Published online Jun 7, 2016. doi: 10.3748/wjg.v22.i21.5060
Peer-review started: December 25, 2015
First decision: January 13, 2016
Revised: January 27, 2016
Accepted: February 22, 2016
Article in press: February 22, 2016
Published online: June 7, 2016
AIM: To investigate catalase (KatA) and alkyl hydroperoxide reductase (AhpC) antibodies of Helicobacter pylori as biomarkers for gastric cancer (GC).
METHODS: This study included 232 cases and 264 controls. Recombinant KatA and AhpC proteins were constructed and the levels of antibodies were tested by indirect enzyme-linked immunosorbent assay (ELISA). Logistic regression was applied to analyze the relationships between KatA, AhpC and GC. The χ2 trend test was used to evaluate the dose-response relationships between serum KatA and AhpC antibody levels and GC. Receiver operating characteristic (ROC) curve was used to evaluate the screening accuracy of KatA and AhpC as biomarkers. Combined analysis was used to observe screening accuracy of predictors for GC.
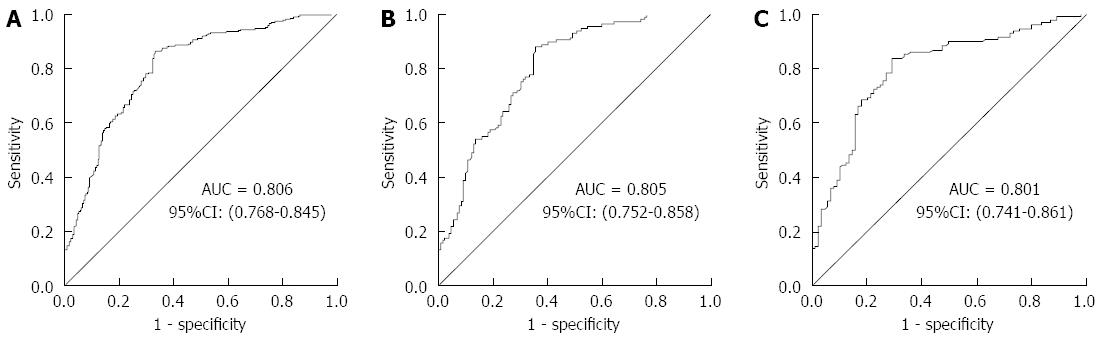
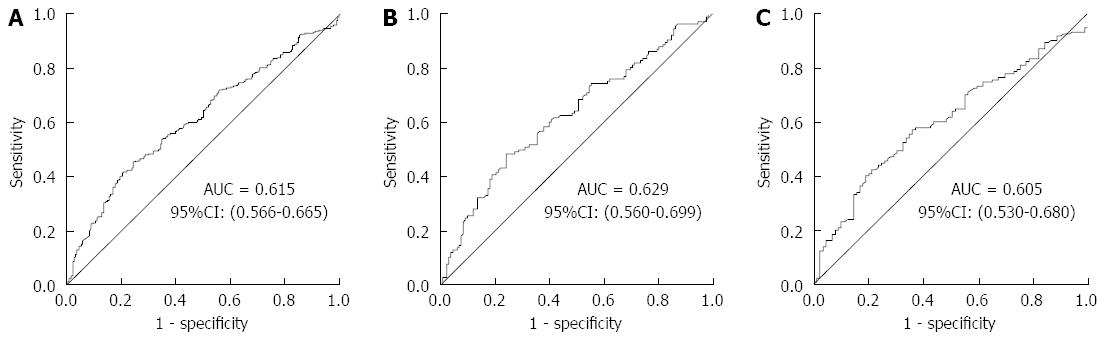
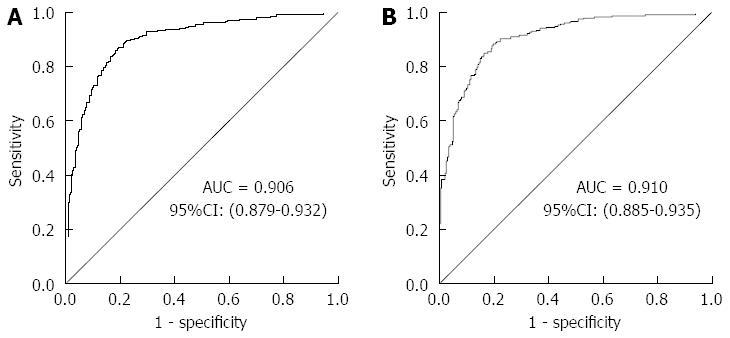
RESULTS: In all subjects, the association between KatA and AhpC and GC risk was significant (P < 0.001) with odds ratio (OR) = 12.84 (95%CI: 7.79-21.15) and OR = 2.4 (95%CI: 1.55-3.73), respectively. KatA and AhpC antibody levels were strongly related to GC risk with a dose-dependent effect (P for trend < 0.001). The area under the ROC (AUC) for KatA was 0.806, providing a sensitivity of 66.81% and specificity of 86.36%; and the AUC for AhpC was 0.615, with a sensitivity of 75.65% and specificity of 45.49%. The AUC was 0.906 for KatA and flagella protein A (FlaA) combined analysis.
CONCLUSION: Serum KatA and AhpC antibodies are associated with GC risk and KatA may serve as a biomarker for GC. KatA/FlaA combined analysis improved screening accuracy.
Core tip: Effective screening methods for gastric cancer (GC) have remained limited to date. The aim of this study was to explore whether serum catalase and alkyl hydroperoxide reductase antibodies of Helicobacter pylori could serve as novel and reliable biomarkers for GC monitoring.
- Citation: Zhang B, Li HL, Fan Q, Guo F, Ren XY, Zhou HB, Zhu JW, Zhao YS, Tian WJ. Serum Helicobacter pylori KatA and AhpC antibodies as novel biomarkers for gastric cancer. World J Gastroenterol 2016; 22(21): 5060-5067
- URL: https://www.wjgnet.com/1007-9327/full/v22/i21/5060.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i21.5060
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer-related death worldwide[1]. Although the overall incidence rate of GC continues to fall, there were still almost 1 million new cases of GC in 2012[2]. Helicobacter pylori (H. pylori) are micro-aerophilic gram-negative bacteria that cause inflammatory reactions by selectively colonizing the gastric mucosa. The International Agency for Research on Cancer has classified H. pylori as a category I carcinogen since 1994[3]. Epidemiological data also support that H. pylori infection is strongly associated with GC[4-6], increasing risk by up to six-fold[7]. In contrast, increasing data shows that H. pylori eradication significantly decreases the development of GC[8,9], particularly in high-risk populations with no precancerous lesions[10]. Eradication of H. pylori seems a reasonable approach for preventing GC. However, nearly 50% of the population worldwide is infected with H. pylori[11]. Mass eradication therapy in the general population may bring about development of antibiotic-resistant strains of H. pylori as well as over-consumption of medical resources. Therefore, there is an urgent and need to identify a reliable screening biomarker for GC.
It is reported that only a small fraction of patients infected with H. pylori have severe clinical outcomes, such as gastric ulcer (10%), atrophic gastritis (5%), and gastric malignancy (2%)[3]. Research indicates that these different outcomes may be associated with the virulence factors of H. pylori[12-14]. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) virulence factors play a crucial role in protecting H. pylori from oxidative stress and maintaining a stable environment for the growth of bacteria[15,16]. Huang et al[17] confirmed that KatA and AhpC were over expressed under the condition of oxidation stress (H2O2) in H. pylori strains isolated from patients with GC, gastritis, or duodenal ulcer. We previously reported that serum flagella protein A (FlaA) antibody of H. pylori may serve as noninvasive biomarker for early detection of GC[18]. In this study, combined analysis was applied to explore the screening value of KatA, AhpC, and FlaA for GC. This study aims to assess the correlations between KatA and AhpC and GC and explore whether they could serve as novel and reliable biomarkers for GC.
This was a hospital-based case-control study, which was approved by the Committee of Human Research of Harbin Medical University, Harbin, China. Two hundred and thirty-two cases of GC were primarily diagnosed by pathology at the Third Affiliated Hospital of Harbin Medical University between April and July 2010. The controls comprised 182 healthy people chosen from the Harbin Xiangfang Center for Disease Control and Prevention and 82 cancer-free people recruited from the neurology department at the Fourth Affiliated Hospital of Harbin Medical University between March and July 2011. All participants gave signed informed consent, and we completed a face-to-face questionnaire that included age, sex, smoking status, and alcohol consumption. Venous blood samples of 5 mL were collected from all participants, centrifuged at 3000 r/min, and stored at -80 °C.
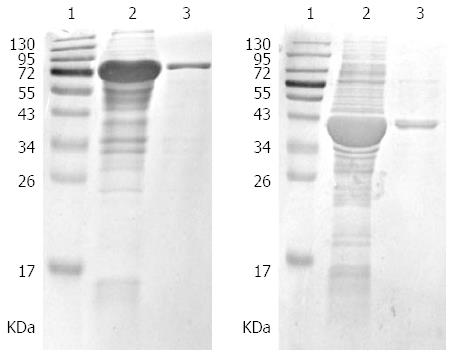
A clinical strain of H. pylori provisionally named H015a was isolated from a GC patient at the Second Affiliated Hospital of Harbin Medical University. Genomic DNA of H015a was extracted as a template using a DNA extraction kit (QIAGEN, Valencia, CA, United States). The katA and ahpC gene coding sequences were obtained from Genbank. Amplification of katA and ahpC gene fragments was implemented by polymerase chain reaction (PCR). The PCR primers were designed using Primer Premier 5.0 software. For katA, the primer sequences were 5’-CCGGAATTCATGGTTAATAAAGATGTGAACA-3’ (forward) and 5’-CCGCTCGAGTTACTTTTTCTTTTTTGTGTGG-3’ (reverse) that generated a 1518 bp fragment. For ahpC, the primer sequences were 5’-CCGGAATTCATGTTAGTTACAAAACTTGCCC-3’ (forward) and 5’-CCGCTCGAGTTAAAGCTTAATGGAATTTTC-3’ (reverse) that generated a 597 bp fragment. EcoRI and XhoI restriction endonuclease sites were incorporated into the forward and reverse primer sequences of these two genes, respectively. Amplification was implemented under the following conditions: 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 90 s followed by a final extension at 72 °C for 7 min. Subsequently, two PCR products were cloned into the cloning vector pMD18-T and transformed into Escherichia coli (E. coli) strain DH5α. The positive clones were screened and cloned into the prokaryotic expression vector pET-32a. The recombinant plasmids katA-pET-32a and ahpC-pET-32a were introduced into E. coli BL21 (DE3) cells for expression of recombinant proteins, respectively. The target sequences of katA and ahpC gene were assayed by the dideoxy chain termination method (Biotechnology firm, BGI, Beijing, China). The recombinant katA-pET-32a-BL21 and ahpC-pET-32a-BL21 strains were cultured in lysogeny broth (LB) with 100 μg/mL ampicillin, and induced at 30 °C by isopropylthio-β-d-galactoside with a final concentration of 1 mmol/L and 0.5 mmol/L, respectively. E. coli cells were harvested after 4 h and disrupted ultrasonically. The suspension and precipitate were collected and protein expression was analyzed by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
A serological test for H. pylori immunoglobulin (Ig)G antibodies has already been completed and described by our group[17].
The recombinant proteins were purified by Ni-NTA His Bind resin (Novagen, Darmstadt, Germany). We used stepwise dialysis to obtain the fusion protein by removing the denaturant (urea) in the purified protein. The dialysis tube was boiled 10 min in buffer (2% NaHCO3 and 1mmol/L EDTA pH 8.0) and EDTA solution (1 mmol/L) sequentially. After cooling, the purified protein was put into the dialysis tube, and both ends were clamped with the dialysis clips. The protein was dialyzed in urea solution (pH 8.3) with a slowly decreasing concentration: 6 mol/L, 4 mol/L, 2 mol/L, 1 mol/L and 0 mol/L. Each dialysis lasted 24 h. Finally, the sample was removed from the dialysis tube and stored at -80 °C until analysis.
An indirect enzyme-linked immunosorbent assay (ELISA) was applied to detect the serum antibodies against H. pylori recombinant KatA and AhpC proteins. Recombinant KatA and AhpC proteins were diluted to 2 μg/mL and 0.25 μg/mL, respectively. Proteins at 100 μL/well were incubated in a 96-well micro-plate (Costar, Washington, DC, United States) at 4 °C overnight and washed three times with phosphate buffer saline, Tween-20 (PBST), followed by blocking with 10% goat serum (AR0009; Boster, Beijing, China) and incubation for 2 h at 37 °C. Serum sample from cases and controls diluted 3200-fold with 10% bovine serum albumin (BSA) was added to the plate at 100 μL/well and incubated for 1 h at 37 °C. Each serum sample was tested in three parallel wells. The plate was again washed three times with PBST. Peroxidase-conjugated goat anti-human IgG (H+L) (ZSGB-Bio, Beijing, China) was diluted 1:5000 with buffer, and 100 μL was added to each well and incubated 30 min at 37 °C. Tetramethylbenzidene (TMB) substrate buffer was added to the plate at 100 μL/well and incubated in the dark for 15 min at 37 °C, Fifty microliters stop solution was added per well to terminate the reaction. Finally, the plate was read at 450 nm absorbance using a micro-plate reader (Biotech Synergy 2, Winooski, Vermont, United States). The determination of serostatus of antibody was based on optical density (OD) value. The optimal cutoff point of OD values was used to classify samples as seropositive or seronegative.
All statistical analyses were conducted using SPSS 22.0 version software (Armonk, NY, United States). Unconditional logistic regression analysis was performed to estimate odds ratio (OR) and 95% confidence interval (CI) for the relationship between GC and antibodies. The χ2 trend test was used to assess dose-response relationships between serum KatA and AhpC antibody levels and GC. In addition, a receiver operating characteristic (ROC) curve was plotted to identify the cutoff point of serum KatA and AhpC antibody results. Sensitivity, specificity, and area under the ROC curve (AUC) with 95%CI were calculated to evaluate the screening value of serum KatA and AhpC antibody levels for GC. Moreover, the optimal cutoff value was determined by the maximum Youden index (Youden index = sensitivity + specificity - 1). Combined analysis was used to observe screening accuracy of predictors for GC. For all tests, P < 0.05 was considered statistically significant.
The characteristics of the study subjects were described in our previous study[18].
Nucleotide homology of the cloned katA gene compared to H. pylori 26695 was 95.52% The homology of ahpC nucleotide was 96.48% compared with H. pylori J99.
A prokaryotic expression system was constructed. After induction by isopropyl beta D thiogalactoside (IPTG), proteins with the expected size were clearly present as inclusion bodies in the ultrasonic precipitation by SDS-PAGE. Finally, the purified fusion proteins were obtained (Figure 1).
As shown in Table 1, an association between KatA and GC risk was observed, with OR = 12.84 (95%CI: 7.79-21.15), 14.59 (6.84-31.13), and 12.15 (5.79-25.51) in all, H. pylori-positive and H. pylori-negative subjects, respectively (P < 0.001). Dose-dependent effects showed that KatA antibody levels were strongly related to GC risk in the three populations mentioned above (P for trend < 0.001) (Table 2). Similarly, a significant association between GC risk and serum positivity of AhpC was observed with OR = 2.40 (95%CI: 1.55-3.73) in all subjects, 2.30 (1.25-4.23) in H. pylori-positive subjects, and 2.04 (1.10-3.78) in H. pylori-negative subjects (P < 0.001) (Table 1). Correspondingly, AhpC antibody level was significantly related to GC risk in a dose-dependent manner (P for trend < 0.001) (Table 2). Moreover, an evident association between GC risk and serum positivity of combination of KatA and AhpC was present, with OR = 11.64 (95%CI: 7.12-19.01), 13.39 (6.29-28.53), and 13.91 (6.74-28.74) in all, H. pylori-positive and H. pylori-negative subjects, respectively (P < 0.001) (Table 1).
| Virulence factors serostatus | All subjects | H. pylori positive subjects | H. pylori negative subjects | |||||||||
| Case | Control | OR (95%CI) | P value1 | Case | Control | OR (95%CI) | P value1 | Case | Control | OR (95%CI) | P value1 | |
| KatA | ||||||||||||
| Negative | 78 (33.62) | 228 (86.36) | 1.0 (Reference) | < 0.001 | 47 (35.61) | 104 (88.14) | 1.0 (Reference) | < 0.001 | 26 (29.21) | 109 (83.85) | 1.0 (Reference) | < 0.001 |
| Positive | 154 (66.38) | 36 (13.64) | 12.84 (7.80-21.15) | 85 (64.39) | 14 (11.86) | 14.59 (6.84-31.13) | 63 (70.79) | 21 (16.15) | 12.15 (5.79-25.51) | |||
| AhpC | ||||||||||||
| Negative | 56 (24.14) | 121 (45.83) | 1.0 (Reference) | < 0.001 | 33 (25.00) | 57 (48.31) | 1.0 (Reference) | < 0.001 | 54 (54.00) | 103 (70.55) | 1.0 (Reference) | < 0.001 |
| Positive | 176 (75.86) | 143 (54.17) | 2.40 (1.55-3.73) | 99 (75.00) | 61 (51.69) | 2.30 (1.25-4.23) | 46 (46.00) | 43 (29.45) | 2.04 (1.10-3.78) | |||
| Combination of KatA and AhpC | ||||||||||||
| Negative | 78 (33.62) | 226 (85.61) | 1.0 (Reference) | < 0.001 | 49 (37.12) | 104 (88.14) | 1.0 (Reference) | < 0.001 | 33 (33.00) | 127 (86.99) | 1.0 (Reference) | < 0.001 |
| Positive | 154 (66.38) | 38 (14.39) | 11.64 (7.12-19.01) | 83 (62.88) | 14 (11.86) | 13.40 (6.29-28.53) | 67 (67.00) | 19 (13.01) | 13.91 (6.74-28.74) | |||
| All subjects | H. pylori positive subjects | H. pylori negative subjects | ||||||||||||
| Antibody level (OD)1 | Case | Control | OR (95%CI)2 | P value for trend | Antibody level (OD) | Case | Control | OR (95%CI)2 | P value for trend | Antibody level (OD) | Case | Control | OR (95%CI)2 | P value for trend |
| KatA | ||||||||||||||
| ≤ 0.4187 | 167 (71.98) | 66 (25.0) | 1.0 (Reference) | < 0.001 | ≤ 0.4152 | 92 (69.70) | 29 (24.58) | 1.0 (Reference) | < 0.001 | ≤ 0.4167 | 66 (74.16) | 32 (24.58) | 1.0 (Reference) | < 0.001 |
| 0.4187-0.5313 | 36 (15.52) | 66 (25.0) | 4.25 (2.49-7.27) | 0.4152-0.5133 | 23 (17.42) | 30 (25.42) | 3.79 (1.78-8.06) | 0.4167-0.5568 | 9 (10.11) | 33 (25.42) | 6.67 (2.70-16.51) | |||
| 0.5313-0.6799 | 18 (7.76) | 66 (25.0) | 9.95 (5.05-19.62) | 0.5133-0.6692 | 9 (6.82) | 30 (25.42) | 9.69 (3.81-24.70) | 0.5568-0.6824 | 11 (21.36) | 33 (25.42) | 7.00 (2.68-18.30) | |||
| > 0.6799 | 11 (4.74) | 66 (25.0) | 15.85 (6.97-36.06) | > 0.6692 | 8 (6.06) | 29 (24.58) | 16.55 (5.51-49.76) | > 0.6824 | 3 (3.39) | 32 (24.58) | 19.89 (4.32-91.70) | |||
| AhpC | ||||||||||||||
| ≤ 0.2168 | 88 (37.93) | 66 (25.00) | 1.0 (Reference) | < 0.001 | ≤ 0.2182 | 51 (38.64) | 29 (24.58) | 1.0 (Reference) | < 0.001 | ≤ 0.2110 | 31 (34.83) | 32 (24.58) | 1.0 (Reference) | < 0.001 |
| 0.2168-0.3265 | 69 (29.74) | 66 (25.00) | 1.26 (0.76-2.11) | 0.2182-0.3433 | 41 (31.06) | 30 (25.42) | 1.10 (0.54-2.25) | 0.2110-0.3310 | 29 (32.58) | 33 (25.42) | 1.44 (0.69-3.00) | |||
| 0.3265-0.4888 | 49 (21.12) | 66 (25.00) | 1.41 (0.82-2.43) | 0.3433-0.4908 | 28 (21.21) | 30 (25.42) | 1.54 (0.70-3.38) | 0.3310-0.4948 | 16 (17.98) | 33 (25.42) | 1.83 (0.80-4.23) | |||
| > 0.4888 | 26 (11.21) | 66 (25.00) | 3.54 (1.84-6.82) | > 0.4908 | 12 (9.09) | 29 (24.58) | 3.40 (1.32-8.73) | > 0.4948 | 13 (14.61) | 32 (24.58) | 3.33 (1.31-8.46) | |||
An ROC curve was plotted to explore the screening value of KatA and AhpC for GC. The AUC for KatA was 0.806 (95%CI: 0.768-0.845), 0.805 (0.751-0.853), and 0.801 (0.741-0.861) in all, H. pylori-positive and H. pylori-negative subjects, respectively (Figure 2). The AUC for AhpC was 0.615 (95%CI: 0.566-0.665) in all subjects, 0.629 (0.560-0.699) in H. pylori-positive subjects, and 0.605 (0.530-0.680) in H. pylori-negative subjects (Figure 3). As shown in Table 3, the optimal cutoff value of KatA and AhpC for GC was 0.3583 and 0.3647 in all subjects, providing a sensitivity of 66.81% and 75.65% and a specificity of 86.36% and 45.49%, respectively. AUC for the combination of KatA and FlaA was 0.906 (95%CI: 0.879-0.932), and the optimal cutoff value was 0.4305 with a sensitivity of 78.88% and a specificity of 89.02% (Figure 4A). For combination of KatA, FlaA, and AhpC, the AUC was 0.910 (95%CI: 0.885-0.935), offering a sensitivity of 80.17% and a specificity of 88.64%, while the optimal cutoff value was 0.4354 (Figure 4B).
| Percentile1 | All subjects | H. pylori positive subjects | H. pylori negative subjects | ||||||
| Critical value (OD)2 | Sensitivity | Specificity | Critical value (OD)2 | Sensitivity | Specificity | Critical value (OD)2 | Sensitivity | Specificity | |
| KatA | |||||||||
| Optimal cutoff point2 | 0.3583 | 66.81% | 86.36% | 0.3557 | 64.39% | 88.14% | 0.3730 | 70.79% | 83.85% |
| 25% | 0.2800 | 46.55% | 93.18% | 0.4152 | 69.70% | 74.58% | 0.2773 | 50.56% | 90.00% |
| 50% | 0.4305 | 75.00% | 71.59% | 0.5133 | 87.12% | 49.15% | 0.4447 | 78.65% | 69.23% |
| 75% | 0.5958 | 90.95% | 36.36% | 0.6692 | 93.94% | 24.58% | 0.6107 | 92.13% | 36.15% |
| 90% | 0.7418 | 97.84% | 16.29% | 0.9042 | 100.00% | 8.47% | 0.7873 | 97.75% | 14.62% |
| AhpC | |||||||||
| Optimal cutoff point2 | 0.3647 | 75.65% | 45.49% | 0.3613 | 75.76% | 48.31% | 0.2330 | 43.82% | 70.77% |
| 25% | 0.1953 | 30.43% | 78.95% | 0.1953 | 30.30% | 80.51% | 0.1917 | 30.34% | 77.69% |
| 50% | 0.2830 | 59.57% | 57.14% | 0.2865 | 59.85% | 60.17% | 0.2913 | 62.92% | 57.69% |
| 75% | 0.4267 | 84.35% | 32.71% | 0.4325 | 83.33% | 33.05% | 0.4313 | 85.39% | 23.85% |
| 90% | 0.5747 | 95.65% | 14.29% | 0.5302 | 93.94% | 13.56% | 0.6410 | 96.63% | 13.85% |
Gastric carcinogenesis is a multifactorial process, and H. pylori infection plays an important role in the initial stage[19]. Patients with malignant tumors are often diagnosed at an advanced stage, and 5-year survival rate is < 10%[20]. Therefore, early detection is a crucial factor for GC prevention. However, it is difficult to diagnose GC any earlier because the symptoms of gastric pre-cancerous and malignant diseases are non-specific and vague. At present, endoscopy is the gold standard for screening GC and is commonly used in the clinic. A large case-control study from Japan indicated that GC mortality was reduced 30% by endoscopic screening compared with no screening[21]. In spite of this finding, limitations of endoscopy, such as the existence of over diagnosis and unwillingness of asymptomatic patients because of pain as well as cost make endoscopy unsuitable for population-based screening. Serological testing is widely available and is a low-cost noninvasive diagnostic method. In the present study, we explored whether serum H. pylori antibody could serve as a biomarker for GC monitoring.
KatA is a ubiquitous enzyme that protects H. pylori cells from extracellular H2O2 attack[22,23] and plays an important role in colonization of gastric mucosa[15]. AhpC is the most abundant and essential antioxidant protein of H. pylori[16], and it protects bacteria from lipid peroxidation and DNA damage[24,25]. We used a commercial ELISA method to detect H. pylori infection status. However, this method may fail to detect prior H. pylori infection in GC patients, and patients positive for anti-CagA (cytotoxin-associated gene A) antibody may have negative results for H. pylori serological testing[26,27]. In order to eliminate these possible influences on our results, H. pylori-negative and overall subjects were also analyzed to observe the associations between GC and the KatA and AhpC antibodies. The results indicated that we should be more vigilant regarding antibody titer and seropositivity. Meanwhile, we found that the median of KatA and AhpC antibody levels were lower in cases group than in the controls (data not shown). This finding implied that the high antibody titer of H. pylori KatA and AhpC may protect against the occurrence of GC.
A Latin American study showed that seropositivity of KatA in a population within a high risk of GC area was higher than that in a low-risk population[28]. Our results confirmed that KatA was associated with GC, and seropositivity of KatA antibody showed a 14.59-fold increased risk of GC. Yan et al[29] found that AhpC antibody of H. pylori may be related to the development of gastric diseases using the gerbil model to simulate human H. pylori infection. In addition, Huang et al[30] indicated that AhpC was expressed in greater amounts in GC than gastritis strains. In our study, there was a significant association between AhpC antibody and GC, based on epidemiology data. Further analysis found that KatA and AhpC antibody levels were strongly related to GC risk in a dose-dependent manner. In order to explore whether KatA and AhpC could serve as biomarkers for GC, ROC curves were plotted to evaluate the screening value of the antibodies. The results showed that the AUC for KatA was 0.806, which was higher than the general standard for diagnosis (AUC ≥ 0.7)[31,32]. Unfortunately, the AUC for AhpC was lower. Generally, a single indicator for screening has a lower screening yield. At this point, we attempted to develop a combined analysis to assess the value of screening. Our previous study found that the sensitivity was 74.1%, and the specificity was 64.4%, while FlaA served as a screening biomarker for GC alone[17]. The combined results for KatA, FlaA, and AhpC showed that the AUC for combination of KatA and FlaA was elevated by 0.10, and sensitivity and specificity were increased by 12.07% and 2.66%, respectively, in all subjects compared to KatA alone. Yet, combination of KatA, FlaA, and AhpC did not improve screening power in the identification of patients with GC compared to combination of KatA and FlaA.
Indirect ELISA method was adopted to detect serum KatA and AhpC antibodies in this study, and this method might be accompanied by the non-specific signal caused by cross-reactivity. In other words, KatA and AhpC will not only react with the corresponding specific antibody but also with the non-specific antibodies in the present study, Because of this non-specific signal, some H. pylori-negative subjects were classified as KatA or AhpC positive.
Some evidence indicates that H. pylori infection increases the risk of non-cardia GC[7,33]. Nine (3.88%) cardia GC cases were included in our study. However, their involvement did not affect the overall results and conclusion.
In conclusion, the data indicate that serum KatA and AhpC antibodies are associated with GC risk and that KatA may serve as a novel biomarker for GC screening. Combined analysis of KatA and FlaA could improve screening accuracy. However, serum AhpC antibody performed poorly as a marker for GC. Our study offers a basis for early diagnosis of GC, and further prospective studies are needed to verify our findings.
Helicobacter pylori (H. pylori) infection is a crucial cause of gastric cancer (GC). Eradication of H. pylori seems a reasonable approach for preventing GC, but it is not feasible in large populations due to financial limitations. Therefore, a sensitive and low-cost screening biomarker for GC is urgently needed.
Invasive endoscopy is the gold standard for GC detection, but it is not suitable for population-based screening. Serological testing is a widely available and noninvasive diagnostic method. In this study, the authors explored the value of serum catalase (KatA) and alkyl hydroperoxide reductase (AhpC) antibodies of H. pylori as biomarkers for GC monitoring.
This study indicated that KatA and AhpC antibodies are associated with GC risk and that KatA may serve as a novel biomarker for GC screening. Besides, combining for KatA and flagella protein A could improve screening accuracy.
These finding offers a basis for early diagnosis of GC.
This is a well-designed study showing that KatA and AhpC antibodies are associated with GC. The methodology is well described. Exploration of KatA and AhpC as biomarkers has important value for GC prevention.
P- Reviewer: Aoyagi K, Vorobjova T S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19772] [Article Influence: 2196.9] [Reference Citation Analysis (17)] |
| 2. | Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 3. | Conteduca V, Sansonno D, Lauletta G, Russi S, Ingravallo G, Dammacco F. H. pylori infection and gastric cancer: state of the art (review). Int J Oncol. 2013;42:5-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Limburg P, Qiao Y, Mark S, Wang G, Perez-Perez G, Blaser M, Wu Y, Zou X, Dong Z, Taylor P. Helicobacter pylori seropositivity and subsite-specific gastric cancer risks in Linxian, China. J Natl Cancer Inst. 2001;93:226-233. [PubMed] [Cited in This Article: ] |
| 5. | El-Omar EM, Oien K, Murray LS, El-Nujumi A, Wirz A, Gillen D, Williams C, Fullarton G, McColl KE. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology. 2000;118:22-30. [PubMed] [Cited in This Article: ] |
| 6. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [Cited in This Article: ] |
| 7. | Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347-353. [PubMed] [Cited in This Article: ] |
| 8. | Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639-642. [PubMed] [Cited in This Article: ] |
| 9. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 850] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 10. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [PubMed] [Cited in This Article: ] |
| 11. | Correa P, Piazuelo MB. Natural history of Helicobacter pylori infection. Dig Liver Dis. 2008;40:490-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci USA. 2005;102:16339-16344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Lahner E, Bernardini G, Santucci A, Annibale B. Helicobacter pylori immunoproteomics in gastric cancer and gastritis of the carcinoma phenotype. Expert Rev Proteomics. 2010;7:239-248. [PubMed] [Cited in This Article: ] |
| 14. | Yan J, Mao YF, Shao ZX. Frequencies of the expression of main protein antigens from Helicobacter pylori isolates and production of specific serum antibodies in infected patients. World J Gastroenterol. 2005;11:421-425. [PubMed] [Cited in This Article: ] |
| 15. | Hazell SL, Evans DJ, Graham DY. Helicobacter pylori catalase. J Gen Microbiol. 1991;137:57-61. [PubMed] [Cited in This Article: ] |
| 16. | O’Riordan AA, Morales VA, Mulligan L, Faheem N, Windle HJ, Kelleher DP. Alkyl hydroperoxide reductase: a candidate Helicobacter pylori vaccine. Vaccine. 2012;30:3876-3884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Huang CH, Chiou SH. Proteomic analysis of upregulated proteins in Helicobacter pylori under oxidative stress induced by hydrogen peroxide. Kaohsiung J Med Sci. 2011;27:544-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Tian W, Jia Y, Yuan K, Huang L, Nadolny C, Dong X, Ren X, Liu J. Serum antibody against Helicobacter pylori FlaA and risk of gastric cancer. Helicobacter. 2014;19:9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719-31.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 287] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 20. | Msika S, Benhamiche AM, Jouve JL, Rat P, Faivre J. Prognostic factors after curative resection for gastric cancer. A population-based study. Eur J Cancer. 2000;36:390-396. [PubMed] [Cited in This Article: ] |
| 21. | Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013;8:e79088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Free C, Lee RM, Ogden J. Young women’s accounts of factors influencing their use and non-use of emergency contraception: in-depth interview study. BMJ. 2002;325:1393. [PubMed] [Cited in This Article: ] |
| 23. | Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006;61:847-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Wang G, Hong Y, Johnson MK, Maier RJ. Lipid peroxidation as a source of oxidative damage in Helicobacter pylori: protective roles of peroxiredoxins. Biochim Biophys Acta. 2006;1760:1596-1603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Wang G, Conover RC, Olczak AA, Alamuri P, Johnson MK, Maier RJ. Oxidative stress defense mechanisms to counter iron-promoted DNA damage in Helicobacter pylori. Free Radic Res. 2005;39:1183-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ekström AM, Held M, Hansson LE, Engstrand L, Nyrén O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 295] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Annibale B, Lahner E, Santucci A, Vaira D, Pasquali A, Severi C, Mini R, Figura N, Delle Fave G. CagA and VacA are immunoblot markers of past Helicobacter pylori infection in atrophic body gastritis. Helicobacter. 2007;12:23-30. [PubMed] [Cited in This Article: ] |
| 28. | Camargo MC, Beltran M, Conde-Glez CJ, Harris PR, Michel A, Waterboer T, Carolina Flórez A, Torres J, Ferreccio C, Sampson JN. Serological response to Helicobacter pylori infection among Latin American populations with contrasting risks of gastric cancer. Int J Cancer. 2015;137:3000-3005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Yan J, Kumagai T, Ohnishi M, Ueno I, Ota H. Immune response to a 26-kDa protein, alkyl hydroperoxide reductase, in Helicobacter pylori-infected Mongolian gerbil model. Helicobacter. 2001;6:274-282. [PubMed] [Cited in This Article: ] |
| 30. | Huang CH, Chuang MH, Lo WL, Wu MS, Wu YH, Wu DC, Chiou SH. Alkylhydroperoxide reductase of Helicobacter pylori as a biomarker for gastric patients with different pathological manifestations. Biochimie. 2011;93:1115-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Zheng J, Ding X, Tian X, Jin Z, Pan X, Yan H, Feng X, Hou J, Xiang H, Ren L. Assessment of different biomarkers provides valuable diagnostic standards in the evaluation of the risk of acute rejection. Acta Biochim Biophys Sin (Shanghai). 2012;44:730-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36. [PubMed] [Cited in This Article: ] |
| 33. | Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |