 0.871 |
| ESD criteria | |||
| Absolute | 201 (78.2) | 142 (80.7) | 0.234 |
| Expended | 42 (16.3) | 20 (11.4) | |
| Beyond expanded | 14 (5.4) | 14 (8.0) | |
| Depth of primary cancer | |||
| Mucosa | 240 (93.4) | 161 (91.5) | 0.456 |
| Submucosa | 17 (6.6) | 15 (8.5) | |
| Metachronous cancer recurrence | 11 (4.3) | 4 (2.3) | 0.262 |
Among total 433 patients, MGC developed in 15 (3.5%) patients; 11 (4.3%) patients in the H. pylori tested group and 4 (2.3%) in the H. pylori non-tested group without significant difference (P = 0.262) (Table 2).
Among 11 patients who developed MGC in the H. pylori tested group, MGC developed in 7 (7.4%) patients of the H. pylori-negative subgroup, 3 (2.5%) patients of the H. pylori-eradicated subgroup, and 1 (2.4%) patient of the H. pylori-persistent subgroup. Although the incidence of MGC was higher in the H. pylori-negative subgroup than other two subgroups, statistical significance was not found among the three subgroups (P = 0.173) (Table 1).
Mean age of patients with MGC was 68.93 years and all patients with MGC were male. No significant differences were observed between MGC group and non-MGC group in terms of age, primary cancer location, and primary lesion size (P > 0.05), and mean follow-up duration was not significantly different between two groups (P = 0.752). Endoscopic mucosal atrophy and intestinal metaplasia were significantly more prevalent in patients with MGC than without (P < 0.05) (Table 3).
| Non-metachronous gastric cancer group (n = 418) | Metachronous gastric cancer group (n = 15) | P value | |
| Sex | |||
| Male | 310 (74.2) | 15 (100) | 0.028 |
| Female | 108 (25.8) | 0 (0) | |
| Age, mean (SD) | 66.95 (9.82) | 68.93 (9.35) | 0.441 |
| Location of primary cancer | |||
| Upper | 35 (8.4) | 2 (13.3) | 0.163 |
| Middle | 115 (27.5) | 7 (46.7) | |
| Lower | 268 (64.1) | 6 (40.0) | |
| Lesion size (cm), mean (SD) | 14.01 (6.10) | 15.60 (13.48) | 0.350 |
| Endoscopic mucosal atrophy | 87 (20.8) | 8 (53.3) | 0.003 |
| Intestinal metaplasia | 117 (28.0) | 10 (66.7) | 0.001 |
| Histology of undifferentiated type | 12 (2.9) | 1 (6.7) | 0.397 |
| SM invasion | 31 (7.4) | 1 (6.7) | 0.913 |
| Non-performance of H. pylori status test | 172 (41.1) | 4 (26.7) | 0.262 |
| Follow-up period (mo), mean period (SD) | 33.72 (22.58) | 35.60 (22.68) | 0.752 |
In the H. pylori tested group, age, primary cancer location, and lesion size were not significantly different between patients with or without MGC and follow-up duration was similar between two groups (33.72 ± 23.64 vs 34.13 ± 25.30, P = 0.997). The patient with MGC showed higher proportion of negative H. pylori status than without (63.6% vs 35.8%, P = 0.061). However, endoscopic mucosal atrophy and intestinal metaplasia were observed significantly more in patients with MGC than without (72.7% vs 30.5%, P = 0.003 and 81.8% vs 42.3%, P = 0.010) (Table 4).
| Non-metachronous gastric cancer group (n = 246) | Metachronous gastric cancer group (n = 11) | P value | |
| Sex | |||
| Male | 178 (72.4) | 11 (100) | 0.042 |
| Female | 68 (29.6) | 0 (0) | |
| Age, mean (SD) | 66.44 (9.66) | 70.45 (8.52) | 0.177 |
| Location of primary cancer | |||
| Upper | 24 (9.8) | 1 (9.1) | 0.290 |
| Middle | 79 (32.1) | 6 (54.5) | |
| Lower | 143 (58.1) | 4 (36.4) | |
| Lesion size (cm), mean (SD) | 13.75 (6.32) | 17.36 (15.45) | 0.458 |
| Endoscopic mucosal atrophy | 75 (30.5) | 8 (72.7) | 0.003 |
| Intestinal metaplasia | 104 (42.3) | 9 (81.8) | 0.010 |
| Histology of undifferentiated type | 8 (3.3) | 0 (0) | 0.543 |
| SM invasion | 16 (6.5) | 1 (9.1) | 0.736 |
| Persistent H. pylori infection | 41 (16.7) | 1 (9.1) | 0.173 |
| H. pylori negative | 88 (35.8) | 7 (63.6) | 0.061 |
| Follow-up period (mo), mean (SD) | 34.13 (25.30) | 33.72 (23.64) | 0.958 |
In the H. pylori test group, endoscopic mucosal atrophy and intestinal metaplasia were found to be significantly associated with the development of MGC by univariate analysis (P < 0.05). Multivariate Cox proportional hazard regression analysis revealed an association with MGC development only for endoscopic mucosal atrophy (HR = 6.080, P = 0.009) (Table 5).
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age ≥ 65 yr | 1.736 | 0.449-6.705 | 0.424 | |||
| Endoscopic mucosal atrophy | 6.080 | 1.569-23.556 | 0.009 | 6.080 | 1.569-23.556 | 0.009 |
| Intestinal metaplasia | 6.144 | 1.300-29.033 | 0.022 | 2.654 | 0.400-17.621 | 0.312 |
| Histology of undifferentiated type | 0.000 | 0.000 | 0.999 | |||
| SM invasion | 1.437 | 0.173-11.942 | 0.737 | |||
| H. pylori negative | 3.142 | 0.895-11.031 | 0.074 | 1.638 | 0.426-6.299 | 0.473 |
| H. pylori eradication | 0.413 | 0.107-1.595 | 0.200 | |||
In all patients, endoscopic mucosal atrophy and intestinal metaplasia were significantly associated with MGC development in univariate analysis and multivariate Cox proportional hazard regression analysis showed an association between intestinal metaplasia and MGC development (HR = 4.67, P = 0.006) (Table 6).
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P valve | HR | 95%CI | P value | |
| Age ≥ 65 yr | 1.317 | 0.442-3.923 | 0.620 | |||
| Endoscopic mucosal atrophy | 4.348 | 1.535-12.320 | 0.006 | 1.966 | 0.526-7.351 | 0.315 |
| Intestinal metaplasia | 5.145 | 1.722-15.373 | 0.003 | 5.145 | 1.722-15.373 | 0.003 |
| Histology of undifferentiated type | 2.417 | 0.293-19.902 | 0.412 | |||
| SM invasion | 0.892 | 0.113-7.007 | 0.913 | |||
| H. pylori non-tested | 0.520 | 0.163-1.660 | 0.270 | |||
In the present study, 15 (3.5%) of the 433 patients developed MGC after ESD for EGC and this result was comparable with previous reports[16,17]. MGC occurs more frequently after ER for EGC than surgery (2.5%-14% vs 1.8%-5%)[16-19]. This increased risk of MGC after ER for EGC can be partly explained by higher proportion of salvaged stomach in ER than surgery. The mean duration of MGC development from ESD of EGC was 35.6 mo and 3 patients developed MGC after 5 years from initial ESD of EGC. A retrospective study of 1526 patients who underwent ESD for EGC reported that 5-year, 7-year, and 10-year cumulative incidence functions of MGC were 9.5%, 13.1%, and 22.7%, respectively[20]. Meticulous examination at surveillance EGD is needed in patients who underwent ER for EGC and EGD should be done with schedule. Further studies are needed to find optimal schedule for surveillance EGD after ER of EGC.
The chronic inflammation of stomach induced by H. pylori infection may lead to mucosal atrophy, intestinal metaplasia, and dysplasia, and risk of developing gastric cancer is increased in patients exhibiting such histologic changes of stomach[21,22]. An animal study has reported that H. pylori eradication decreased polyp formation, inflammatory cell infiltration, and cellular proliferation in the gastric mucosa and suggested that H. pylori eradication could diminish mucosal alterations related to gastric carcinogenesis[23]. However, the preventative effect of H. pylori eradication on MGC development after ESD for EGC is still on debate. In the present study, the incidence of MGC after ESD of EGC was not significantly different between the H. pylori-negative subgroup, the H. pylori-eradicated subgroup, and the H. pylori-persistent subgroups and H. pylori eradication had no preventive effect on the development of MGC after ESD of EGC. However, the H. pylori-negative subgroup showed higher tendency towards development of MGC after ESD of EGC than other two subgroups without statistical significance and this might be due to significantly higher proportion of endoscopic mucosal atrophy and intestinal metaplasia in the H. pylori-negative subgroup than other two subgroups. The higher proportion of patients with intestinal metaplasia in the H. pylori-negative subgroup might have led to false negative result in the H. pylori status test.
In the present study, the development of MGC was compared according to performance of H. pylori status test and no significant difference in the development of MGC after ESD of EGC was observed between two groups during follow-up. As H. pylori status test and H. pylori eradication failed to show preventive effect on development of MGC after ESD of EGC in the present study, further large scaled prospective studies are needed to clarify the effect of H. pylori status test and H. pylori eradication on MGC development in patients who underwent ESD for EGC.
The mucosal atrophy of stomach has been previously reported to contribute to the development of MGC[15]. A study of 100 patients who underwent ESD for EGC reported that the frequency of severe atrophy assessed by histology was higher in the group that developed cancer compared to the group that did not and severity of atrophy was the only independent risk factor of MGC development after H. pylori eradication[24]. In the present study, endoscopic mucosal atrophy and intestinal metaplasia were observed more frequently in patients with MGC than in those without. Furthermore, multivariate analysis showed that endoscopic mucosal atrophy in the H. pylori tested group and intestinal metaplasia in all patients as a risk factor of MGC development after ESD of EGC.
The effect of H. pylori eradication on improvement of mucosal atrophy remains unclear in previous studies[25-28]. A study of 544 patients with EGC reported the preventive effect of H. pylori eradication on development of MGC after ER of EGC even in patients with corpus atrophy[29]. However, a large-scale, randomized, and controlled study about 1630 healthy carriers of H. pylori infection in China reported that the H. pylori carriers with precancerous state defined as presence of mucosal atrophy, intestinal metaplasia, or dysplasia had no preventive effect of H. pylori eradication on development of gastric cancer[30]. The ineffectiveness of H. pylori eradication on MGC development after ESD of EGC in the present study might have been due to irreversible mucosal atrophic change. Further studies to clarify the effect of H. pylori eradication on MGC development according to status of gastric mucosa is needed.
In the present study, all patients who developed MGC were male. A previous study reported that male gender was one of risk factors for MGC development of ESD of EGC[20]. However, male gender was not found as a risk factor of MGC in the present study and further study with longer follow up duration is needed to clarify the effect of gender on development of MGC after ESD of EGC.
The present study has several limitations. First, its retrospective nature study makes selection bias inevitable, as was reflected by differences in the baseline characteristics of patients including atrophy and intestinal metaplasia status. Second, relatively small patients of MGC were included for the analysis, and if more patients with MGC had been included, it is possible that H. pylori eradication might have been found to influence MGC development. Third, determination of H. pylori infection status was inadequate, and thus, false negative and positive results were possibly included. Forth, we did not examine other causes of mucosal atrophy.
In conclusion, H. pylori eradication and H. pylori status test seems to have no preventive effect on the development of MGC after ESD for EGC. The risk factors of MGC development after ESD of EGC were gastric mucosal atrophy in H. pylori tested group and intestinal metaplasia in all patients.
This work was supported by the 2015 Yeungnam University Research Grant.
Helicobacter pylori (H. pylori) infection is related to the development of gastritis, atrophy, intestinal metaplasia, dysplasia, and gastric cancer. The odds for development of gastric cancer reported to increase by 2-4 folds in the patients with H. pylori infection in previous studies. The effect of H. pylori eradication on development of metachronous gastric cancer (MGC) after endoscopic resection (ER) of early gastric cancer (EGC) is still on debate. Studies about the effect of H. pylori status test on development of MGC after ER of EGC has been scarce. In this study, we evaluated the effect of H. pylori status test and H. pylori eradication on the occurrence of MGC after endoscopic submucosal dissection (ESD) of EGC and risk factors of MGC.
Studies about the preventive role of H. pylori eradication in the development of MGC after ER of EGC showed conflicting results.
In this study, H. pylori status test and H. pylori eradication seems to have no preventive effect on the development of MGC after ESD for EGC. The risk factors of MGC development were endoscopic gastric mucosal atrophy in H. pylori tested group alone and intestinal metaplasia in all patients.
Due to retrospective nature of the study, further prospective studies to clarify the effect of H. pylori status test and H. pylori eradication on the occurrence of MGC after ESD of EGC and risk factors of MGC are needed.
Early gastric cancer: An adenocarcinoma that is restricted to the mucosa or submucosa of stomach, irrespective of lymph node metastasis.
To provide the comments from peer reviewers that most represent the characteristics, values and significance of the article, and allow the readers to have an objective point of view toward the article.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mohammadi M, Wang WH S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Kim SG. Endoscopic treatment for early gastric cancer. J Gastric Cancer. 2011;11:146-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Wang KK, Prasad G, Tian J. Endoscopic mucosal resection and endoscopic submucosal dissection in esophageal and gastric cancers. Curr Opin Gastroenterol. 2010;26:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 496] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 4. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 443] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 5. | Kwon YH, Heo J, Lee HS, Cho CM, Jeon SW. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment Pharmacol Ther. 2014;39:609-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Parsonnet J, Vandersteen D, Goates J, Sibley RK, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst. 1991;83:640-643. [PubMed] [Cited in This Article: ] |
| 7. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1302] [Cited by in F6Publishing: 1203] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 8. | Yamagata H, Kiyohara Y, Aoyagi K, Kato I, Iwamoto H, Nakayama K, Shimizu H, Tanizaki Y, Arima H, Shinohara N. Impact of Helicobacter pylori infection on gastric cancer incidence in a general Japanese population: the Hisayama study. Arch Intern Med. 2000;160:1962-1968. [PubMed] [Cited in This Article: ] |
| 9. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3126] [Cited by in F6Publishing: 3021] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 10. | Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] [Cited in This Article: ] |
| 11. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. [PubMed] [Cited in This Article: ] |
| 12. | Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169-1179. [PubMed] [Cited in This Article: ] |
| 13. | Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639-642. [PubMed] [Cited in This Article: ] |
| 14. | Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62:1425-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Maehata Y, Nakamura S, Fujisawa K, Esaki M, Moriyama T, Asano K, Fuyuno Y, Yamaguchi K, Egashira I, Kim H. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer. 2006;9:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37:990-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Takeda J, Toyonaga A, Koufuji K, Kodama I, Aoyagi K, Yano S, Ohta J, Shirozu K. Early gastric cancer in the remnant stomach. Hepatogastroenterology. 1998;45:1907-1911. [PubMed] [Cited in This Article: ] |
| 19. | Nicholls JC. Stump cancer following gastric surgery. World J Surg. 1979;3:731-736. [PubMed] [Cited in This Article: ] |
| 20. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Nakajima T, Sekiguchi M, Mori G, Taniguchi H, Sekine S. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy. 2015;47:1113-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 587] [Cited by in F6Publishing: 580] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 22. | Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 23. | Maruta F, Sugiyama A, Ishizone S, Miyagawa S, Ota H, Katsuyama T. Eradication of Helicobacter pylori decreases mucosal alterations linked to gastric carcinogenesis in Mongolian gerbils. J Gastroenterol. 2005;40:104-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Shiotani A, Uedo N, Iishi H, Yoshiyuki Y, Ishii M, Manabe N, Kamada T, Kusunoki H, Hata J, Haruma K. Predictive factors for metachronous gastric cancer in high-risk patients after successful Helicobacter pylori eradication. Digestion. 2008;78:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Zhou LY, Lin SR, Ding SG, Huang XB, Zhang L, Meng LM, Cui RL, Zhu J. The changing trends of the incidence of gastric cancer after Helicobacter pylori eradication in Shandong area. Chin J Dig Dis. 2005;6:114-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536-1540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, Sumii M, Tanaka S, Yoshihara M, Chayama K. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002;16:1449-1456. [PubMed] [Cited in This Article: ] |
| 28. | Sung JJ, Lin SR, Ching JY, Zhou LY, To KF, Wang RT, Leung WK, Ng EK, Lau JY, Lee YT. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000;119:7-14. [PubMed] [Cited in This Article: ] |
| 29. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 850] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 30. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 1107] [Article Influence: 55.4] [Reference Citation Analysis (0)] |