Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9727
Peer-review started: June 27, 2016
First decision: July 29, 2016
Revised: August 23, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: November 28, 2016
To explore the influence of Infliximab (IFX) on cancer progression in a murine model of colonic cancer associated to chronic colitis.
AOM/DSS model was induced in C57BL/6 mice. Mice were injected with IFX (5 mg/kg) during each DSS cycle while control mice received saline. Body weight, occult blood test and stool consistency were measured to calculate the disease activity index (DAI). Mice were sacrificed at week 10 and colons were analyzed macroscopically and microscopically for number of cancers and degree of inflammation. MTT assay was performed on CT26 to evaluate the potential IFX role on metabolic activity and proliferation. Cells were incubated with TNF-α or IFX or TNF-α plus IFX, and cell vitality was evaluated after 6, 24 and 48 h. The same setting was used after pre-incubation with TNF-α for 24 h.
IFX significantly reduced DAI and body weight loss in mice compared with controls, preserving also colon length at sacrifice. Histological score was also reduced in treated mice. At macroscopic analysis, IFX treated mice showed a lower number of tumor lesions compared to controls. This was confirmed at microscopic analysis, although differences were not statistically significant. In vitro, IFX treated CT26 maintained similar proliferation ability at MTT test, both when exposed to IFX alone and when associated to TNF-α.
IFX did not increase colonic cancer risk in AOM-DSS model of cancer on chronic colitis nor influence directly the proliferation of murine colon cancer epithelial cells.
Core tip: We report our results on the potential role of Infliximab on cancer progression in AOM-DSS murine model of colorectal cancer associated to chronic colitis. AOM/DSS model was induced in C57BL/6 mice. Mice were injected with Infliximab during each DSS cycle while control mice received saline. Mice were sacrificed at week 10 and colons were analyzed macroscopically and microscopically for number of cancers and degree of inflammation. MTT assay was performed on CT26 to evaluate the potential influence of Infliximab (IFX) role on metabolic activity and proliferation. This study demonstrates that beside its well-known healing capacity, IFX does not increase proliferative cancer cells ability and colorectal cancer risk in AOM-DSS model of tumor on chronic colitis.
- Citation: Lopetuso LR, Petito V, Zinicola T, Graziani C, Gerardi V, Arena V, Caristo ME, Poscia A, Cammarota G, Papa A, Cufino V, Sgambato A, Gasbarrini A, Scaldaferri F. Infliximab does not increase colonic cancer risk associated to murine chronic colitis. World J Gastroenterol 2016; 22(44): 9727-9733
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9727
Inflammatory bowel disease (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, relapsing inflammatory disorder of the digestive tract resulting from a loss of homeostasis between the intestinal immune system and the gut microbiota in genetically-predisposed individuals[1]. Inappropriate mucosal immune responses, due to disruption of the epithelial barrier separating microorganisms from underlying tissues and/or dysregulated tolerance to the intestinal microbiota, likely contribute to the development and perpetuation of IBD[2-5]. In this scenario, long history of colonic IBD is associated with colonic cancer progression[6].
Colorectal cancer (CRC) is an important worldwide medical problem as it is the third most commonly diagnosed serious form of cancer in men and in women and represents the third leading cause of cancer death for men and for women and the second leading cause of cancer mortality overall[7]. Sporadic cases are the most frequent. UC patients have a 20-fold higher risk to develop CRC compared to general population. This risk is higher especially in pancolitis: 30% of patients with pancolitis develop CRC after 30 years of disease[7,8]. In addition, UC patients with CRC have an overall poor prognosis and limited therapeutic options are available. Mechanistically, CRC in chronic colitis has a different pathogenesis compared to sporadic or familial cancer[6]. Experimental observations provide full support for the role of inflammation in IBD-related colorectal carcinogenesis. The administration of agents that cause colitis in healthy or genetically modified rodents accelerates the development of CRC[9]. Mice genetically predisposed to develop IBD also develop CRC, especially in the presence of microbial colonization[10]. Although there is little doubt that chronic inflammation promotes colon cancer, the mechanisms involved are unclear. Tumor Necrosis Factor (TNF)-α and other cytokines involved in the development of chronic gastric inflammatory conditions play a key role in triggering early epithelial alterations observed in intestinal metaplasia and in promoting the progression to epithelial dysplasia[11,12]. Moreover, proinflammatory mediators represent critical factors in promoting the growth of neoplastic lesions, inducing epithelial cell proliferation and neoangiogenesis[12,13].
