Published online Dec 14, 2015. doi: 10.3748/wjg.v21.i46.13020
Peer-review started: May 14, 2015
First decision: June 2, 2015
Revised: June 23, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: December 14, 2015
AIM: To investigate the preventive effect of kefir on colitis induced with dextran sulfate sodium (DSS) in rats.
METHODS: Twenty-four male Wistar-albino rats were randomized into four groups: normal control, kefir-control, colitis, and kefir-colitis groups. Rats in the normal and kefir-control groups were administered tap water as drinking water for 14 d. Rats in the colitis and kefir-colitis groups were administered a 3% DSS solution as drinking water for 8-14 d to induce colitis. Rats in the kefir-control and kefir-colitis groups were administered 5 mL kefir once a day for 14 d while rats in the normal control and colitis group were administered an identical volume of the placebo (skim milk) using an orogastric feeding tube. Clinical colitis was evaluated with reference to the disease activity index (DAI), based on daily weight loss, stool consistency, and presence of bleeding in feces. Rats were sacrificed on the 15th day, blood specimens were collected, and colon tissues were rapidly removed. Levels of myeloperoxidase (MPO), tumor necrosis factor (TNF)-α, interleukin (IL)-10, malondialdehyde, and inducible nitric oxide synthase (iNOS) were measured in colon tissue.
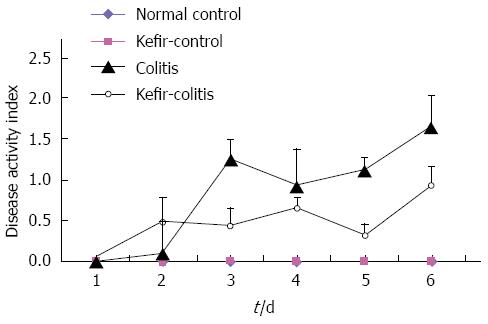
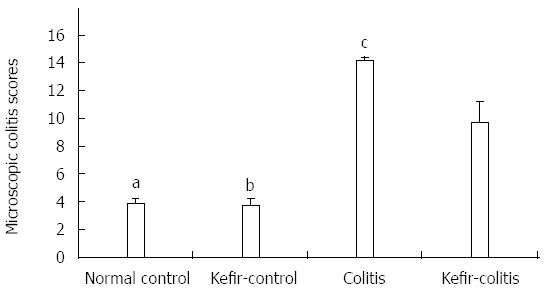
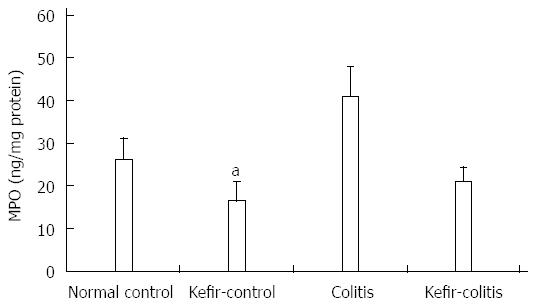
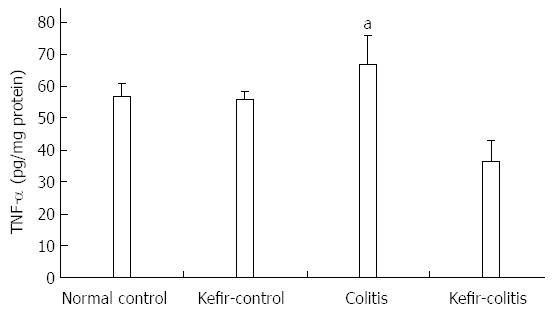
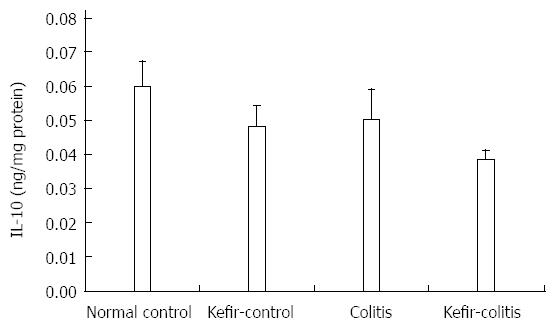
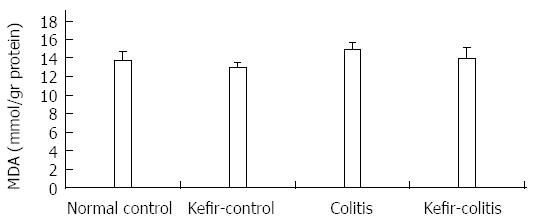
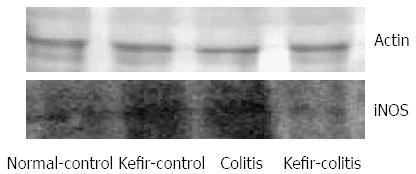
RESULTS: The DAI was lower in the kefir-colitis group than in the colitis group (on the 3rd and 5th days of colitis induction; P < 0.01). The DAI was also significantly higher in the colitis group between days 2 and 6 of colitis induction when compared to the normal control and kefir-control groups. The DAI was statistically higher only on the 6th day in the kefir-colitis group when compared to that in the normal control groups. Increased colon weight and decreased colon length were observed in colitis-induced rats. Mean colon length in the colitis group was significantly shorter than that of the kefir-control group. Kefir treatment significantly decreased histologic colitis scores (P < 0.05). MPO activity in the colitis group was significantly higher than in the kefir-control group (P < 0.05). Kefir treatment significantly reduced the DSS colitis-induced TNF-α increase (P < 0.01). No statistically significant differences were observed among groups for IL-10 and MDA levels. Colon tissue iNOS levels in the colitis group were significantly higher than those in the control and kefir-colitis groups (P < 0.05).
CONCLUSION: Kefir reduces the clinical DAI and histologic colitis scores in a DSS-induced colitis model, possibly via reduction of MPO, TNF-α, and iNOS levels.
Core tip: The present study aimed to determine the pathophysiology for the preventive effect of kefir on colitis induced with dextran sulfate sodium in rats. The results show that kefir reduces the clinical disease activity index and histologic colitis scores in an experimental colitis model induced with 3% dextran sulfate sodium. The mechanisms of these beneficial effects of kefir include reductions in myeloperoxidase, tumor necrosis factor-α, and inducible nitric oxide synthase levels. Indeed, the results of this study show that the therapeutic administration of kefir may have a place in the clinical treatment of inflammatory bowel diseases.
- Citation: Senol A, Isler M, Sutcu R, Akin M, Cakir E, Ceyhan BM, Kockar MC. Kefir treatment ameliorates dextran sulfate sodium-induced colitis in rats. World J Gastroenterol 2015; 21(46): 13020-13029
- URL: https://www.wjgnet.com/1007-9327/full/v21/i46/13020.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i46.13020
The pathogenesis of inflammatory bowel disease (IBD) is not well understood, though there is strong evidence of an abnormal mucosal immune system response against intestinal lumen antigens in genetically sensitive individuals[1-5]. An intestinal microflora balance in favor of pathogenic bacterial species is observed in patients with IBD[6,7], and studies suggest that enteric flora strongly influence disease occurrence and progression[8-10]. Broad-spectrum antibiotics affect intestinal flora and so aid the treatment of ulcerative colitis and Crohn’s disease. The elimination of pathogens responsible for the disease, inhibition of bacterial secretory products and secondary bacterial invasion can contribute towards the efficacy of antibacterial treatment[11].
Intestinal microflora may also be modified with probiotic treatments, and in recent years, studies have shown that modification of the intestinal microflora with probiotic treatments could in fact be a new therapeutic modality.
The probiotic bacteria Lactobacillus salivarius has been determined to significantly reduce inflammation indicators in the colon and proinflammatory cytokine production in splenocytes in interleukin (IL)-10 knockout mice[12]. It has furthermore been reported that spontaneous development of intestinal inflammation can be prevented by Escherichia coli ssp.[13], and DNA from the probiotic VSL#3 reduces production of tumor necrosis factor (TNF)-α and interferon (IFN)-γ in the colon, and attenuates histologic colitis scores in IL-10 knockout mice[14].
It has been shown that p75 and p40 proteins, which are obtained from Lactobacillus rhamnosus GG culture supernatant, inhibit epithelial injury to the colon stimulated by TNF[15]. Probiotics stimulate mucin gene expression in colonic epithelial cells[16-18]. It has also been reported that some lactobacillus and bifidobacteria produce antioxidant substances[19,20].
Kefir, a natural probiotic beverage, is a fermented milk product of Caucasian origin. Fermentation is facilitated by a complex combination of yeast and bacteria, and the combination of this complex includes lactobacillus, streptococcus, acetobacteria, and yeasts.
The aim of this study was to investigate the protective effects of kefir in dextran sulfate sodium (DSS)-induced colitis in rats. This study also was proposed to determine the activity of myeloperoxidase (MPO), concentrations of malondialdehyde (MDA), TNF-α, IL-10, and inducible nitric oxide synthase (iNOS) in colon tissue.
This study was performed at the Experimental Research and Biochemistry Laboratories, Medical School of Suleyman Demirel University, with the approval of the ethical committee of Medical School of Suleyman Demirel University (protocol no: 08.08.2007.06/09). All animals received care in compliance with the “Principles of Laboratory Animal Care”(http://www.nap.edu/catalog/5140.html).
Twenty-four 18-wk-old Wistar-albino male rats weighing between 198-264 g were used. Animals were housed in a temperature-controlled room (23 ± 2 °C) with illumination (12 h each of light and darkness) during the study period. Animals were placed in separate cages and fed with standard food. Cables were laid in the cages to prevent coprophagy.
A commercial kefir culture (Danisco®, Poland) was used in this study and contents included Lactobacillus lactis subs., Leuconostoc subs., Streptococcus thermophilus, Lactobasillus subs. and yeast of kefir. Fermentation was obtained by incubation at 26 °C for 16 h.
Colitis was induced by administering a 3% DSS solution (3% [w/v] DSS, MW = 40.00 Fluka-RA10585; Sigma-Aldrich, St. Louis, MO, United States) to animals in lieu of normal tap water for a period of 7 d.
The DSS solution was prepared daily and daily water intake was recorded. Either kefir or placebo (skim milk) was administered orogastrically with a 4F feeding tube between 9 am and 10 am. Control of whether or not the tube was in the trachea was performed by putting the extended edge into water. Anesthetic agents were not used during the procedure.
Rats were randomly divided into four groups of six animals each. Group I was the normal control group, and a once daily dose of placebo (5 mL skim milk) was administered orogastrically for 14 d. Animals in this group had access to normal tap water for the full 14-d study period. Group II was the kefir-control group, and once daily dose of kefir (5 mL) was administered orogastrically for 14 d. Animal in this group had access to normal tap water for to full 14-d study period. Group III was the colitis group, and a once-daily dose of placebo (5 mL skim milk) was administered orogastrically for 14 d; they had drinking water with 3% DSS on the 8th and 14th days to stimulate colitis. Group IV was the kefir-colitis group, and a once-daily dose of kefir (5 mL) was administered orogastrically for 14 d; they had drinking water with 3% DSS on the 8th and 14th days to stimulate colitis.
Body weight was recorded daily and general condition and stool features were recorded three times a day during the study period. On the 15th day, between 9 am and 10 am, ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg) were administered intraperitoneally and weight was measured under anesthesia. A midline incision was made using a sterile technique and blood was collected from the cardiac ventricle. Blood serum was separated and stored at -80 °C.
The full colon between the ileocecal junction and the anus was swiftly removed and opened longitudinally along the mesenteric fixation line. Colons were washed in PBS in a frozen water tank and lightly dried with filter paper. The length and weight of the entire colons were then measured.
A distal colon segment of 10 cm proximal to the anus was separated into two pieces: proximal and distal. The distal segment was placed in a formalin solution for histopathologic examination. The proximal segment was used for Western blot analysis, biochemical examinations, and cytokine determination.
Loss of body weight, stool consistency, and presence of bleeding in feces were noted. The detailed information is listed in Table 1[21].
| Score | Weight loss (%) | Stool consistency | Occult/gross bleeding |
| 0 | None | Normal | Negative |
| 1 | 1-5 | ||
| 2 | 5-10 | Loose stool | |
| 3 | 10-20 | ||
| 4 | > 20 | Diarrhea | Gross bleeding |
Histopathologic examinations were performed by a blinded, unbiased pathologist. After tissue processing, sections 5 μm thick were cut from paraffin blocks and stained with hematoxylin eosin. Histopathologic evaluation was performed according to criteria presented in Table 2. A score between 0-30 was obtained[22].
| Mucosal epithelium | |
| Ulceration; none (0); mild surface (1); moderate (2); extensive-full thickness (3) | |
| Crypts | |
| Mitotic activity; lower third (0); mild mid-third (1); moderate mid-third (2); upper third (3) | |
| Neutrophilic infiltrate | |
| Mucus depletion | |
| Lamina propria | |
| Plasmacytoid infiltrate | |
| Neutrophilic infiltrate | |
| Vascularity | |
| Fibrin deposition | |
| None (0); mucosal (1); submucosal (2); transmural (3) | |
| Submucosal | |
| Neutrophilic infiltrate | |
| Edema | |
Colonic tissue was homogenized, and levels of MPO, TNF-α, and IL-10 were measured by enzyme-linked immunosorbent assay (ELISA), MDA by HPLC, and iNOS by Western blot.
MPO concentration in homogenized supernatants was determined with an ELISA kit (AG-K6631-061109; Immunodiagnostic Systems, United Kingdom) according to the manufacturer’s instructions. The level expressed as ng/mg protein. TNF-α and IL-10 concentrations were also determined with ELISA kits (ASSAYPRO LLC, St. Charles, MO, United States; Biosource of Thermo Fisher Scientific, Waltham, MA, United States), and levels are expressed as pg/mg protein.
MDA was determined by HPLC using a Thermo Finnigan Spectra system with a diode array detector (Thermo Fisher Scientific). Alkaline supernatants, which were obtained from colonic tissue, were incubated in a water bath for 30 min at 60 °C (for the hydrolysis of protein-bound MDA). After the cooling of examples, 30% perchlorate was added to precipitate the proteins. The mixture was centrifuged at 2800 ×g; 250 μL of supernatant was transferred to microcentrifuge tubes and then 25 μL dinitrophenyl hydrazine (5 mmol/L) was added. Finally, 50 μL samples were left in the dark for 30 min, then injected into the HPLC system. Results are presented as mmol/g protein.
For Western blotting analyses, 80 μg protein for each sample was separated by SDS-PAGE and transferred to nitrocellulose membrane. The membrane was probed with anti-iNOS antibody (1:1000) and then incubated with horseradish peroxidase-conjugated anti-mouse antibody (1:5000) at room temperature. Immunoreaction was visualized with an enhanced chemiluminescence system. Total protein levels were determined by the Lowry method[23].
Data are presented as the mean ± SD. The importance of quantitative data differentiation between groups was determined by one-way analysis of followed by a Tukey’s post-hoc test where appropriate. Kruskall-Wallis analysis was used for evaluating the importance of microscopic colitis score differences between experimental groups. If this analysis showed significant differentiation, the relevant group was investigated using the Mann-Whitney U test. P < 0.05 was considered statistically significant.
The disease activity index (DAI) in the colitis group was statistically higher than that of the kefir-colitis group between days 3 and 5 of colitis induction (P < 0.01). The DAI was also significantly higher in the colitis group between days 2 and 6 of colitis induction when compared to the normal control and kefir-control groups. The DAI was statistically higher only on the 6th day in the kefir-colitis group when compared to that in the normal control groups. The DAI was not evaluated on the 7th day (the day of the procedure) as animals were starved for 12 h prior. The DAI process is presented in Figure 1.
Differences in the length and weights of rats are presented in Table 3. Kefir administration did not have a significant effect on either the length or weight of normal rats. However, increased colon weight and decreased colon length were observed in colitis-induced rats. The colon weight increase was greater in the kefir-colitis group than in the colitis group. Nonetheless, no significant difference was observed among the groups in terms of colon weights. Mean colon length in the colitis group was significantly shorter than that of the kefir-control group (P < 0.05).
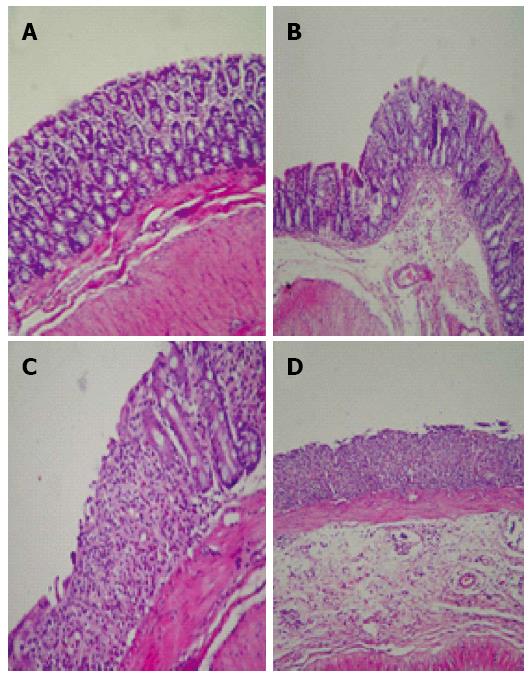
Kefir treatment significantly reduced histologic colitis scores (P < 0.05; Figure 2). The criteria used in histologic evaluation were the same as those generally used in DSS colitis. According to these criteria, kefir treatment appears to improve the histology of colitis. However, submucosal inflammation was observed in the kefir-treated groups (Figure 3).
DSS increased MPO activity in colonic tissue. Treatment with kefir eliminated this increase in DSS-induced MPO activity. MPO activity in the colitis group was significantly higher than that of the kefir-control group (P < 0.05; Figure 4).
Induction of colitis increased TNF-α concentrations in colonic tissue, though levels were not significantly different when compared to either the normal control or kefir-control groups. Elevated TNF-α levels induced by DSS colitis were, however, significantly reduced by kefir treatment (P < 0.01; Figure 5).
No statistically significant differences were observed among the groups for IL-10 levels (Figure 6).
MDA levels in colon tissue were higher in the colitis group than other groups, but the differences were not statistically significant (Figure 7).
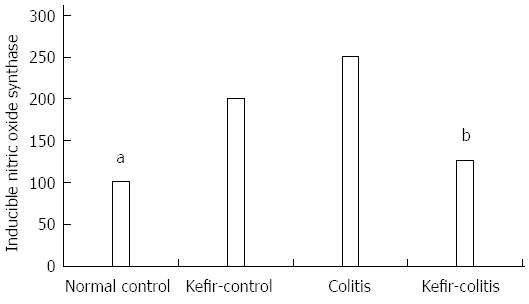
iNOS expression as determined by Western blot is shown in Figure 8. Colon iNOS expression was significantly higher in the colitis group than in either the normal control or kefir-control groups (P < 0.05; Figure 9).
Efficacy of probiotic treatment in Crohn’s disease[24], pouchitis[25,26], and ulcerative colitis[7,27,28] has been previously shown. The period of remission was significantly shortened in ulcerative colitis patients when treated with a preparation of VSL#3, a probiotic combination of eight bacteria species, and balsalazin, than when a single- treatment of balsalazide or mesalazine was administered. In addition, remission induction rate was increased and improved endoscopic and histologic findings were obtained[9,10]. In another study, 187 patients with ulcerative colitis in remission were evaluated at 6 and 12 mo after treatment with either Lactobacillus GG, Lactobacillus GG + mesalazine, or mesalazine exclusively, and the results showed that Lactobacillus GG was as effective as mesalazine in maintaining remission in these patients[29,30].
In the present study, DSS-induced colitis model was the preferred experimental model. In rodents, DSS-induced colitis is a well-established model that reflects the major clinical and histopathologic features of human ulcerative colitis. DSS impairs the barrier function of epithelial cells in the intestine[31], and luminal bacterial antigens activate the inherent immune response by reaching lamina propria easier and stimulate inflammation[32]. Inflammatory changes, such as weight loss, hemorrhagic diarrhea, and histopathologic erosion in crypts, are observed with the addition of DSS to fresh drinking water in rodents[21].
In the literature, colitis models induced by a 1.5%-5% DSS solution have been reported. The severity of colitis increases concordantly with DSS concentration[33-36]. In the present study, a 3% DSS solution resulted in mild-medium severity colitis.
The present study found that kefir treatment reduces clinical DAI in DSS-induced colitis and that this reduction was statistically significant on the 3rd and 5th days of colitis induction. We further observed that kefir-treatment reduces histologic colitis scores, supporting clinical findings. However, we observed submucosal inflammation due to kefir-treatment in both control and colitis-induced rats. It is well known that there is continuous and persistent “intestinal inflammatory response” in the intestinal mucosa (physiologic inflammation)[37]. Our hypothesis in this context is that kefir might augment physiologic inflammation. We found that colon weights were greater in kefir-treated and colitis-induced rats than in colitis-induced but not kefir-treated rats. Kefir-induced submucosal inflammation could be the reason for this increased colon weight.
MPO in neutrophil granules converts hydrogen peroxide to hypochloric acid in the presence of chlorine ions. Hypochloric acid is both a potent oxidant as well as an antimicrobial agent. In the presence of inflammation, leukocyte infiltration of colonic mucosa and release of free oxygen radicals from activated leukocytes induces proinflammatory cytokine release and tissue injury progress[38]. Some investigators have reported that an increase in free oxygen radicals induces nuclear factor (NF)-κB, which in turn elevates TNF-α production and further contributes to the inflammatory process[39].
In the present study, MPO levels in the colitis group were two times higher than in the control and kefir-control groups, and statistically different from the kefir-control group. MPO levels in the kefir-colitis group were in line with those in the control group. These findings could provide evidence for the protective effect of kefir against tissue injury by reducing elevated MPO levels in inflammation.
Cytokines play an important role in the modulation of the immune system. In the pathogenesis of IBD, it is accepted that the pro- and anti-inflammatory cytokine balance is altered[40]. Colon biopsies in IBD show an increase in proinflammatory cytokine levels including IL-1, IL-2, IL-6, IL-8, IFN-γ, and TNF, as well as a decrease in anti-inflammatory cytokines such as IL-4[31,40]. Obermeimer et al[40] reported that production of the proinflammatory cytokines TNF and IFN-γ is observed during chronic processes, such as in the DSS-induced colitis model.
Oxidative stress, cytokines (IL-1, IL-6, and TNF-α), bacteria, and viruses may augment the inflammatory process by stimulating NF-κB activation. A study evaluating the effect of probiotics for the prevention of relapse in patients with ulcerative colitis showed that a probiotic preparation of three bifidobacteria strains inhibited proinflammatory cytokine (TNF-α, IL-1β) expression by impeding NF-κB activation[41].
A study investigating the effect of a probiotic combination including Lactobacillus and Bifidobacterium spp. in a rat model of colitis induced by 4% DSS showed that TNF-α levels in colon mucosa were significantly elevated in the colitis group when compared to both the control and probiotic-colitis groups[42]. In the present study, we also found that tissue TNF-α levels were significantly higher in colitis-induced rats compared to the control groups. Our results furthermore show that kefir treatment lessens the increase in tissue TNF-α concentration that occurs with DSS-induced colitis, which supports the concept that inhibition of proinflammatory cytokine production is one of the most important beneficial effects of this treatment.
IL-10 is synthesized by T-cells, B cells, and monocytes activated by lipopolysaccharides[43]. It inhibits proinflammatory cytokines synthesized by activated macrophages during inflammation. IL-10 plays a role in modulation of T-cell activation and also downregulation of the acute inflammatory response[44,45]. In recent years, the anti-inflammatory effects of IL-10 in IBD have been studied and indicate a role in the immune modulation of the gastrointestinal tract, as evidenced by alleviation of enterocolitis by giving IL-10 to IL-10-knockout rats[46]. In our model, no significant difference in IL-10 levels was observed between the colitis and control groups. Another study examining the cytokine levels in colonic tissue in a 4% DSS-induced model of chronic colitis found TNF-α levels to be significantly increased in the colitis-induced group, although no significant difference in serum IL-10 levels was observed[47].
Activated neutrophils and macrophages produce reactive oxygen products, and oxidative stress subsequently increases in inflamed intestinal mucosa in IBD[48]. In these conditions, excessive amounts of toxic reactive oxygen products exceed the capacity of intestinal antioxidant defense systems, and oxidative injury leads to increased mucosal injury in IBD patients[49]. In oxidatively injured hepatic and renal tissue with CCL4, kefir suppressed the reduction in glutathione and glutathione peroxidase levels and significantly alleviates elevated MDA levels, an indicator of lipid peroxidation[50]. MDA production is a strong indicator of lipid peroxidation and is thus used to evaluate oxidative stress. In the 3% DSS-induced colitis model used in the present study, colonic tissue MDA levels were elevated, though not significantly, compared to those in the control group. In this study, MDA concentration was measured in the full thickness of the colonic tissue. However, inflammation in this model concerns the mucosa, and so MDA levels measured in full thickness colonic tissue may be somewhat reduced.
In general, the antioxidant effects of probiotics are well established. In a study on 5% DSS-induced colitis, Bifidobacterium infantis DSM 15139 spp significantly reduced both MDA and MPO levels[51]. This experimental model most closely resembles the model used in the present study, as Bifidobacterium infantis was administered 7 d prior to colitis induction and administration continued for 7 d after induction.
Nitric oxide (NO) is a biologic mediator with short-term effects and a broad functional spectrum, including thrombocyte and leucocyte activation, regulation of vessel reactivity, regulation of cell growth, and non-specific immune reactions[52]. Elevated iNOS production has been previously demonstrated in both clinical and experimental IBD studies[39,53]. Activation of iNOS augments NO synthesis.
Inflammatory cytokines such as IL-1, IFN-γ, and TNF-α stimulate significant NO production in the intestinal epithelium, macrophages, and other cells[40]. In different experimental colitis models-as well as in patients with ulcerative colitis-increased NO levels have been observed in the lavage fluid of the colon[40,54,55]. Various studies have reported that excessive NO production in the colonic mucosa results in increased oxidative stress and subsequent tissue injury[54-56]. Compatible with these findings, iNOS inhibition has shown positive effects in certain animals with intestinal inflammation[57,58]. In a study on mice with chronic colitis induced with 5% DSS, inhibition of TNF-α and IFN-γ by anti-cytokine anticores downregulated NO levels and alleviated colitis[58].
In our study, iNOS levels were higher in the colitis group when compared to the other groups. Kefir reduced iNOS levels in DSS-induced colitis; however, iNOS levels were also elevated in the kefir-control group. Some studies in the literature have indicated that some probiotic species may in fact increase NO and iNOS levels.
L. rhamnosus GG has been found to induce iNOS, which promotes low level NO production through activation of NF-κB in j774 macrophages and human T84 intestinal epithelial cells. Researchers have reported that L. rhamnosus GG has a protective effect towards increased NO levels in the gastrointestinal tract[59]. According to our findings, kefir increases iNOS levels in the normal colon, but reduces increased iNOS levels in the presence of inflammation.
In conclusion, in an experimental model of 3% DSS-induced colitis, kefir not only reduces clinical disease activity, but also histologic colitis scores. Reduced MPO, TNF-α, and iNOS levels are among the positive effects of kefir. The results of this study promise hope that kefir may have a place in the clinical treatment of IBD.
An intestinal microflora balance in favor of pathogenic bacterial species is observed in patients with inflammatory bowel disease (IBD), and studies suggest that enteric flora strongly influence disease occurrence and progression. Intestinal microflora may also be modified with probiotic treatments, and in recent years, studies have shown that realignment of the composition of the intestinal microflora with probiotic treatments could in fact be a new therapeutic modality.
Probiotics inhibit the proliferation of pathogenic microorganisms in the intestine and prevent the attachment of microorganisms to epithelial cells by competing for hanging regions in the intestinal mucosa, improving the structure of the host immune system and mucosa gate system via interactions with immune cells in the bowel, and modulating apoptosis. Beneficial effects of various probiotic microorganisms have been demonstrated in both an experimental colitis model and in clinical inflammatory intestinal diseases.
The overall results of this study showed that kefir treatment reduces the clinical disease activity index and histologic colitis scores in an experimental colitis model induced with 3% dextran sulfate sodium (DSS). Mechanisms for the beneficial effects of kefir include reduction in levels of myeloperoxidase, tumor necrosis factor-α, and inducible nitric oxide synthase. Indeed, the results of this study show that the therapeutic administration of kefir may have a place in the clinical treatment of IBD.
This study was designed to evaluate the preventive effect of kefir on colitis induced with DSS in rats.
Dextran sulfate is a polyanionic derivative of dextran produced by esterification of dextran with chlorosulphonic acid. DSS is the sodium salt, which is a white to off-white powder freely soluble in water and salt solutions to form a stable, clear solution. By first interfering with intestinal barrier function, and next stimulating local inflammation, DSS is often used to induce a form of mouse colitis that mimics the clinical and histologic features of IBDs. Probiotics are living microorganisms that, when taken by mouth, benefit your health by improving the balance of bacteria in the intestines. Kefir, a natural probiotic beverage, is a fermented milk product of Caucasian origin. Fermentation is facilitated by a complex combination of yeast and bacteria, and the combination of this complex includes lactobacillus, streptococcus, acetobacteria, and yeasts.
The present study aimed to find a pathophysiologic mechanism for the preventive effect of kefir on colitis induced with DSS in rats. The results show that kefir reduces the clinical disease activity index and histologic colitis scores in an experimental colitis model induced with 3% DSS. Beneficial effects mechanisms of kefir include reduction in myeloperoxidase, tumor necrosis factor-α, and inducible nitric oxide synthase levels.
P- Reviewer: Dou W S- Editor: Yu J L- Editor: Filipodia E- Editor: Liu XM
| 1. | Dotan I, Rachmilewitz D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr Opin Gastroenterol. 2005;21:426-430. [PubMed] [Cited in This Article: ] |
| 2. | Sartor RB. Review article: Role of the enteric microflora in the pathogenesis of intestinal inflammation and arthritis. Aliment Pharmacol Ther. 1997;11 Suppl 3:17-22; discussion 22-23. [PubMed] [Cited in This Article: ] |
| 3. | Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620-1633. [PubMed] [Cited in This Article: ] |
| 4. | Farrell RJ, LaMont JT. Microbial factors in inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:41-62. [PubMed] [Cited in This Article: ] |
| 5. | Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1-4. [PubMed] [Cited in This Article: ] |
| 6. | Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl. 2001;29-40. [PubMed] [Cited in This Article: ] |
| 7. | Seksik P, Sokol H, Lepage P, Vasquez N, Manichanh C, Mangin I, Pochart P, Doré J, Marteau P. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24 Suppl 3:11-18. [PubMed] [Cited in This Article: ] |
| 8. | Isler M. İnflamatuar b arsak hastalığı ve probiyotikler. Güncel Gastroenteroloji. 2005;9:3: 134-140. [Cited in This Article: ] |
| 9. | Ardizzone S, Bianchi Porro G. Inflammatory bowel disease: new insights into pathogenesis and treatment. J Intern Med. 2002;252:475-496. [PubMed] [Cited in This Article: ] |
| 10. | Guslandi M. Probiotics for chronic intestinal disorders. Am J Gastroenterol. 2003;98:520-521. [PubMed] [Cited in This Article: ] |
| 11. | Ohkusa T, Sato N. Antibacterial and antimycobacterial treatment for inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:340-351. [PubMed] [Cited in This Article: ] |
| 12. | McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O’Sullivan GC, Kiely B, Collins JK. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975-980. [PubMed] [Cited in This Article: ] |
| 13. | Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107-1114. [PubMed] [Cited in This Article: ] |
| 14. | Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, De Simone C, Madsen K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358-1373. [PubMed] [Cited in This Article: ] |
| 15. | Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562-575. [PubMed] [Cited in This Article: ] |
| 16. | Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002;18:586-590. [PubMed] [Cited in This Article: ] |
| 17. | Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613-G626. [PubMed] [Cited in This Article: ] |
| 18. | Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G315-G322. [PubMed] [Cited in This Article: ] |
| 19. | Gotteland M, Cruchet S, Verbeke S. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment Pharmacol Ther. 2001;15:11-17. [PubMed] [Cited in This Article: ] |
| 20. | Bing SR, Kinouchi T, Kataoka K, Kuwahara T, Ohnishi Y. Protective effects of a culture supernatant of Lactobacillus acidophilus and antioxidants on ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug. Microbiol Immunol. 1998;42:745-753. [PubMed] [Cited in This Article: ] |
| 21. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] [Cited in This Article: ] |
| 22. | Stucchi AF, Shofer S, Leeman S, Materne O, Beer E, McClung J, Shebani K, Moore F, O’Brien M, Becker JM. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1298-G1306. [PubMed] [Cited in This Article: ] |
| 23. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] [Cited in This Article: ] |
| 24. | Borruel N, Carol M, Casellas F, Antolín M, de Lara F, Espín E, Naval J, Guarner F, Malagelada JR. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659-664. [PubMed] [Cited in This Article: ] |
| 25. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114. [PubMed] [Cited in This Article: ] |
| 26. | Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment Pharmacol Ther. 2003;17:509-515. [PubMed] [Cited in This Article: ] |
| 27. | Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635-639. [PubMed] [Cited in This Article: ] |
| 28. | Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539-1546. [PubMed] [Cited in This Article: ] |
| 29. | Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567-1574. [PubMed] [Cited in This Article: ] |
| 30. | Isaacs K, Herfarth H. Role of probiotic therapy in IBD. Inflamm Bowel Dis. 2008;14:1597-1605. [PubMed] [Cited in This Article: ] |
| 31. | Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382-389. [PubMed] [Cited in This Article: ] |
| 32. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [PubMed] [Cited in This Article: ] |
| 33. | Korenaga D, Takesue F, Kido K, Yasuda M, Inutsuka S, Honda M, Nagahama S. Impaired antioxidant defense system of colonic tissue and cancer development in dextran sulfate sodium-induced colitis in mice. J Surg Res. 2002;102:144-149. [PubMed] [Cited in This Article: ] |
| 34. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] [Cited in This Article: ] |
| 35. | Jungbeck M, Daller B, Federhofer J, Wege AK, Wimmer N, Männel DN, Hehlgans T. Neutralization of LIGHT ameliorates acute dextran sodium sulphate-induced intestinal inflammation. Immunology. 2009;128:451-458. [PubMed] [Cited in This Article: ] |
| 36. | Camuesco D, Comalada M, Rodríguez-Cabezas ME, Nieto A, Lorente MD, Concha A, Zarzuelo A, Gálvez J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol. 2004;143:908-918. [PubMed] [Cited in This Article: ] |
| 37. | Lammers KM, Brigidi P, Vitali B, Gionchetti P, Rizzello F, Caramelli E, Matteuzzi D, Campieri M. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 2003;38:165-172. [PubMed] [Cited in This Article: ] |
| 38. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [PubMed] [Cited in This Article: ] |
| 39. | Head KA, Jurenka JS. Inflammatory bowel disease Part 1: ulcerative colitis--pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8:247-283. [PubMed] [Cited in This Article: ] |
| 40. | Obermeier F, Kojouharoff G, Hans W, Schölmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238-245. [PubMed] [Cited in This Article: ] |
| 41. | Cui HH, Chen CL, Wang JD, Yang YJ, Cun Y, Wu JB, Liu YH, Dan HL, Jian YT, Chen XQ. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol. 2004;10:1521-1525. [PubMed] [Cited in This Article: ] |
| 42. | Peña JA, Rogers AB, Ge Z, Ng V, Li SY, Fox JG, Versalovic J. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect Immun. 2005;73:912-920. [PubMed] [Cited in This Article: ] |
| 43. | Nanda Kumar NS, Balamurugan R, Jayakanthan K, Pulimood A, Pugazhendhi S, Ramakrishna BS. Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate. J Gastroenterol Hepatol. 2008;23:1834-1839. [PubMed] [Cited in This Article: ] |
| 44. | Mosmann TR. Cytokine secretion patterns and cross-regulation of T cell subsets. Immunol Res. 1991;10:183-188. [PubMed] [Cited in This Article: ] |
| 45. | Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815-3822. [PubMed] [Cited in This Article: ] |
| 46. | Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263-274. [PubMed] [Cited in This Article: ] |
| 47. | Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16:297-304. [PubMed] [Cited in This Article: ] |
| 48. | Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S-15S. [PubMed] [Cited in This Article: ] |
| 49. | Babbs CF. Oxygen radicals in ulcerative colitis. Free Radic Biol Med. 1992;13:169-181. [PubMed] [Cited in This Article: ] |
| 50. | Güven A, Güven A, Gülmez M. The effect of kefir on the activities of GSH-Px, GST, CAT, GSH and LPO levels in carbon tetrachloride-induced mice tissues. J Vet Med B Infect Dis Vet Public Health. 2003;50:412-416. [PubMed] [Cited in This Article: ] |
| 51. | Osman N, Adawi D, Molin G, Ahrne S, Berggren A, Jeppsson B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006;6:31. [PubMed] [Cited in This Article: ] |
| 52. | de Belder AJ, Radomski MW. Nitric oxide in the clinical arena. J Hypertens. 1994;12:617-624. [PubMed] [Cited in This Article: ] |
| 53. | Miller MJ, Thompson JH, Zhang XJ, Sadowska-Krowicka H, Kakkis JL, Munshi UK, Sandoval M, Rossi JL, Eloby-Childress S, Beckman JS. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995;109:1475-1483. [PubMed] [Cited in This Article: ] |
| 54. | Lundberg JO, Hellström PM, Lundberg JM, Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet. 1994;344:1673-1674. [PubMed] [Cited in This Article: ] |
| 55. | Ferretti M, Gionchetti P, Rizzello F, Venturi A, Stella P, Corti F, Mizrahi J, Miglioli M, Campieri M. Intracolonic release of nitric oxide during trinitrobenzene sulfonic acid rat colitis. Dig Dis Sci. 1997;42:2606-2611. [PubMed] [Cited in This Article: ] |
| 56. | Rachmilewitz D, Stamler JS, Karmeli F, Mullins ME, Singel DJ, Loscalzo J, Xavier RJ, Podolsky DK. Peroxynitrite-induced rat colitis--a new model of colonic inflammation. Gastroenterology. 1993;105:1681-1688. [PubMed] [Cited in This Article: ] |
| 57. | Hogaboam CM, Jacobson K, Collins SM, Blennerhassett MG. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am J Physiol. 1995;268:G673-G684. [PubMed] [Cited in This Article: ] |
| 58. | Neilly PJ, Gardiner KR, Rowlands BJ. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1996;38:475. [PubMed] [Cited in This Article: ] |
| 59. | Korhonen R, Korpela R, Saxelin M, Mäki M, Kankaanranta H, Moilanen E. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation. 2001;25:223-232. [PubMed] [Cited in This Article: ] |