Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9445
Peer-review started: July 22, 2016
First decision: July 21, 2016
Revised: August 11, 2016
Accepted: August 23, 2016
Article in press: August 23, 2016
Published online: November 14, 2016
Patients with advanced hepatocellular carcinoma (HCC) showing portal vein tumor thrombosis (PVTT) have an extremely poor prognosis. According to treatment guidelines, the only option for HCC patients with PVTT is sorafenib chemotherapy. However, in Asia, various treatments have been attempted and possible prolongation of overall survival has been repeatedly reported. We herein report the first case of a patient with an initially unresectable advanced HCC with PVTT who underwent curative hepatectomy after sorafenib and transcatheter arterial chemoembolization (TACE) showing complete histological response. Two months after induction with sorafenib, a significant decrease in serum alpha-fetoprotein level was observed and computed tomography imaging showed a significant decrease in tumor size. Because of remaining PVTT, TACE and curative resection were performed. The combination of sorafenib and TACE may be an effective treatment for HCC patients with PVTT.
Core tip: Patients with advanced hepatocellular carcinoma (HCC) showing portal vein tumor thrombosis (PVTT) have an extremely poor prognosis. The only proposed treatment option for HCC patients with PVTT is sorafenib chemotherapy. However, in Asia, various treatments have been attempted and possible prolongation of overall survival has been repeatedly reported. Here we report the first case of a patient with an initially unresectable advanced HCC and PVTT who underwent curative hepatectomy after sorafenib and transcatheter arterial chemoembolization (TACE) showing complete histological response. The combination of sorafenib and TACE may be an effective treatment for HCC patients with PVTT.
- Citation: Takano M, Kokudo T, Miyazaki Y, Kageyama Y, Takahashi A, Amikura K, Sakamoto H. Complete response with sorafenib and transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma. World J Gastroenterol 2016; 22(42): 9445-9450
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9445.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9445
Patients with advanced hepatocellular carcinoma (HCC) showing portal vein tumor thrombosis (PVTT) have an extremely poor prognosis[1,2]. The median survival of untreated HCC with PVTT has been reported to be 2.7-6 mo[2,3]. According to the American Association for the Study of the Liver Disease/Barcelona Clinic for Liver Cancer Staging System and treatment guidelines, the only proposed treatment option for HCC patients with PVTT is sorafenib chemotherapy[4]. In Asia, various treatments including hepatectomy and transcatheter arterial chemoembolization (TACE) have been attempted and possible prolongation of overall survival (OS) has been repeatedly reported[5]. We herein report the first case of a patient with an initially unresectable advanced HCC with PVTT who underwent curative hepatectomy after sorafenib and TACE, showing complete histological response.
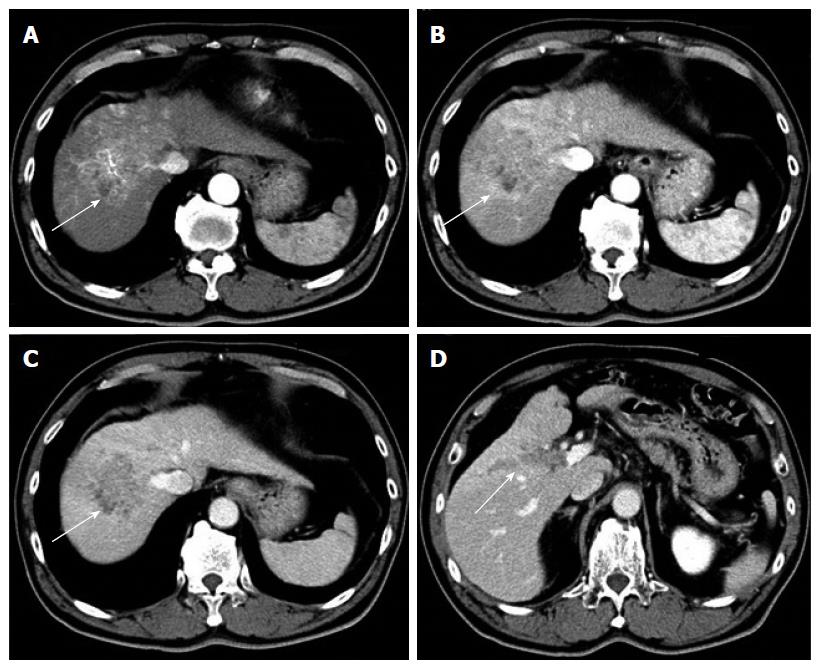
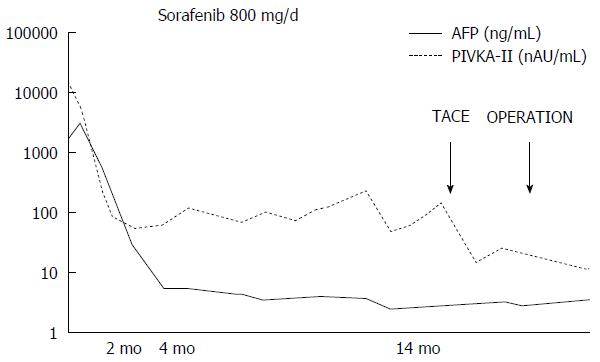
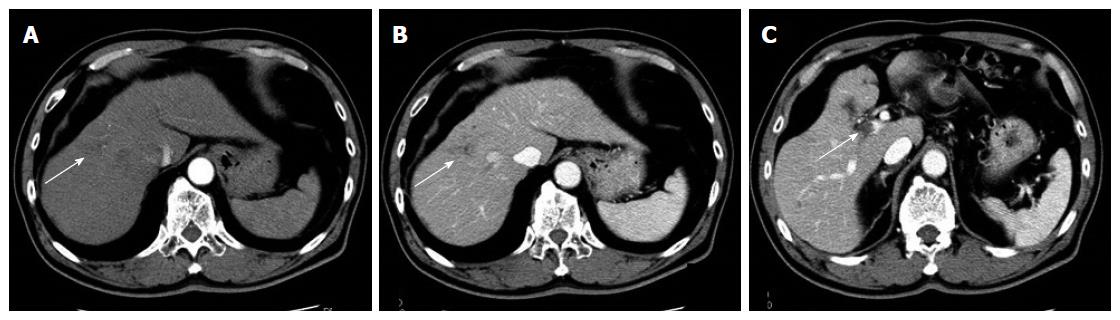
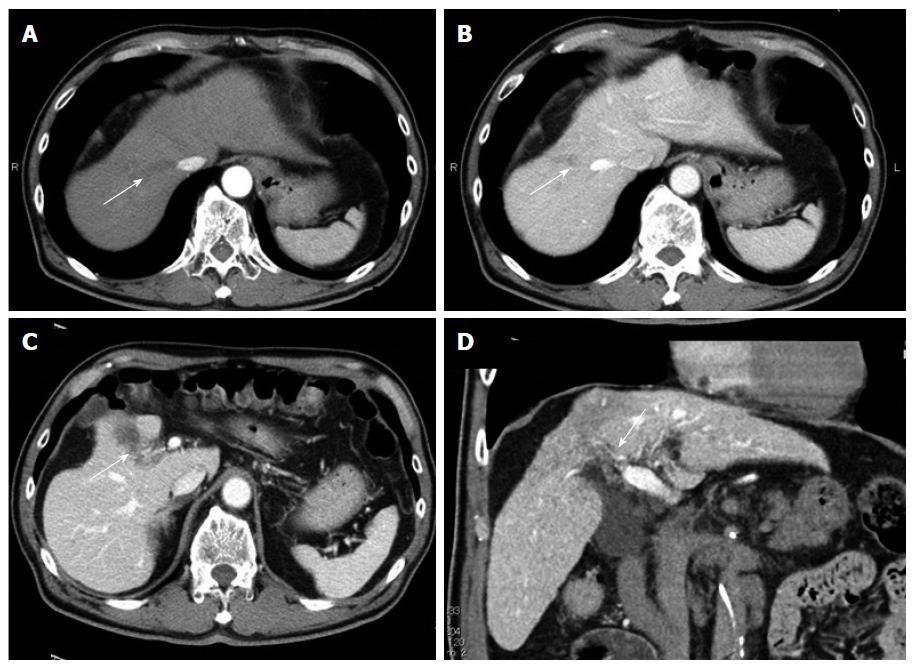
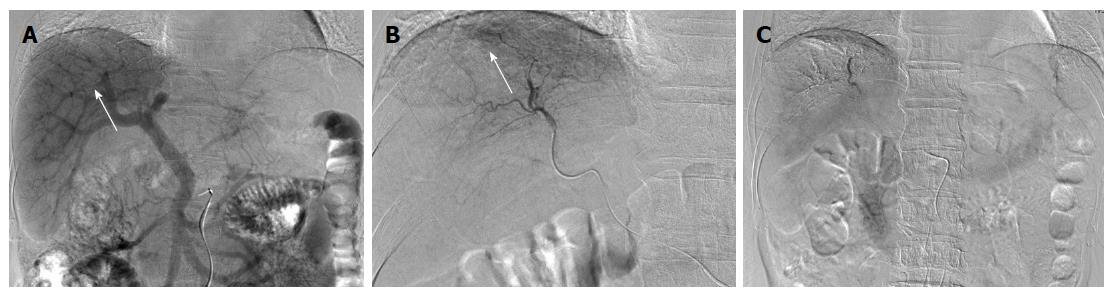
A 67-year-old man was diagnosed with HCC through abdominal ultrasound during examination for elevated liver enzymes by his family doctor and was referred to our hospital. The patient had no history of alcohol abuse, hepatitis B or C infection. Serum α-fetoprotein (AFP) level and protein induced by vitamin K absence or antagonist-II level were 1736 ng/mL (normal range: < 10 ng/mL) and 15388 mAU/mL (normal range: < 40 mAU/mL), respectively. Contrast-enhanced computed tomography (CT) scan revealed the presence of an 8.7 cm × 6 cm tumor in the right paramedian sector, showing early enhancement in the arterial phase and wash-out in the late phase together with PVTT limited to the first-order branch and invading the right portal vein (Figure 1). Right hepatectomy was considered to be necessary for curative resection. Although the patient’s liver function was Child-Pugh A, the patient’s indocyanine green retention rate at 15 min was 21% (normal range; < 10%) and right hepatectomy was considered to be intolerable according to our institutional criteria[6]. Therefore, sorafenib was orally administered twice daily at a dose of 800 mg. During sorafenib treatment, the patient have no adverse event. Two months later, a significant decrease in serum AFP level was observed (195 ng/mL) (Figure 2). The CT scan showed a significant decrease in tumor size (3 cm); however, PVTT remained in the right portal vein (Figure 3). Four months later, serum AFP level decreased to within normal range (4.5 mg/mL), and 14 months later, CT scan revealed the residual PVTT in right portal vein (Figure 4). Portography revealed filling defect in S8 and digital subtraction ateriography showed irregular shaped tumor stain. Thus TACE was performed with 30 mg of mirpulatin, 3 mL of lipiodol and gelatin sponge particle (Figure 5), followed by right paramedian sectionectomy. During the operation, neither the main tumor nor the PVTT was identified through intraoperative ultrasound. The operation time was 318 min and the estimated blood loss was 762 mL. The patient’s postoperative course was uneventful, and he was discharged from hospital on postoperative day 11. Pathological examination revealed complete necrosis without viable tumor cells both in the scar of PVTT and the main tumor. To date, no recurrence has been observed after 12 mo of follow-up.
Sorafenib has been reported to prolong survival in patients with unresectable or advanced HCC; however, complete response (CR) was not achieved in these reports[4,7,8]. In Asian countries, including Japan, liver resection and TACE have been reported to improve the prognosis of patients with HCC with PVTT. In the presence of PVTT, TACE is theoretically contraindicated in Western countries because of the potential risk of hepatic insufficiency that results from ischemia following TACE. However, recent studies demonstrate that TACE can be safely performed in the presence of adequate collateral circulation around the occluded portal vein[9,10]. A median OS period after treatment with TACE was reported to be 5.6-16.5 mo in patients with HCC accompanied by PVTT[11-13]. A median OS period after treatment with surgery was reported to be 6-19.9 mo[14-16]. Furthermore, Minagawa et al[16] reported a high survival rate in these patients with the combination of TACE followed by hepatic resection. In the very recent paper, liver resection is associated with prolongation of overall survival of HCC with PVTT[13]. Thus, TACE and surgery are the common choices of the treatment for HCC in Japan. Anticoagulants (e.g., low molecular weight heparin, warfarin and oral anticoagulant) has been reported to be effective for portal vein thrombosis[17]. However, evidence is limited concerning the effect of anticoagulants other than anti-cancer treatment for PVTT. After treatment of HCC with PVTT with sorafenib, an OS period of 6.2-12.3 mo has been reported[13,18]. Although CR after sorafenib treatment is rare, to the best of our knowledge, 10 cases of HCC patients with PVTT who achieved CR after treatments including sorafenib have been reported (Table 1)[19-27]. Four of the 10 cases underwent hepatectomy and had confirmed histological CR. Five of the 10 cases only underwent sorafenib treatment, and the other cases had other combined treatment modalities. The median time to normalized level of serum AFP was 4.5 mo (range, 2.75-6.5 mo). The median time to CR is 8 mo (range, 6-16.5 mo). All cases including ours, showed disappearance of the main tumor and PVTT. We herein report the first case of histologically confirmed CR of HCC with PVTT after sorafenib and TACE. Combination of sorafenib and TACE may be an effective treatment for HCC patients with PVTT.
| Age | Sex | Etiology | Extrahepatic metastasis | Pre-AFP | Post-AFP | Sorafenib dose | Duration (mo) | Time to CR (mo) | Resection | Other therapy | Follow-up period (mo) |
| 54 | Male | Hepatitis C | Lung | 52347 | 30.2 | 800-400 | 5 | 5 | + | EBRT | 14 |
| 83 | Male | None | Lung | 41948 | W.N.L. | 800-400-200-100 | 34 | 8 | - | TACE RFA HAI | 34 |
| 59 | Male | Hemochromatosis | Little omentum LN | 866 | W.N.L. | 800 | 6 | 6 | + | None | 16 |
| 57 | Male | Hepatitis B | None | 17000 | W.N.L. | 800-400 | 12 | 12 | + | None | 12 |
| 74 | Male | Hepatitis C | ND | 3300 | W.N.L. | 400 | 8 | 8 | - | None | 24 |
| 84 | ND | Hepatitis C | None | 353 | W.N.L. | 800 | 12 | 6 | - | None | 12 |
| 69 | Male | Hepatitis C | None | n.d. | ND | 800-400-200 | 62 | 23 | - | None | 62 |
| 74 | Male | None | ND | 33058 | 2 | 800-400-200 | 19 | 19 | - | None | 19 |
| 68 | Male | Hepatitis C | Dissemination | 4773 | 45.7 | 800-400 | 28 | 24 | + | None | 40 |
| 48 | Male | Hepatitis C | None | 135835 | W.N.L. | 800 | 9 | 4 | - | None | 31 |
| 67 | Male | None | None | 3385 | W.N.L. | 800 | 14 | 14 | + | TACE | 26 |
A 67-year-old man had no symptom.
On physical examination, he had a palpable mass in right upper quadrant of the abdomen.
Hepatocellular carcinoma, metastatic liver tumor, intrahepatic cholangiocarcinoma, malignant lymphoma and liver hemangioma.
The patient have elevated hematological value for alkaline phosphatase (316 IU/L), Glutamic-oxaloacetic transaminase (80 IU/L), glutamic pyruvic transaminase (89 IU/L), γ-glutamyltranspeptidase (338 IU/L), α-fetoprotein (1736.3 ng/mL), protein induced by vitamin K absence or antagonist-II (15388 mAU/mL).
Contrast-enhanced computed tomography scan revealed the presence of an 8.7 cm × 6 cm tumor in the right paramedian sector, showing early enhancement in the arterial phase and wash-out in the late phase together with portal vein tumor thrombosis limited to the first-order branch and invading the right portal vein.
Histological examination after sorafenib chemotherapy and transcatheter arterial chemoembolization showed complete necrosis without viable tumor cells both in the scar of portal vein tumor thrombosis and the main tumor.
The patient received a sorafenib chemotherapy and transcatheter arterial chemoembolization.
Sorafenib chemotherapy is associated with prolongation of overall survival of advanced hepatocellular carcinoma (HCC), compared with best supportive care. However, complete response after sorafenib treatment with or without other treatments is very rare.
Portal vein tumor thrombosis is a form of venous thrombosis affecting the hepatic portal vein, caused by tumor invasion.
This case report presents a new choice of treatment for advanced hepatocellular carcinoma accompanying with portal vein tumor thrombosis. Combination of sorafenib and transcatheter arterial chemoembolization may be an effective treatment for HCC patients with portal vein tumor thrombosis.
The authors have described a case of advanced hepatocellular carcinoma with portal vein thrombosis that showed complete response after sorafenib and transcatheter arterial chemoembolization. The article provides another choice of treatment for advanced hepatocellular carcinoma.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bester L, Gatselis NK, Ji JS, Somma F S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Kim JM, Kwon CH, Joh JW, Park JB, Ko JS, Lee JH, Kim SJ, Park CK. The effect of alkaline phosphatase and intrahepatic metastases in large hepatocellular carcinoma. World J Surg Oncol. 2013;11:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [PubMed] [Cited in This Article: ] |
| 3. | Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, De Santis M, Manenti F. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233-238. [PubMed] [Cited in This Article: ] |
| 4. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3249] [Cited by in F6Publishing: 3477] [Article Influence: 289.8] [Reference Citation Analysis (3)] |
| 5. | Shaohua L, Qiaoxuan W, Peng S, Qing L, Zhongyuan Y, Ming S, Wei W, Rongping G. Surgical Strategy for Hepatocellular Carcinoma Patients with Portal/Hepatic Vein Tumor Thrombosis. PLoS One. 2015;10:e0130021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589-594. [PubMed] [Cited in This Article: ] |
| 7. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9502] [Article Influence: 593.9] [Reference Citation Analysis (1)] |
| 8. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3854] [Cited by in F6Publishing: 4336] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 9. | Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007;18:1517-1526; quiz 1527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Kim JH, Yoon HK, Kim SY, Kim KM, Ko GY, Gwon DI, Sung KB. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29:1291-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 12. | Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima O. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 14. | Lee JM, Jang BK, Lee YJ, Choi WY, Choi SM, Chung WJ, Hwang JS, Kang KJ, Kim YH, Chauhan AK. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin Mol Hepatol. 2016;22:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561-7567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 209] [Cited by in F6Publishing: 218] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 16. | Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233:379-384. [PubMed] [Cited in This Article: ] |
| 17. | Harding DJ, Perera MT, Chen F, Olliff S, Tripathi D. Portal vein thrombosis in cirrhosis: Controversies and latest developments. World J Gastroenterol. 2015;21:6769-6784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 75] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Kudo M, Ikeda M, Takayama T, Numata K, Izumi N, Furuse J, Okusaka T, Kadoya M, Yamashita S, Ito Y. Safety and efficacy of sorafenib in Japanese patients with hepatocellular carcinoma in clinical practice: a subgroup analysis of GIDEON. J Gastroenterol. 2016; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 19. | Kitajima T, Hatano E, Mitsunori Y, Taura K, Fujimoto Y, Mizumoto M, Okajima H, Kaido T, Minamiguchi S, Uemoto S. Complete pathological response induced by sorafenib for advanced hepatocellular carcinoma with multiple lung metastases and venous tumor thrombosis allowing for curative resection. Clin J Gastroenterol. 2015;8:300-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Shinoda M, Kishida N, Itano O, Ei S, Ueno A, Kitago M, Abe Y, Hibi T, Yagi H, Masugi Y. Long-term complete response of advanced hepatocellular carcinoma treated with multidisciplinary therapy including reduced dose of sorafenib: case report and review of the literature. World J Surg Oncol. 2015;13:144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Irtan S, Chopin-Laly X, Ronot M, Faivre S, Paradis V, Belghiti J. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int. 2011;31:740-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Wang SX, Byrnes A, Verma S, Pancoast JR, Rixe O. Complete remission of unresectable hepatocellular carcinoma treated with reduced dose of sorafenib: a case report. Target Oncol. 2010;5:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Sacco R, Bargellini I, Gianluigi G, Bertini M, Bozzi E, Altomare E, Battaglia V, Romano A, Bertoni M, Capria A. Complete response for advanced liver cancer during sorafenib therapy: case report. BMC Gastroenterol. 2011;11:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Abbadessa G, Rimassa L, Pressiani T, Carrillo-Infante C, Cucchi E, Santoro A. Optimized management of advanced hepatocellular carcinoma: four long-lasting responses to sorafenib. World J Gastroenterol. 2011;17:2450-2453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Kee KM, Hung CH, Wang JH, Lu SN. Serial changes of clinical parameters in a patient with advanced hepatocellular carcinoma with portal vein thrombosis achieving complete response after treatment with sorafenib. Onco Targets Ther. 2014;7:829-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Kogame M, Kanayama M, Matsui T, Kikuchi Y, Ishii K, Igarashi Y. Sustained complete response of hepatocellular carcinoma with portal vein tumor thrombus following discontinuation of sorafenib: A case report. Oncol Lett. 2014;7:50-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Gamstätter T, Weinmann A, Schadmand-Fischer S, Spies PR, Niederle IM, Schuchmann M, Galle PR, Wörns MA. AFP measurement in monitoring treatment response of advanced hepatocellular carcinoma to sorafenib: case report and review of the literature. Onkologie. 2011;34:538-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |