Published online Aug 7, 2016. doi: 10.3748/wjg.v22.i29.6673
Peer-review started: March 2, 2016
First decision: April 14, 2016
Revised: May 21, 2016
Accepted: June 13, 2016
Article in press: June 13, 2016
Published online: August 7, 2016
Gut microbiota plays a key role in the pathogenesis of alcoholic liver disease (ALD). Consumption of alcohol leads to increased gut permeability, small intestinal bacterial overgrowth, and enteric dysbiosis. These factors contribute to the increased translocation of microbial products to the liver via the portal tract. Subsequently, bacterial endotoxins such as lipopolysaccharide, in association with the Toll-like receptor 4 signaling pathway, induce a gamut of damaging immune responses in the hepatic milieu. Because of the close association between deleterious inflammation and ALD-induced microbiota imbalance, therapeutic approaches that seek to reestablish gut homeostasis should be considered in the treatment of alcoholic patients. To this end, a number of preliminary studies on probiotics have confirmed their effectiveness in suppressing proinflammatory cytokines and improving liver function in the context of ALD. In addition, there have been few studies linking the administration of prebiotics and antibiotics with reduction of alcohol-induced liver damage. Because these preliminary results are promising, large-scale randomized studies are warranted to elucidate the impact of these microbiota-based treatments on the gut flora and associated immune responses, in addition to exploring questions about optimal delivery. Finally, fecal microbiota transplant has been shown to be an effective method of modulating gut microbiota and deserve further investigation as a potential therapeutic option for ALD.
Core tip: Alcoholic liver disease (ALD) brings about imbalance in the gut microbiota which results in deleterious immune responses that affect the liver. However, there is little research on therapy that targets this aspect of ALD pathophysiology. This review summarizes ALD-induced changes in gut microbiota and its associated inflammatory effects, and explores the gamut of latest research on microbiota-based treatments for ALD, which include probiotics, prebiotics, antibiotics, and fecal microbiota transplant.
- Citation: Sung H, Kim SW, Hong M, Suk KT. Microbiota-based treatments in alcoholic liver disease. World J Gastroenterol 2016; 22(29): 6673-6682
- URL: https://www.wjgnet.com/1007-9327/full/v22/i29/6673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i29.6673
The human gut microbiota is composed with trillions of bacteria which total 1-2 kg in mass. These microorganisms in the human gut, in maintaining a close relationship with the host, play an important role in promoting metabolism and digestion[1,2]. Recent research has suggested that each person hosts a distinctive, personalized gut microbiota which may consist of Bacteroides, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia. However, a comprehensive understanding of the human gut microbiota and its variations across different geographical regions has yet to be established[3]. Because the gut is connected to the liver by the portal tract, the disturbance of intestinal microbiota can lead to disease, especially in the liver. Indeed, a number of liver conditions, including alcoholic liver disease (ALD), have been associated with qualitative and quantitative changes in the gut microbiota[4,5].
This review focuses on the ALD-induced changes in the intestinal microbiota and the concomitant immune responses that damage the liver. Further, novel therapeutic approaches such as probiotics are proposed for the management of ALD in promoting the reestablishment of gut homeostasis.
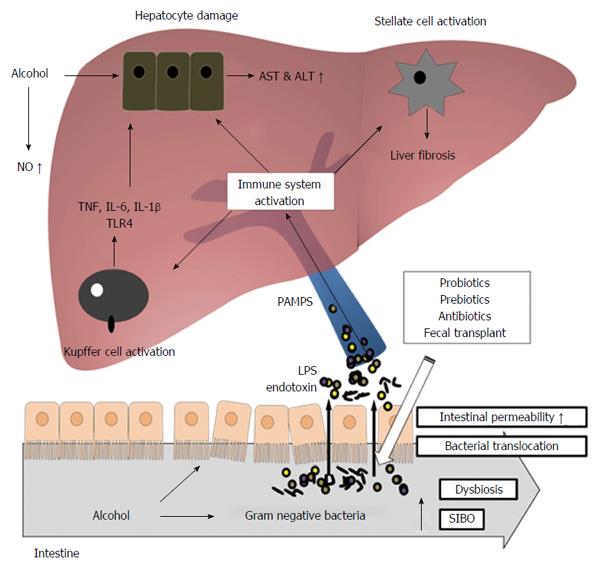
ALD is one of the causes for chronic liver disease, which encompasses a wide spectrum of liver pathologies including steatosis, fibrosis, alcoholic hepatitis, cirrhosis, and hepatocellular carcinoma[6,7]. Consumption of alcohol may induce small intestinal bacterial overgrowth (SIBO), particularly of Gram negative bacteria[8]. Further, the damage caused by alcohol on the intestinal lumen results in the increased translocation of microbial products, which include endotoxin, bacterial DNA, and pathogen-associated molecular patterns (PAMPs), from the gut to the liver. Toxic metabolites such as PAMPs, in turn, result in the triggering of the inflammatory cascade, and the induction of reactive oxygen species and other factors in various cells of the liver[9,10]. Figure 1 depicts this process of alcohol consumption bringing about changes in the intestinal milieu and inducing consequent downstream immune responses in the liver. Expectedly, liver dysfunction has been observed to be closely associated with SIBO and the increased translocation of bacterial products[11-14].
A potential clinical implication of advanced ALD in the gut-liver axis is the promotion of bacterial infections, often manifesting as subacute bacterial peritonitis, hepatic encephalopathy, or severe systemic infections[15]. However, selective gut decontamination with rifaximin has been found to improve the conditions of patients with hepatic encephalopathy, highlighting the possible benefits of judicious antibiotic usage in treating conditions associated with advanced ALD[16,17].
Lipopolysaccharide (LPS), a bacterial endotoxin, plays a particularly integral role in the pathophysiology of ALD. Previous studies have revealed that levels of LPS are elevated in the plasma of both ALD patients and experimental animal models of ALD[18,19]. Further, it has been seen that mice deficient in LPS receptor Toll-like receptor 4 (TLR4) or LPS co-receptor CD14 are resistant to alcohol-induced liver injury, underscoring the significance of LPS in context of ALD[20,21]. It is worth noting that plasma endotoxin levels were not dissimilar between TLR4 deficient and wild type mice, which suggests that TLR4 signaling pathway is not involved in the modulation of gut leakiness.
As suggested above, TLR4 is also an important component in hepatic inflammation associated with ALD and, therefore, a brief discussion on TLR4 is merited. Toll receptors have gained attention in the immunology field when TLR4 was demonstrated to induce the expression of genes that mediate inflammatory responses[22]. Additionally, a Tlr4 gene mutation in the mouse model was found to be hyporesponsive to LPS[23]. Eventually, 10 proteins that are structurally similar to TLR4 were identified and classified under the TLR family[24]. The physiological function of TLRs has been identified largely through genetic approaches; TLRs were soon identified as crucial components in the mammalian detection of microbial invasion, of which TLR-mediated recognition of specific pathogenic components is integral[25].
In the context of ALD, it has been found that both chronic and acute alcohol consumption cause the expression of TLR4 and its co-receptors to increase[20,26-29]. As previously mentioned, TLR4 deficient mice were found to be protected from ALD, suggesting the key role of TLR4 in the pathogenesis of ALD[30,31].
The TLR4 pathway is activated when LPS in portal and systematic circulation is initially bound by CD14 and LPS-binding protein (LBP), and the resultant complex binds to TLR4. The resulting signaling cascade causes the activation of Kupffer cells and multiple pro-inflammatory cytokines and, ultimately, the onset of alcohol-induced liver injury[9,32-34]. The increased recruitment and activation of inflammatory cells and pro-inflammatory cytokines result in the modulation of hepatocyte function. Namely, liver cells respond to inflammation by the production of acute phase reactants, which include serum amyloid A, LBP, fibrinogen, C-reactive protein, and ceruloplasmin[35-37]. One potential outcome of such LPS-induced alteration of hepatocyte function is cholestasis, in which the bile flow is decreased by impairment of hepatocytes[38]. Other possible consequences include the exacerbation of alcohol-induced liver injury by the enhancement of LPS-induced signal transduction by LBP, an acute phase reactant, as confirmed in a study utilizing mouse model[39]. Figure 1 summarizes the overall role of PAMPs, LPS, TLR4, and other immune components in alcohol-induced damage to the liver.
Additionally to LPS, other bacterial products including bacterial DNA can also translocate from the intestine to other organs and extraintestinal space. Indeed, bacterial DNA was observed to be elevated in the plasma of ALD patients[40]. Bacterial DNA, in turn, may be recognized by TLR9 which results in the sensitization of liver to injury induced by LPS[41]. Summarily, translocation of bacterial products from the intestinal lumen to other organs such as the liver contribute significantly to the pathogenesis of ALD[6].
In normal homeostasis, PAMPs such as LPS are scavenged by Kupffer cells and hepatocytes in the liver and are consequently metabolized[42-44]. Nullification of LPS, in particular, is carried out by several possible mechanisms: first, molecules can bind to LPS, preventing it from activating TLR4. Alternately, enzymes can degrade the lipid A moiety of LPS, thereby decreasing the activity of the endotoxin[45]. Still other mechanisms exist, including those involving serum lipoproteins and chylomicrons, resulting in the detoxification of LPS[46-48].
In summary, a number of processes contribute to the clearance of LPS and prevent significant inflammatory cell activation. In the context of ALD, however, LPS clearance by hepatocytes is found to be significantly impaired; it is unlikely that hepatocytes constantly exposed to alcohol retain the capability of detoxificating LPS[49].
Chronic alcohol ingestion, in addition to causing intestinal overgrowth of bacteria, may also lead to enteric dysbiosis, in which the physiological composition of microbes becomes imbalanced[50,51]. Several studies have demonstrated the role of heavy alcohol consumption in the breakdown of this balance. In mice, for instance, intragastric feeding of alcohol for three weeks led to the dominance of Bacteroidetes in the cecum, while in control mice, Firmicutes are the dominant species[52]. In addition, Akkermansia and Bacteroides became more numerous, while Lactobacillus decreased in number[52]. Further, in rats that underwent daily alcohol gavage for 10 wk, an alteration in the mucosa-associated microbiota composition was observed in the colon[53].
In context of humans, consumption of excessive alcohol was also found to be associated with significant changes in microbial composition in the intestinal system. Namely, an increase of Prevotellaceae in the feces was observed for patients with alcoholic liver cirrhosis compared to healthy controls[54]. On the other hand, a significant decrease of Bacteroidaceae was found for ALD patients when compared with the control group[55]. Another study revealed that excessive alcohol consumption over long period leads to the reduction of Bacteriodetes and Firmicutes, and the elevation of Gram negative bacteria such as Actinobacteria and Proteobacteria[56]. Proteobacteria, in particular, includes a number of pathogenic species such as Salmonella, Vibrio, Helicobacter, and Escherichia. A separate study also implicated increase of Proteobacteria and decrease of Bacteroidetes, specifically in the context of ALD patients[4]. In a genetic study, it was demonstrated that the amount of Enterobactericaea bacterial DNA was increased in the feces of patients with ALD compared to those of the control group[57]. Finally, in studying ascites from ALD patients, 50% of samples were found to contain Enterobactericaea, Clostridium leptum, or Lactobacillus[57,58].
Immediate abstinence from alcohol is the most critical and effective treatment for patients with ALD[59,60]. Abstinence was found to improve both the survival and prognosis of ALD patients; it can also stop the progression of disease to liver cirrhosis by bringing about histologic improvement and reduction in portal pressure[61,62]. As emphasized above, the pathophysiology of ALD has been found to be clearly linked with the overgrowth of intestinal bacteria. In addition, enteric dysbiosis has been demonstrated to be associated with ALD. Consequentially, reestablishing the balance of microbes through the administration of probiotics, prebiotics, antibiotics, or fecal microbiota transplantation (FMT) may be effective in preventing bacterial translocation and deleterious inflammatory responses that may result from ALD-associated changes in gut microbiota, and may forestall the progression of disease to serious conditions such as cirrhosis, fibrosis, or hepatocellular carcinoma[63]. Therefore, further discussion on these microbiota-based treatments as potential therapy for ALD patients is merited.
Probiotics are defined as monocultures or mixed cultures of microorganisms that can be administered to potentially improve the properties of the gut microbiota. Specifically, probiotics promote an anti-inflammatory milieu in which the intestinal barrier integrity is upheld while bacterial translocation and endotoxin production are inhibited[8]. Four potential mechanisms have been proposed through which probiotics bring about their beneficial effects[64]: first, probiotic bacteria such as Lactobacillus reuteri may produce antimicrobial agents that suppress the growth, epithelial binding, and invasion of pathogenic bacteria[65]. Second, probiotic bacteria may enhance intestinal barrier function by promoting intestinal epithelial cell survival and growth[66]. Third, the immune system may be modulated to suppress the release of proinflammatory cytokines such as TNF-α[67] and to induce the release of protective cytokines such as IL-10[68] and TGF-β. Finally, probiotic microorganisms may induce the expression of microopiod and cannabinoid receptors, conferring analgesic properties in the context of intestinal pain[69].
In the clinical setting, the administration of probiotics has been demonstrated to be effective in reducing endotoxemia and improving liver function, a result observed in a study enrolling cirrhotic patients[70]. These beneficial effects of probiotics have been further confirmed in larger study involving patients with alcohol-induced liver injury, in which a probiotic preparation containing Bifidobacterium bifidum and Lactobacillus plantarum was utilized[71]. The reestablishment of microbiota balance through probiotics also has been shown to restore neutrophil dysfunction in patients with compensated alcoholic cirrhosis, in which Lactobacillus casei Shirota was administered[72,73]. In another study enrolling alcoholic hepatitis patients, intake of Lactobacillus subtilis and Streptococcus faecium led to the reduction of gut-derived microbial LPS[74], which, as discussed earlier, plays an integral role in the pathophysiology of ALD (Table 1).
| Patients | Enroll criteria or alcohol amount | Treatment | Results | Ref. |
| Compensated LC | Liver biopsy | Escherichia coli Nissle | Lactobacillus and Bifidobacterium sp. ↑ | [70] |
| [alcohol: 22 (56.4%)] | Biochemical study | (2.5-25 × 109 for 42 d) | Proteus hausei and Citrobacter sp. ↓ | |
| Age = 53 | Endotoxin level | Morganella sp. and endotoxemia ↓ | ||
| M/F = 1.8:1 | Stool microbiota | improvement of liver functions | ||
| Alcohol-related psychosis | Consumed 750 mL of Russian vodka (40% ethanol, daily) | Bifidobacterium bifidum | Bifidobacteria and Lactobacilli ↑ | [71] |
| [66 (73.3%)] | (0.9 × 108 CFU for 5 d) | AST and ALT ↓ | ||
| Age = 42.3 ± 1.1 | Lactobacillus plantarum 8PA3 (0.9 × 109 CFU for 5 d) | |||
| All males | ||||
| LC | LC | Lactobacillus casei Shirota | Neutrophil phagocytic capacity ↑ | [72] |
| [alcohol: 12 (48%)] | (19.5 × 109 CFU for 28 d) | sTNFR1 ↓ | ||
| Age = 51.2 ± 1.8 | sTNFR2 ↓ | |||
| M/F = 2:1 | IL10 ↓ | |||
| TLR4 ↓ | ||||
| AH [60 (51.3%)] | AST/ALT > 1 | Lactobacillus subtilis, Streptococcus faecium | Serum LPS level ↓ | [74] |
| Age = 52.7 ± 11.3 | AST and ALT level ↑ | (1500 mg/d for 7 d) | TNF-α↓ | |
| M/F = 5.3:1 | Alcohol intake | |||
| > 40 g/d for female | ||||
| > 60 g/d for male |
In two separate studies utilizing mouse, the efficacy of probiotics has been implicated directly in the context of ALD. When a probiotics diet consisting of Lactobacillus rhamnosus and Lactobacillus acidophilus was administered to mouse model of ALD for four weeks, TLR4 levels were found to be significantly lower in the probiotics group compared to the control group[75,76]. The TLR4 pathway has been previously described as the central component through which ALD-induced changes in microbiota bring about eventual inflammatory responses that damage the liver. The study also demonstrated that the administration of probiotics led to the decrease in deleterious cytokines such as IL-1β and TNF-α[75]; this result aligns with the finding that probiotics inhibit the TLR-4 pathway, which is known to induce release of pro-inflammatory cytokines (Table 2).
| Animal model | Alcohol amount | Treatment | Results | Ref. |
| 6-wk-old male 10 C57BL/6 mice | Lieber-DeCarli liquid diet with 10% alcohol for 6 wk | Lactobacillus rhamnosus R0011, Lactobacillus acidophilus R0052 | TLR-4 ↓ | [75] |
| (1 mg/mL per day for 4 wk) | IL-1β↓ | |||
| 4-wk-old male 20 C57BL/6 mice (10 Normal diet, 10 High-fat diet) | Oral administration 5 g/kg per day, twice/wk, for 9 wk | Lactobacillus rhamnosus R0011, Lactobacillus acidophilus R0052 | In normal diet groups | [76] |
| (1 mg/mL per day for 2 wk) | TNF-α↓ | |||
| IL-1↓ | ||||
| TLR4 ↓ | ||||
| TLR4/GADPH ↓ | ||||
| In high-fat diet groups: IL-10 ↑ | ||||
| Male Sprague-Dawley rats | Dose gradually increased every 2 to 3 d up to a maximum of 8 g/kg per day by 2 wk | Oats (10 g/kg per day) | Tight junctions in colon ↓ | [79] |
| Disorganization of actin cytoskeleton ↓ | ||||
| 6 g/kg per day for final 10 wk | Oxidative stress ↓ | |||
| NO overproduction ↓ | ||||
| Oxidative tissue damage ↓ | ||||
| Nitrotyrosine ↓ | ||||
| Carbonyl ↓ |
In addition to probiotics, other treatments such as prebiotics, antibiotics, and FMT might help promote the reestablishment of gut homeostasis, and also deserve attention as potential treatment options for ALD. Prebiotics are identified as ingredients that support the growth and activity of a selection of microbes in the gastrointestinal-tract, resulting in health benefits for the host[77]. As complex carbohydrates that are not metabolized by pancreatic and intestinal enzymes[52], prebiotics reach the large bowel and act as substrates for advantageous gut bacteria such as Bifidobacteria and Lactobacilli, which promotes the body’s resistance to invading pathogens[78]. In a study utilizing rats, the administration of prebiotics has been demonstrated to decrease the liver damage caused by alcohol[79]. Further, in cirrhotic patients, prebiotics intake was found to be very effective in treating subclinical hepatic encephalopathy[80]. Beyond this, however, studies that explore the potential effectiveness of prebiotics in the context of ALD are few and limited. Synbiotics, in which probiotics and prebiotics are synergistically co-administered[81], also warrant study as potential treatment for ALD.
Antibiotics, on the other hand, are antimicrobials which may reduce the population of deleterious bacteria, decreasing the amount of LPS released and diminishing the associated inflammatory response. In a preliminary study involving a small number of ALD patients, treatment with antibiotics (neomycin and norfloxacin) led to an improvement in the Child-Pugh score, a measure of severity of chronic liver disease[82]. As previously noted, rifaximin, a broad-spectrum antibiotic, has been administered to treat hepatic encephalopathy and was found to improve not only the prognosis of patients[16] but also cirrhosis-related thrombocytopenia[83]. The latter finding might be related to the reduction of endotoxemia resulting from intestinal decontamination, and highlight the therapeutic potential of antibiotics in treating ALD (Table 3).
| Patients | Enroll criteria or alcohol amount | Treatment | Results | Ref. |
| HE | ≥ two episodes of overt HE (Conn score ≥ 2) | Rifaximin | Episode of encephalopathy ↓ | [16] |
| [alcohol 140 (46.8%)] | LC (MELD ≤ 25) | (1100 mg/d, for 6 mo) | (HR = 0.42) | |
| Age = 56 ± 10 | ||||
| M/F = 1.2:1 | ||||
| LC with subclinical HE | Psychometric tests | Lactulose (45 mL/d for 8 wk) | Number of the abnormal psychometric test ↓ | [80] |
| [alcohol 36 (48%)] | -Trail making test A | |||
| Age = 62.0 ± 7.3 | -Wechsler adult intelligence scale | Prevalence of subclinical HE ↓ | ||
| M/F = 1.2:1 | -Symbol digit | |||
| -Block design tests | ||||
| LC | Laboratory investigations | Norfloxacin (800 mg/d) | Small-intestinal motor activity ↑ | [82] |
| [alcohol 12 (35.3%)] | -Liver biopsy | Neomycin (1500 mg/d) alternating periods of 15 d for 6 mo | Transit time ↓ | |
| Age = 57.6 | -Endoscopy | Small intestinal bacterial overgrowth ↓ | ||
| M/F = 0.8:1 | Child-Pugh Score ↓ | |||
| TC | For LC | Rifaximin | Platelet count ↑ | [83] |
| [alcohol 13 (56.5%)] | -Liver biopsy | (1200 mg/d, for 4 wk) | Endotoxin ↓ | |
| Age = 58 ± 3 | -Laboratory findings | IL-1 ↓ | ||
| M/F = 11.5:1 | For hematological indices | IL-6 ↓ | ||
| -Platelet count ≤ 150000/μL | TNF-α↓ |
Finally, FMT refers to the transfer of fecal material, which contains the microflora of an healthy individual, to a diseased recipient[84]. The main mechanism of FMT likely involves the establishment of non-pathogenic bacterial strains in the gut and production of the antimicrobial components (e.g., bacteriocins) produced by these microbes[84]. When FMT was first introduced to the medical community, it understandably attracted both interest and significant controversy. However, FMT is becoming increasingly accepted as a legitimate and effective therapeutic approach in addressing various conditions characterized by microbiota imbalance; for instance, FMT has been found to be highly valuable in treating ulcerative colitis, in which FMT successfully modulated the gut microbiota and minimized colonic inflammatory responses[85]. Other studies also highlight the potential of FMT in addressing both gastrointestinal and non-gastrointestinal diseases in which therapeutic modulation of gut microbiota might be beneficial[86,87]. However, despite showing promise as a cost-effective method of promoting gut homeostasis, FMT has not yet been studied as a potential therapeutic option for ALD.
Changes in gut microbiota are an important factor in the pathogenesis of ALD and can be considered a novel therapeutic target for ALD. Thus far, a number of preliminary studies have been carried out to evaluate the effectiveness of probiotics, prebiotics, and antibiotics in modulating the gut flora and treating ALD patients. In both clinical and non-clinical settings, the administration of probiotics has been found to be effective in reducing harmful immune responses and improving liver function in the context of ALD. However, to confirm these results, randomized clinical trials of large sample size are necessary to further elucidate the role of probiotics in the treatment of ALD. In addition, questions on which patient population should be treated and which bacterial strains should be utilized need to be answered before probiotics gain widespread acceptance as clinical therapy for ALD.
While research on the efficacy of prebiotics and antibiotics in treating ALD is limited, prebiotics have been associated with reduction of alcohol-induced liver damage in rats and antibiotics have been implicated in improving the conditions of ALD patients. In consideration of these promising results, these approaches warrant further clinical studies to clarify their impact on the gut flora and associated immune responses. Synbiotics, which combine prebiotics and probiotics synergistically, also remain as an interesting area of exploration for the treatment of ALD.
Recently, FMT has gained attention as an effective method of addressing the imbalance of gut microbiota, which is associated with various medical conditions. FMT should be explored as a potential therapeutic approach for ALD. In addition to evaluating the efficacy of FMT in improving symptoms associated with ALD, investigation is required for unsolved questions on protocol, which include the route of administration, the optimal time of delivery, and the most effective amount of microflora. Finally, long-term clinical benefits and the safety of FMT should be evaluated in a clinical setting involving a large number of randomized ALD patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cichoz-Lach H, John JA S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59:328-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut. 2013;62:1591-1601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 3. | Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM. Enterotypes of the human gut microbiome. Nature. 2011;473:174-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4310] [Cited by in F6Publishing: 4470] [Article Influence: 343.8] [Reference Citation Analysis (0)] |
| 4. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 493] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 5. | Vassallo G, Mirijello A, Ferrulli A, Antonelli M, Landolfi R, Gasbarrini A, Addolorato G. Review article: Alcohol and gut microbiota - the possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment Pharmacol Ther. 2015;41:917-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Chen P, Schnabl B. Host-microbiome interactions in alcoholic liver disease. Gut Liver. 2014;8:237-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756-17772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 286] [Cited by in F6Publishing: 304] [Article Influence: 30.4] [Reference Citation Analysis (5)] |
| 8. | Malaguarnera G, Giordano M, Nunnari G, Bertino G, Malaguarnera M. Gut microbiota in alcoholic liver disease: pathogenetic role and therapeutic perspectives. World J Gastroenterol. 2014;20:16639-16648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 96] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 10. | Hritz I, Velayudham A, Dolganiuc A, Kodys K, Mandrekar P, Kurt-Jones E, Szabo G. Bone marrow-derived immune cells mediate sensitization to liver injury in a myeloid differentiation factor 88-dependent fashion. Hepatology. 2008;48:1342-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453-460. [PubMed] [Cited in This Article: ] |
| 12. | Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem. 2009;284:24192-24203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218-224. [PubMed] [Cited in This Article: ] |
| 14. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8061] [Cited by in F6Publishing: 8304] [Article Influence: 461.3] [Reference Citation Analysis (0)] |
| 15. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 506] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 16. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in F6Publishing: 784] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 17. | Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 473] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 18. | Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162-169. [PubMed] [Cited in This Article: ] |
| 19. | Rivera CA, Bradford BU, Seabra V, Thurman RG. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am J Physiol. 1998;275:G1252-G1258. [PubMed] [Cited in This Article: ] |
| 20. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 21. | Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737-4742. [PubMed] [Cited in This Article: ] |
| 22. | Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3900] [Cited by in F6Publishing: 3726] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 23. | Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085-2088. [PubMed] [Cited in This Article: ] |
| 24. | Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588-593. [PubMed] [Cited in This Article: ] |
| 25. | Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3-9. [PubMed] [Cited in This Article: ] |
| 26. | Dai Q, Pruett SB. Different effects of acute and chronic ethanol on LPS-induced cytokine production and TLR4 receptor behavior in mouse peritoneal macrophages. J Immunotoxicol. 2006;3:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Pruett SB, Fan R. Ethanol inhibits LPS-induced signaling and modulates cytokine production in peritoneal macrophages in vivo in a model for binge drinking. BMC Immunol. 2009;10:49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243-1249. [PubMed] [Cited in This Article: ] |
| 29. | Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320-1327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Romics L, Kodys K, Dolganiuc A, Graham L, Velayudham A, Mandrekar P, Szabo G. Diverse regulation of NF-kappaB and peroxisome proliferator-activated receptors in murine nonalcoholic fatty liver. Hepatology. 2004;40:376-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 32. | Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Yajima S, Morisaki H, Serita R, Suzuki T, Katori N, Asahara T, Nomoto K, Kobayashi F, Ishizaka A, Takeda J. Tumor necrosis factor-alpha mediates hyperglycemia-augmented gut barrier dysfunction in endotoxemia. Crit Care Med. 2009;37:1024-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S-171S. [PubMed] [Cited in This Article: ] |
| 35. | Mackiewicz A, Kushner I, Baumann H. Acute phase proteins molecular biology, biochemistry, and clinical applications. Boca Raton: CRC Press 1993; . [Cited in This Article: ] |
| 36. | Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1942] [Cited by in F6Publishing: 1859] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 37. | Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431-1433. [PubMed] [Cited in This Article: ] |
| 38. | Navaneethan U, Jayanthi V, Mohan P. Pathogenesis of cholangitis in obstructive jaundice-revisited. Minerva Gastroenterol Dietol. 2011;57:97-104. [PubMed] [Cited in This Article: ] |
| 39. | Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gäbele E, Isayama F, Thurman RG. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963-2969. [PubMed] [Cited in This Article: ] |
| 40. | Francés R, Benlloch S, Zapater P, González JM, Lozano B, Muñoz C, Pascual S, Casellas JA, Uceda F, Palazón JM. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Romics L, Dolganiuc A, Kodys K, Drechsler Y, Oak S, Velayudham A, Mandrekar P, Szabo G. Selective priming to Toll-like receptor 4 (TLR4), not TLR2, ligands by P. acnes involves up-regulation of MD-2 in mice. Hepatology. 2004;40:555-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137-149. [PubMed] [Cited in This Article: ] |
| 43. | Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Roth J, McClellan JL, Kluger MJ, Zeisberger E. Attenuation of fever and release of cytokines after repeated injections of lipopolysaccharide in guinea-pigs. J Physiol. 1994;477:177-185. [PubMed] [Cited in This Article: ] |
| 45. | Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin Exp Res. 1996;20:900-907. [PubMed] [Cited in This Article: ] |
| 46. | McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349-351. [PubMed] [Cited in This Article: ] |
| 47. | Vreugdenhil AC, Snoek AM, van ‘t Veer C, Greve JW, Buurman WA. LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction. J Clin Invest. 2001;107:225-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209-5216. [PubMed] [Cited in This Article: ] |
| 49. | Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcohol Clin Exp Res. 2005;29:172S-179S. [PubMed] [Cited in This Article: ] |
| 50. | Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, Stärkel P, Belzer C, Hellerbrand C, Tsukamoto H. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 51. | Hartmann P, Chen WC, Schnabl B. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Front Physiol. 2012;3:402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 563] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 53. | Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 54. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 701] [Article Influence: 53.9] [Reference Citation Analysis (1)] |
| 55. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 1108] [Article Influence: 100.7] [Reference Citation Analysis (1)] |
| 56. | Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 57. | Tuomisto S, Pessi T, Collin P, Vuento R, Aittoniemi J, Karhunen PJ. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014;14:40. [PubMed] [Cited in This Article: ] |
| 58. | Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21:1691-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 110] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 59. | Borowsky SA, Strome S, Lott E. Continued heavy drinking and survival in alcoholic cirrhotics. Gastroenterology. 1981;80:1405-1409. [PubMed] [Cited in This Article: ] |
| 60. | Pessione F, Ramond MJ, Peters L, Pham BN, Batel P, Rueff B, Valla DC. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003;23:45-53. [PubMed] [Cited in This Article: ] |
| 61. | Veldt BJ, Lainé F, Guillygomarc’h A, Lauvin L, Boudjema K, Messner M, Brissot P, Deugnier Y, Moirand R. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93-98. [PubMed] [Cited in This Article: ] |
| 62. | Luca A, García-Pagán JC, Bosch J, Feu F, Caballería J, Groszmann RJ, Rodés J. Effects of ethanol consumption on hepatic hemodynamics in patients with alcoholic cirrhosis. Gastroenterology. 1997;112:1284-1289. [PubMed] [Cited in This Article: ] |
| 63. | Suk KT, Kim MY, Baik SK. Alcoholic liver disease: treatment. World J Gastroenterol. 2014;20:12934-12944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 64. | Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620-1633. [PubMed] [Cited in This Article: ] |
| 65. | Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009;9:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 66. | Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 567] [Cited by in F6Publishing: 560] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 67. | Borruel N, Carol M, Casellas F, Antolín M, de Lara F, Espín E, Naval J, Guarner F, Malagelada JR. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659-664. [PubMed] [Cited in This Article: ] |
| 68. | McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O’Sullivan GC, Kiely B, Collins JK. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975-980. [PubMed] [Cited in This Article: ] |
| 69. | Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 502] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 70. | Lata J, Novotný I, Príbramská V, Juránková J, Fric P, Kroupa R, Stibůrek O. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. Eur J Gastroenterol Hepatol. 2007;19:1111-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 340] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 72. | Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 73. | Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 74. | Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD, Cheon GJ, Choi DH, Ham YL, Shin DH. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27:1300-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 75. | Bang CS, Hong SH, Suk KT, Kim JB, Han SH, Sung H, Kim EJ, Kim MJ, Kim MY, Baik SK. Effects of Korean Red Ginseng (Panax ginseng), urushiol (Rhus vernicifera Stokes), and probiotics (Lactobacillus rhamnosus R0011 and Lactobacillus acidophilus R0052) on the gut-liver axis of alcoholic liver disease. J Ginseng Res. 2014;38:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Hong M, Kim SW, Han SH, Kim DJ, Suk KT, Kim YS, Kim MJ, Kim MY, Baik SK, Ham YL. Probiotics (Lactobacillus rhamnosus R0011 and acidophilus R0052) reduce the expression of toll-like receptor 4 in mice with alcoholic liver disease. PLoS One. 2015;10:e0117451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Ouwehand AC, Derrien M, de Vos W, Tiihonen K, Rautonen N. Prebiotics and other microbial substrates for gut functionality. Curr Opin Biotechnol. 2005;16:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Cummings JH, Macfarlane GT. Gastrointestinal effects of prebiotics. Br J Nutr. 2002;87 Suppl 2:S145-S151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Tang Y, Forsyth CB, Banan A, Fields JZ, Keshavarzian A. Oats supplementation prevents alcohol-induced gut leakiness in rats by preventing alcohol-induced oxidative tissue damage. J Pharmacol Exp Ther. 2009;329:952-958. [PubMed] [Cited in This Article: ] |
| 80. | Watanabe A, Sakai T, Sato S, Imai F, Ohto M, Arakawa Y, Toda G, Kobayashi K, Muto Y, Tsujii T. Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997;26:1410-1414. [PubMed] [Cited in This Article: ] |
| 81. | Ohshima T, Kojima Y, Seneviratne CJ, Maeda N. Therapeutic Application of Synbiotics, a Fusion of Probiotics and Prebiotics, and Biogenics as a New Concept for Oral Candida Infections: A Mini Review. Front Microbiol. 2016;7:10. [PubMed] [Cited in This Article: ] |
| 82. | Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-Term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001;96:1251-1255. [PubMed] [Cited in This Article: ] |
| 83. | Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxaemia. Liver Int. 2012;32:467-475. [PubMed] [Cited in This Article: ] |
| 84. | Borody TJ, Campbell J. Fecal microbiota transplantation: techniques, applications, and issues. Gastroenterol Clin North Am. 2012;41:781-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 85. | Tian Z, Liu J, Liao M, Li W, Zou J, Han X, Kuang M, Shen W, Li H. Beneficial Effects of Fecal Microbiota Transplantation on Ulcerative Colitis in Mice. Dig Dis Sci. 2016;61:2262-2271. [PubMed] [Cited in This Article: ] |
| 86. | Konturek PC, Haziri D, Brzozowski T, Hess T, Heyman S, Kwiecien S, Konturek SJ, Koziel J. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J Physiol Pharmacol. 2015;66:483-491. [PubMed] [Cited in This Article: ] |
| 87. | Ianiro G, Bibbò S, Gasbarrini A, Cammarota G. Therapeutic modulation of gut microbiota: current clinical applications and future perspectives. Curr Drug Targets. 2014;15:762-770. [PubMed] [Cited in This Article: ] |