In this setting, anti-TNF-α drugs, such as Infliximab (IFX), represent a crucial therapeutic tool for modulating the immune response and the clinical course in IBD[14]. IFX is a monoclonal chimeric antibody. Its clinical efficacy and ability to provide mucosal healing in CD as well as in UC are well demonstrated[14]. However, there are concerns regarding the impact of TNF-α blockade on the incidence of malignancy. In particular, while the curative role of IFX on chronic intestinal inflammation is supposed to reduce CRC risk, a secondary neoplasm is one of the most feared sequelae of the immune system manipulation in general[15,16].
The combination of Azoxymethane (AOM) with exposure to dextran sodium sulphate (DSS) in mice is a well-known CRC model for studying the progression of CRC on chronic colitis and related mechanisms of action. In the present study, we explored the influence of IFX on cancer progression in AOM-DSS murine model of CRC cancer associated to chronic colitis. We showed that IFX significantly reduced disease activity index and body weight loss, preserving colon length at sacrifice. Histological analysis revealed an improved mucosal healing in IFX treated mice. At macroscopic analysis, IFX administration leaded to a lower number of tumor lesions compared with controls, confirmed at microscopic analysis, although differences were not statistically significant. In vitro, IFX did not modify the proliferation ability of a murine colon tumor cell line, confirming the in vivo data. Taken together, our data suggest that beside its well-known healing capacity, IFX does not increase proliferative cancer cells ability and CRC risk in AOM-DSS model of tumor on chronic colitis.
All experiments were approved by the Local Ethics Committee for Animal Research Studies at the Catholic University of Rome (protocol number F42/2009). The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation.
The experimental chronic colitis associated to the development of CRC was obtained in female mice C57BL/6. The animals were treated with an intraperitoneal (i.p.) injection of 10 mg/kg of Azoxymethane (AOM, Sigma-Aldrich, Munich, Germany). After one week (time 0) mice were exposed to three cycles of one week with Dextran Sodium Sulphate 2% (DSS, Molecular Weight 36-44 kDa, MP Biomedical Aurora, OH, United States) in tap water, separated by 2 wk of recovery as already shown[17,18]. Animals were divided into two groups. At the third day of each cycle of DSS the first group (IFX) was treated with IFX 5 mg/kg given intravenously in 200 μL of saline, instead the control group received a saline solution infusion. Animals were evaluated every day, by measuring weight, fecal consistence and fecal occult blood, as suggested[19,20]. Disease Activity Index (DAI) was calculated as reported[19,20]. Mice were sacrificed after three weeks from last DSS cycle. Each experiment was performed using a total number of 10 mice.
At sacrifice, ulcerated and polypoid tumors were counted, photographed and marked for further analysis along with the histologic evaluation. Tumor incidence was expressed as percentage. Colon and final ileum were then fixed in formalin and then embedded in paraffin.
Intestinal inflammation and proliferative alterations, such as hyperplasia, aberrant crypt foci (ACF), gastrointestinal intraepithelial neoplasia (GIN), low grade dysplasia adenoma LGA, high grade dysplasia adenoma (HGA) and adenocarcinoma were evaluated by a single trained gastrointestinal pathologist in a blinded fashion, using a validated semiquantitative scoring system as described[18,19,21].
In order to examine the direct effects of IFX on intestinal epithelial cells proliferation and vitality, the 3-(4,5-Dimethylthiazol-2Y)-2,5Diphenyltetrazolium Bromide (MTT) test was performed on CT26 (ATCC, Teddington, United Kingdom), a murine colon cancer intestinal epithelial cell line, according to manufacturer’s instructions. Briefly, MTT, a salt of tetrazolium, is transformed to insoluble crystals by the succinate-tetrazolium reductase, active only in vital cells. Crystals amount is directly proportional to the number of metabolically active cells and was measured by spectrometry at 540 nm wave length (reference wave length = 630 nm). Cells were incubated with TNF-α (25 ng/mL) or IFX (100 μg/mL) or TNF-α (25 ng/mL) plus IFX (100 μg/mL), and cell vitality was evaluated after 6, 24 and 48 h. The same setting was used after pre-incubation with TNF-α (25 ng/mL) for 24 h, followed by two washings in saline. Data are expressed as percentage vs control. Each analysis was repeated three times.
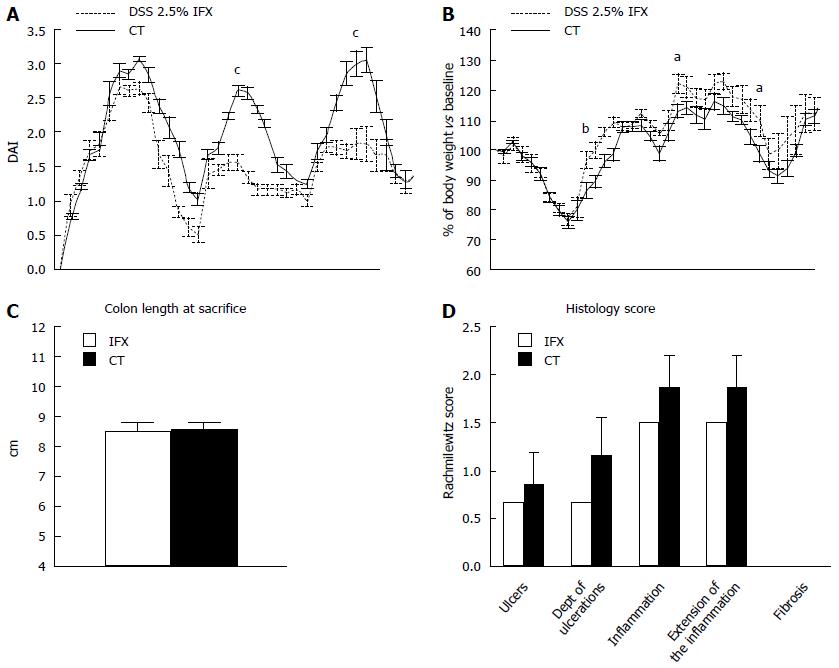
We first explore the impact of IFX on clinical and histological activity in chronic colitis. IFX treated animals showed a significantly lower clinical activity index, expressed by DAI score (Figure 1A) and body weight loss when compared with controls, especially in the 18th, 44th and 54th d (P < 0.05) (Figure 1B). At the sacrifice, we did not observe statistically significant differences in colon length (Figure 1C). The histological examination reported a reduction of the Rachmilewitz score in treated animals compared with controls, with a reduction in ulcer number and depth, inflammation grade and extension. However, this difference was not statistically significant. Fibrosis was absent in both groups (Figure 1D).
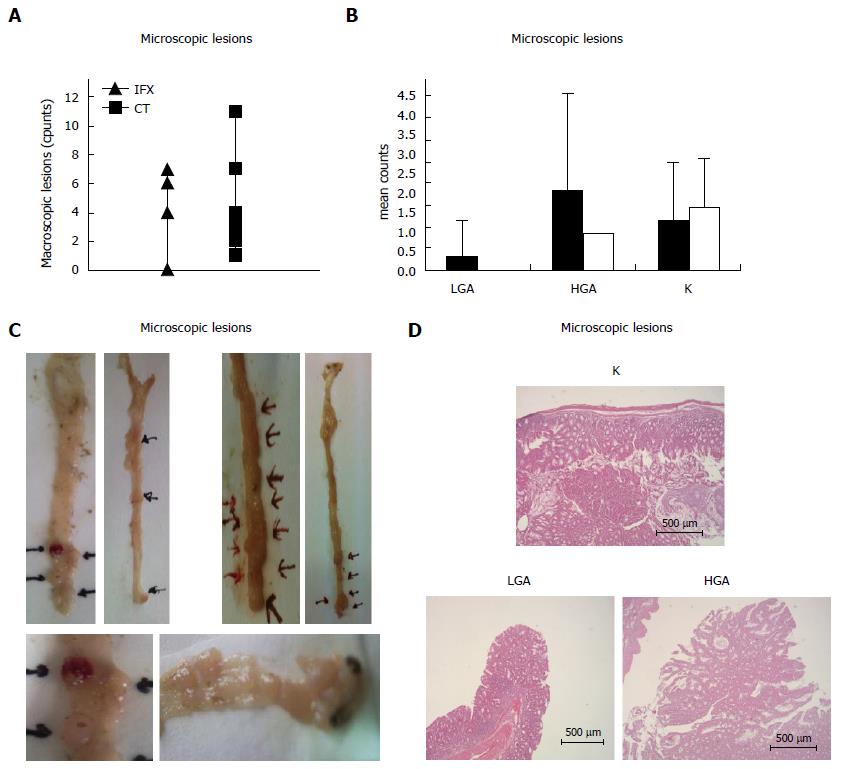
At sacrifice, the presence of neoplastic lesions was verified and evaluated in each animal. All animals showed at least 1 macroscopic tumor. Treated animals showed a lower number of tumors in comparison with controls, with an average of 4,5 tumors per each mouse compared with 4,4 tumors in the control group. The difference was not statistically significant (P > 0.05) (Figure 2A). At microscopic analysis, animals treated with IFX showed a slightly lower number of adenocarcinoma (K) with an average of 1,4 tumors per treated mouse compared with 1, 16 tumors per animal in the control group. Furthermore, in treated mice we found a non-significant higher prevalence of high grade dysplasia adenoma (HGA) compared with controls. All differences were not statistically significant (P > 0.05) (Figure 2B).
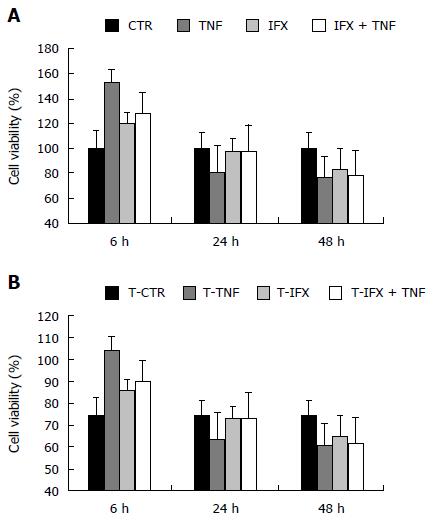
In order to confirm these data, we performed an in vitro MTT assay, indirect expression of IFX effect on cancer cell proliferation. IFX did not significantly modify the metabolic activity of CT26 cells either directly or in association with TNF-α. On the contrary, TNF-α increased the proliferation of the neoplastic cells during the first 6 h of stimulation (Figure 3A). Pre-stimulation with TNF-α did not result in any significant variation from the first MTT assay setting (Figure 3B).
We previously showed that IFX is able to exert a local effect on intestinal mucosa[22,23]. At the same time, its potential role on the progression of colonic cancer associated to chronic colitis is crucially relevant for IBD. Our previous studies suggested that IFX is able to reduce leukocyte infiltrate, inflammatory cytokine, and adaptive and innate immunity chemokine levels, together with a reparation of the intestinal epithelial layer at scratch assay[23]. We thus expected an IFX effect on the modulation of colonic tumor progression.
Many murine models of sporadic and inflammation-related colon carcinogenesis have been developed in the last decade, including chemically induced colon cancer models, genetically engineered murine models, and xenotransplants. Among these, AOM/DSS model has proven to dramatically shorten the latency time for induction of CRC and to rapidly recapitulate the aberrant crypt foci-adenoma-carcinoma sequence that occurs in human CRC. Because of its high reproducibility, as well as the simple and affordable mode of application, the AOM/DSS has become an outstanding model for studying colon carcinogenesis. That is the main reason for choosing this model, instead of that reported by Kim et al[24].
In the present study, we firstly confirmed the IFX ability to reduce the clinical and histological activity of chronic colitis. This was reflected by a reduced DAI and body weight loss, as well as histological score in treated mice. Interestingly, all animals exposed to AOM and DSS expressed macroscopic tumors as expected. IFX treated animals showed a lower number of tumors compared with controls, although this difference was not statistically significant. For this reason the overall message of our experimental setting does not differ significantly from what was previously proposed in a different model[24]. Of note, microscopic analysis showed that treatment with IFX is associated to an increased prevalence of pre-neoplastic lesions (LGA and HGA) compared with controls, together with a slightly and not significant decrease of cancer lesions. This suggests that the control of chronic inflammation provided by IFX can be able to move cancer progression to a pre-cancer condition. This finding is somehow comforting if taken together with new evidences suggesting that colonic cancer risk in IBD and in particular in ulcerative colitis (UC) is lowering in recent years[25-29]. Moreover, since IBD-associated CRC can affects patients at a younger age than sporadic CRC[8,25,30], the finding that IFX do not dramatically move the incidence of colonic cancer in experimental colitis, makes the use of this compound in younger age even less problematic. Nonetheless, murine cancer model cannot be perfectly assimilated to what really happens in humans, since other mechanisms of action could occur. This represents a major limitation for our study. However, in vitro MTT assay supported the idea that IFX has not a potential proliferative role on cancer cells, since it does not increase their metabolic activity.
Overall, our study sustains a safe action of TNF-α block in chronic colitis without increasing the associated tumorigenic risk.
Colorectal cancer (CRC) is an important worldwide medical problem as it is the third most commonly diagnosed serious form of cancer in men and in women and represents the third leading cause of cancer death for men and for women and the second leading cause of cancer mortality overall.
The authors showed that influence of infliximab (IFX) significantly reduced disease activity index and body weight loss, preserving colon length at sacrifice. Histological analysis revealed an improved mucosal healing in IFX treated mice. At macroscopic analysis, IFX administration leaded to a lower number of tumor lesions compared with controls, confirmed at microscopic analysis, although differences were not statistically significant. In vitro, IFX did not modify the proliferation ability of a murine colon tumor cell line, confirming the in vivo data.
Taken together, these data suggest that beside its well-known healing capacity, IFX does not increase proliferative cancer cells ability and CRC risk in AOM-DSS model of tumor on chronic colitis.
This study sustains a safe action of TNF-α block in chronic colitis without increasing the associated tumorigenic risk.
They show that IFX does not affect the development of murine colitis-associated cancer and proliferation of intestinal epithelial cells. Based on these results, the authors conclude that IFX does not increase colonic cancer risk in colitis-associated cancer. This study suggests an important message that anti-TNF-α therapy may not affect the increase cancer risk in inflammatory bowel disease patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hayashi S, Yoshida H S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Bamias G, Corridoni D, Pizarro TT, Cominelli F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine. 2012;59:451-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2894] [Cited by in F6Publishing: 3117] [Article Influence: 183.4] [Reference Citation Analysis (8)] |
| 3. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3465] [Cited by in F6Publishing: 3323] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 4. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1693] [Cited by in F6Publishing: 1713] [Article Influence: 131.8] [Reference Citation Analysis (1)] |
| 5. | Lopetuso LR, Petito V, Zambrano D, Orlando D, Dal Lago A, Serrichhio L, Papa A, Gasbarrini A, Scaldaferri F. Gut Microbiota: A Key Modulator of Intestinal Healing in Inflammatory Bowel Disease. Dig Dis. 2016;34:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 738] [Cited by in F6Publishing: 779] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 7. | Potter MB. Strategies and resources to address colorectal cancer screening rates and disparities in the United States and globally. Annu Rev Public Health. 2013;34:413-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [PubMed] [Cited in This Article: ] |
| 9. | Sussman DA, Santaolalla R, Strobel S, Dheer R, Abreu MT. Cancer in inflammatory bowel disease: lessons from animal models. Curr Opin Gastroenterol. 2012;28:327-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Hale LP, Greer PK. A novel murine model of inflammatory bowel disease and inflammation-associated colon cancer with ulcerative colitis-like features. PLoS One. 2012;7:e41797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 587] [Cited by in F6Publishing: 580] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 12. | Lopetuso LR, Chowdhry S, Pizarro TT. Opposing Functions of Classic and Novel IL-1 Family Members in Gut Health and Disease. Front Immunol. 2013;4:181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369-373. [PubMed] [Cited in This Article: ] |
| 14. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 683] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 15. | First MR, Peddi VR. Malignancies complicating organ transplantation. Transplant Proc. 1998;30:2768-2770. [PubMed] [Cited in This Article: ] |
| 16. | Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 17. | Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965-973. [PubMed] [Cited in This Article: ] |
| 18. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 1160] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 19. | Scaldaferri F, Sans M, Vetrano S, Graziani C, De Cristofaro R, Gerlitz B, Repici A, Arena V, Malesci A, Panes J. Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease. J Clin Invest. 2007;117:1951-1960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585-595.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Lopetuso LR, Petito V, Cufino V, Arena V, Stigliano E, Gerardi V, Gaetani E, Poscia A, Amato A, Cammarota G. Locally injected Infliximab ameliorates murine DSS colitis: differences in serum and intestinal levels of drug between healthy and colitic mice. Dig Liver Dis. 2013;45:1017-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 23. | Petito V, Lopetuso LR, Arena V, Stigliano E, Boninsegna A, Bibbò S, Poscia A, Alfieri S, Rosa F, Amato A. Direct effect of infliximab on intestinal mucosa sustains mucosal healing: exploring new mechanisms of action. Dig Liver Dis. 2016;48:391-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila). 2010;3:1314-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 503] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 26. | Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Schleck CD, Tremaine WJ, Melton LJ, Munkholm P. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130:1039-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 27. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-774, 774.e1-e4; quiz e12-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 28. | Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724-2729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 374] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 29. | Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 389] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 30. | Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839-3848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 147] [Cited by in F6Publishing: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |