Published online Nov 28, 2015. doi: 10.3748/wjg.v21.i44.12722
Peer-review started: March 20, 2015
First decision: July 17, 2015
Revised: August 17, 2015
Accepted: September 13, 2015
Article in press: September 14, 2015
Published online: November 28, 2015
A 66-year-old female presented with the main complaint of defecation trouble and abdominal distention. With diagnosis of rectal cancer, cSS, cN0, cH0, cP0, cM0 cStage II, Hartmann’s operation with D3 lymph node dissection was performed and a para-aortic lymph node and a disseminated node near the primary tumor were resected. Histological examination showed moderately differentiated adenocarcinoma, pSS, pN3, pH0, pP1, pM1 (para-aortic lymph node, dissemination) fStage IV. After the operation, the patient received chemotherapy with FOLFIRI regimen. After 12 cycles of FOLFIRI regimen, computed tomography (CT) detected an 11 mm of liver metastasis in the postero-inferior segment of right hepatic lobe. With diagnosis of liver metastatic recurrence, we performed partial hepatectomy. Histological examination revealed moderately differentiated adenocarcinoma as a metastatic rectal cancer with cut end microscopically positive. After the second operation, the patient received chemotherapy with TS1 alone for 2 years. Ten months after the break, CT detected a 20 mm of para-aortic lymph node metastasis and a 10 mm of lymph node metastasis at the hepato-duodenal ligament. With diagnosis of lymph node metastatic recurrences, we performed lymph node dissection. Histological examination revealed moderately differentiated adenocarcinoma as metastatic rectal cancer in para-aortic and hepato-duodenal ligament areas. After the third operation, we started chemotherapy with modified FOLFOX6 regimen. After 2 cycles of modified FOLFOX6 regimen, due to the onset of neutropenia and liver dysfunction, we switched to capecitabine alone and continued it for 6 mo and then stopped. Eleven months after the break, CT detected two swelling 12 mm of lymph nodes at the left supraclavicular region. With diagnosis of Virchow lymph node metastatic recurrence, we started chemotherapy with capecitabine plus bevacizumab regimen. Due to the onset of neutropenia and hand foot syndrome (Grade 3), we managed to continue capecitabine administration with extension of interval period and dose reduction. After 2 years and 2 mo from starting capecitabine plus bevacizumab regimen, Virchow lymph nodes had slowly grown up to 17 mm. Because no recurrence had been detected besides Virchow lymph nodes for this follow up period, considering the side effects and quality of life, surgical resection was selected. We performed left supraclavicular lymph node dissection. Histological examination revealed moderately differentiated adenocarcinoma as a metastatic rectal cancer. After the fourth operation, the patient selected follow up without chemotherapy. Now we follow up her without recurrence and keep her quality of life high.
Core tip: A 66-year-old female who had para-aortic lymph node metastasis and peritoneal dissemination of rectal cancer underwent Hartmann’s operation. Beginning from stage IV, liver metastasis, para-aortic and hepato-duodenal ligament lymph node and Virchow lymph node recurrence were detected during follow up period. According to the previous reports, the resection of these severe recurrences is controversial. We conducted four operations during 8 years for Stage IV rectal cancer and its recurrences. Finally, there is no recurrence radiologically. It should be considered that surgical resection may bring longer term survival especially in cases with difficulty in management of chemotherapy.
- Citation: Takeshita N, Fukunaga T, Kimura M, Sugamoto Y, Tasaki K, Hoshino I, Ota T, Maruyama T, Tamachi T, Hosokawa T, Asai Y, Matsubara H. Successful resection of metachronous para-aortic, Virchow lymph node and liver metastatic recurrence of rectal cancer. World J Gastroenterol 2015; 21(44): 12722-12728
- URL: https://www.wjgnet.com/1007-9327/full/v21/i44/12722.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i44.12722
In recent decades, great progress has been achieved in the management of colorectal cancer, especially in the development of new chemotherapeutic agents and surgical technique. The prognosis of patients with stage IV colorectal cancer and its recurrence used to be very poor, but depending on metastatic site, surgical resection combined with chemotherapy may bring longer term survival nowadays. Now, we herein report a case of four times successful resections during 8 years for Stage IV rectal cancer and its liver, para-aortic, hepato-duodenal ligament and Virchow lymph node metastases.
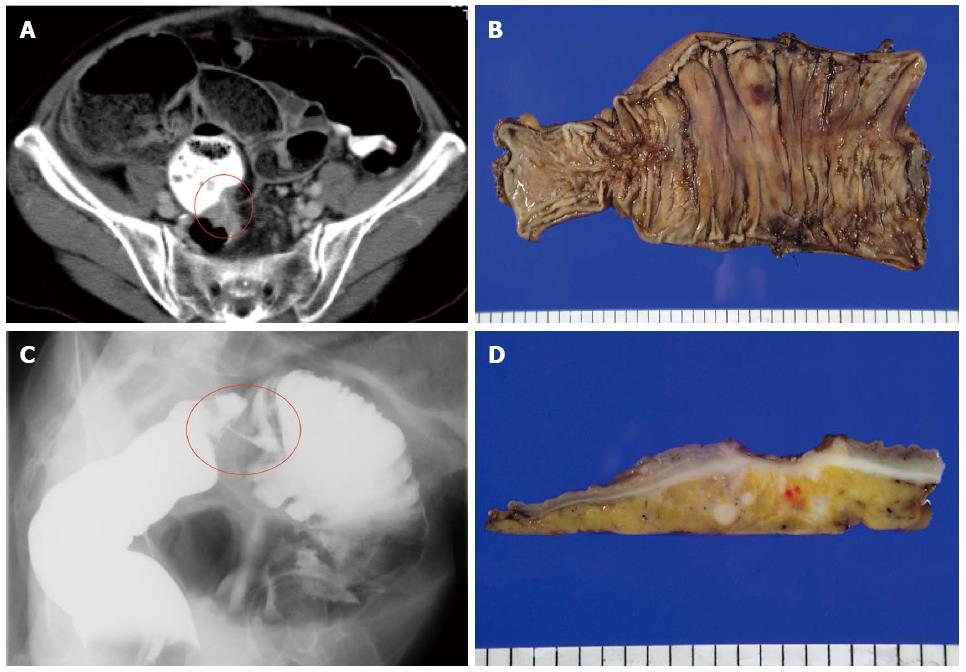
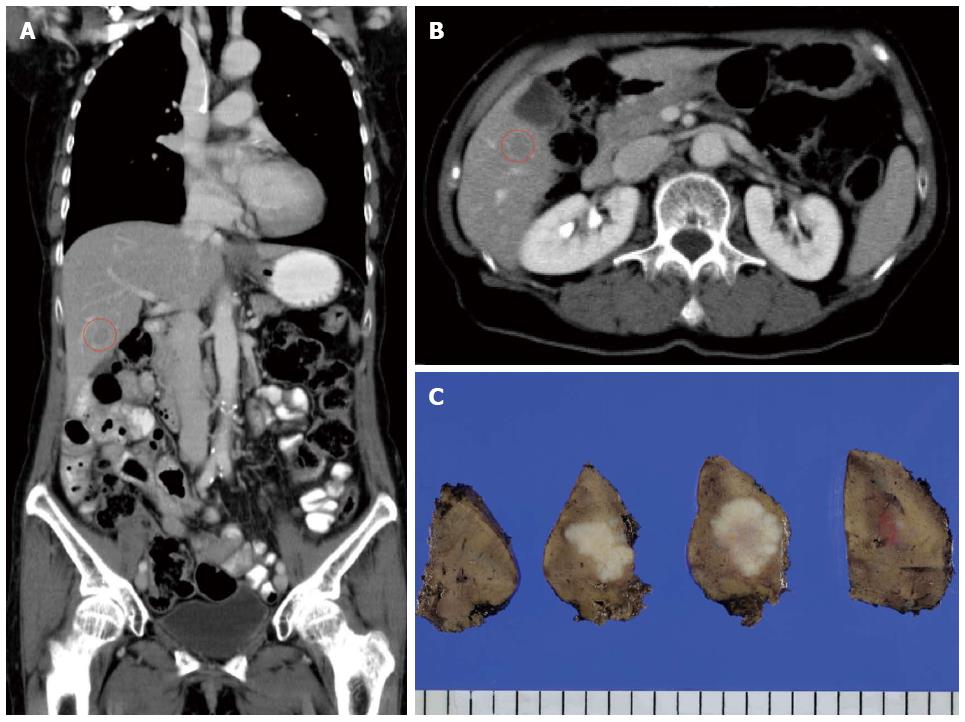
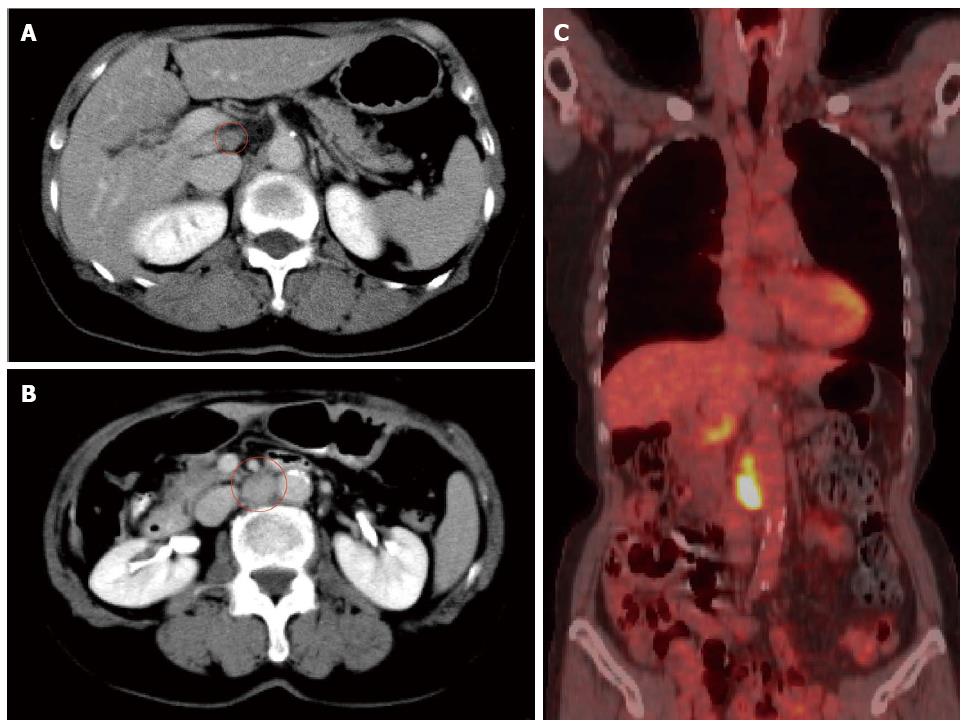
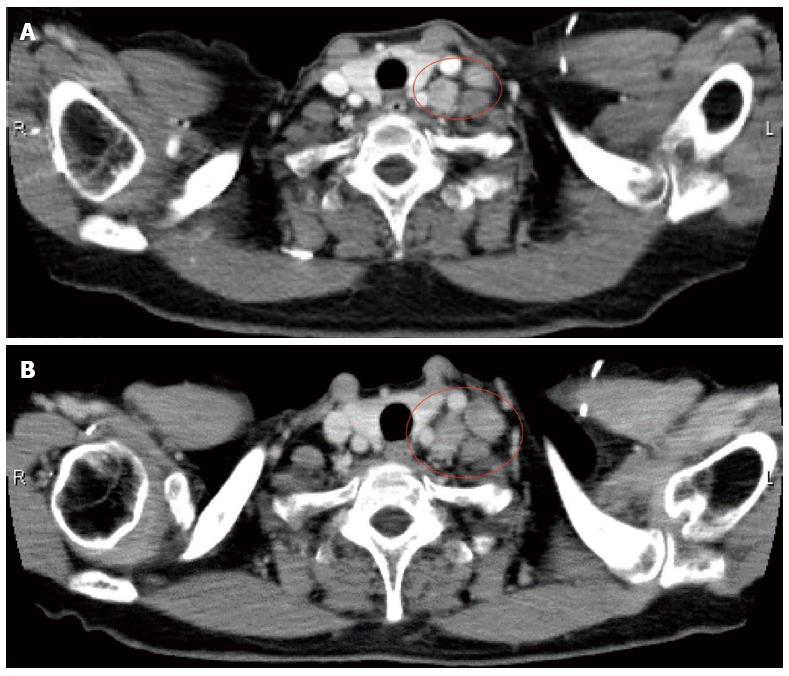
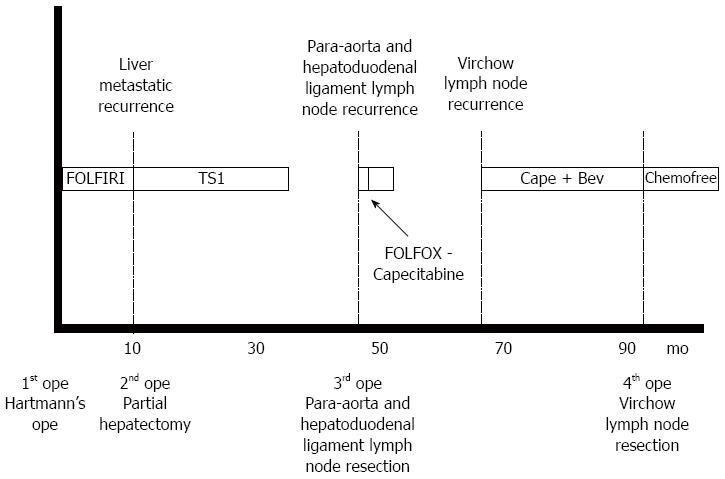
A 66-year-old female presented with the main complaint of defecation trouble and abdominal distention. Abdominal X-ray showed much of colon gas and computed tomography (CT) showed hypertrophy and obstruction of rectum and severe colonic dilatation (Figure 1A). No metastasis was revealed in the liver and lungs. Radiographical examination revealed rectosigmoid obstruction and a decompressive tube couldn’t pass the stenosis (Figure 1B). Mild dehydration was shown, but other laboratory tests and serum levels of carcino-embryonic antigen and carbohydrate antigen 19-9 were all within normal limits. The preoperative diagnosis was rectal cancer, cSS, cN0, cH0, cP0, cM0 cStage II by the Japanese Classification of Colorectal Carcinoma (JCCC)[1]. Hartmann’s operation with D3 lymph node dissection was performed and a swelling para-aortic lymph node and a disseminated node near the primary tumor were resected. R0 (microscopically negative margin) surgery was achieved. Histological examination showed moderately differentiated adenocarcinoma, pSS, pN3, pH0, pP1, pM1 (para-aortic lymph node, dissemination) fStage IV by JCCC (1) (Figure 1C and D). After the operation, the patient received chemotherapy with FOLFIRI regimen (starting doses: irinotecan at 150 mg/m2, folinic acid at 200 mg/m2, fluorouracil (5-FU) bolus at 400 mg/m2 on day 1 and 5-FU continuous intravenous infusion at 2400 mg/m2 on days 1-2, every two weeks). Between 12 cycles of FOLFIRI regimen, due to the onset of neutropenia (Grade 3), we managed to continue FOLFIRI regimen with extension of interval period and dose reduction. After 12 cycles of FOLFIRI regimen (one year after first operation), CT detected an 11 mm of liver metastasis in the postero-inferior segment of right hepatic lobe (Figure 2A and B). With diagnosis of liver metastatic recurrence, we performed partial hepatectomy. Histological examination revealed moderately differentiated adenocarcinoma as a metastatic rectal cancer with cut end microscopically positive (R1) (Figure 2C). After the second operation, the patient received chemotherapy with TS1 alone for 2 years. For 2 years, due to the onset of neutropenia (Grade 3), we managed to continue TS1 administration with extension of interval period and dose reduction. Finally, because of no recurrence, TS1 administration was stopped by mutual consultation between the patient and us. Ten months after the break, CT detected a 20 mm of para-aortic lymph node metastasis and a 10 mm of lymph node metastasis at the hepato-duodenal ligament (Figure 3A and B). Positron emission tomography was performed and detected the recurrences at the same lesions (Figure 3C). With diagnosis of lymph node metastatic recurrences, we performed lymph node dissection (4 years after first operation). R0 surgery was achieved. Histological examination revealed moderately differentiated adenocarcinoma as metastatic rectal cancer in para-aortic and hepato-duodenal ligament areas. After the third operation, we started chemotherapy with modified FOLFOX6 regimen (starting doses: oxaliplatin at 85 mg/m2, intravenous infusion folinic acid at 200 mg/m2, bolus 5-FU at 400 mg/m2 on day 1 and continuous intravenous infusion of 5-FU at 2400 mg/m2 on days 1-2, every two weeks). After 2 cycles of modified FOLFOX6 regimen, due to the onset of neutropenia and liver dysfunction, we switched to capecitabine alone and continued it for 6 mo and then stopped. Eleven months after the break, CT detected two swelling 12 mm of lymph nodes at the left supraclavicular (Figure 4A). With diagnosis of Virchow lymph node metastatic recurrence, we started chemotherapy with capecitabine plus bevacizumab regimen (5 years and 6 mo after first operation). Due to the onset of neutropenia and hand foot syndrome (Grade 3), we managed to continue capecitabine administration with extension of interval period and dose reduction. After 2 years and 2 mo from starting capecitabine plus bevacizumab regimen, Virchow lymph nodes had slowly grown up to 17 mm (Figure 4B). Because no recurrence had been detected besides Virchow lymph nodes for this follow up period, considering the side effects and quality of life, surgical resection was selected by mutual consultation between the patient and us. We performed left supraclavicular lymph node dissection (8 years after first operation). R0 surgery was achieved. Histological examination revealed moderately differentiated adenocarcinoma as a metastatic rectal cancer. After the fourth operation, the patient selected follow up without chemotherapy. Now we follow up her without recurrence and keep her quality of life high. Figure 5 shows the clinical course.
As a result of the significant advances in the clinically available chemotherapeutic agents, such as 5-FU, leucovorin and oxaliplatin combination chemotherapy (FOLFOX) and 5-FU, leucovorin and irinotecan combination chemotherapy (FOLFIRI), the median survival time of patients with metastatic colorectal cancer improved to > 20 mo in a recently published report[2]. Furthermore, recently, bevacizumab and anti-EGFR monoclonal antibody have been reported to improve the survival times of patients[3,4]. Many kinds of chemotherapeutic agents are available, but it is important to manage side effects and keep quality of life high[5].
In colorectal cancer, up to about 20% of all patients present with stage IV disease at initial diagnosis[6,7]. Liver, lung and peritoneum are the most frequently observed metastatic site. It was reported that sites of metastasis were liver 85%, lung 4% and peritoneum 10%[7]. For patients with resectable metastatic lesion, R0 surgery was thought to lead to improvement of prognosis. In the cases of liver or lung metastasis, this policy has been well acceptable and 5 year survival rates were reported to be above 60%[8,9]. Peritoneal carcinomatosis is thought to result from peritoneal spread of cancer cells or seeding of the peritoneum during surgery, and it has been associated with a median survival time of 7 mo[10]. Recently, the combination of multimodality therapy and cytoreduction surgery has been accepted and using this approach, median survival exceeding 60 mo has been reported[11]. Although in the limited cases, surgical resection was acceptable option for the patient with peritoneal carcinomatosis from colorectal cancer.
Extra-mesenteric lymph node metastasis such as para-aortic lymph node and lateral pelvic lymph node metastasis are thought to be poor prognostic risk factors and the indication of surgical resection for these conditions is controversial[12]. On the other hand, para-aortic lymph node recurrence after curative surgery for colorectal cancer is more rare condition. It was reported that isolated para-aortic lymph node recurrence was identified in 1.3% of the patients who underwent curative surgery for colorectal cancer, and the median survival time from para-aortic lymph node recurrence for the resected patients was 34 mo, whereas it was 12 mo for those who did not undergo resection[13]. Lymph node metastasis outside the mesenteric lesion may represent metastasis from metastasis. Traditionally, metastatic disease in the hilar or para-aortic lymph nodes has been considered a strong relative contraindication to surgery due to poor prognosis. However, more recently, with more effective chemotherapy, the role of resection of these extra hepatic lymph node metastasis has been reconsidered. The patients of colorectal liver metastasis with lymph node metastasis in hepato-duodenal ligament and retro-pancreatic area had a 5-year survival greater than 30%. These data may have important implications to help select patients for surgical resection. On the other hand, these patients with lymph node metastasis in para-aortic area had median survival of only 17 mo and every patient experienced a recurrence. On the basis of these data, surgical resection for the cases with para-aortic lymph node metastasis should be more controversial[14]. Left supraclavicular lymph node is often called Virchow lymph node. The lymphatic vessels including the thoracic duct arrive at the Virchow lymph node, and this is recognized as being “final destination” of lymph nodes[15]. The metastasis to this lesion is thought to be extremely poor prognostic risk factor and the evidence of the treatment option for this is insufficient. There are some reports about synchronous or metachronous metastasis of Virchow lymph node which were treated by chemotherapy or chemoradiotherapy and achieved good prognosis[16,17].
In this case, para-aortic lymph node metastasis and peritoneal dissemination of rectal cancer were revealed in first operation. Beginning from stage IV, three times operations were done for liver metastatic recurrence, para-aortic and hepato-duodenal ligament lymph node recurrence and Virchow lymph node recurrence. According to previous reports, the resection of liver metastatic recurrence was acceptable, but the indication of the extrahepatic disease, such as para-aortic, hepato-duodenal ligament and Virchow lymph node is controversial. Furthermore, para-aortic and Virchow lymph node recurrences may be thought to be extremely poor prognostic factors. Meanwhile, this patient had difficulty with the management of chemotherapeutic side effects. In fact, extension of interval period and dose reduction of chemotherapy was necessary and her quality of life was low due to the onset of neutropenia and other side effects. As to Virchow lymph node metastasis, during the period of chemotherapy which had been continued for two years, other new metastatic lesion had not revealed and the target lesion had been slowly increasing. Although we could change the regimen and continue other chemotherapy, the patient didn’t wish to suffer new side effect and preferred surgical resection. It was not easy to propose the option of surgical resection, but we thought that the option of surgery could be acceptable when adequate informed consent about the risks, benefits and other therapeutic options are obtained from the patient. After the R0 surgery of Virchow lymph node recurrence, the necessity of chemotherapy may be controversial. The patient understood the risk of recurrence and preferred the follow up without chemotherapy with keeping her quality of life high. In this case, we conducted four times operations during 8 years for Stage IV rectal cancer and its recurrences. As a result, now, there is no recurrence lesion radiologically.
Although surgery alone for sever metastatic recurrence is rarely selected due to the advance of multimodality therapy, it should be considered that surgical resection may bring longer term survival especially in cases with difficulty in management of side effects or necessity of dose reduction chemotherapy. In the recurrent lesion without enough data about long term prognosis, such as para-aortic and Virchow lymph node, we hope that the reports like our approach will be accumulate and make use in decision of therapeutic options in future.
A female patient underwent four times operations during 8 years for Stage IV rectal cancer and its liver, para-aortic, hepatoduodenal ligament and Virchow lymph node metastasis.
Rectal cancer and its recurrence.
Computed tomography showed liver, para-aortic, hepato-duodenal ligament and Virchow lymph node metastatic recurrence.
Histological examination showed moderately differentiated adenocarcinoma.
Surgical resection and chemotherapy were performed.
According to previous reports, the resection of liver metastatic recurrence was acceptable, but the indication of the extrahepatic disease, such as para-aortic, hepato-duodenal ligament and Virchow lymph node is controversial. There are some reports about synchronous or metachronous metastasis of Virchow lymph node which were treated by chemotherapy or chemoradiotherapy (not by surgical resection) and achieved good prognosis.
Left supraclavicular lymph node is often called Virchow lymph node. The lymphatic vessels including the thoracic duct arrive at the Virchow lymph node, and this is recognized as being the end of lymph nodes.
Although surgery alone for sever metastatic recurrence is rarely selected due to the advance of multimodality therapy, it should be considered that surgical resection may bring longer term survival especially in cases with difficulty in management of side effects or necessity of dose reduction chemotherapy.
This paper is good.
P- Reviewer: Brajuskovic GN, Marickar YMF S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma. 2rd English ed. Tokyo: Kanehara and Co 2009; . [Cited in This Article: ] |
| 2. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2181] [Cited by in F6Publishing: 2147] [Article Influence: 102.2] [Reference Citation Analysis (1)] |
| 3. | Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502-3508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 484] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2901] [Cited by in F6Publishing: 3021] [Article Influence: 201.4] [Reference Citation Analysis (1)] |
| 5. | Zedan AH, Hansen TF, Fex Svenningsen A, Vilholm OJ. Oxaliplatin-induced neuropathy in colorectal cancer: many questions with few answers. Clin Colorectal Cancer. 2014;13:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Mella J, Biffin A, Radcliffe AG, Stamatakis JD, Steele RJ. Population-based audit of colorectal cancer management in two UK health regions. Colorectal Cancer Working Group, Royal College of Surgeons of England Clinical Epidemiology and Audit Unit. Br J Surg. 1997;84:1731-1736. [PubMed] [Cited in This Article: ] |
| 7. | Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999;6:651-657. [PubMed] [Cited in This Article: ] |
| 8. | Ko S, Jo H, Yun S, Park E, Kim S, Seo HI. Comparative analysis of radiofrequency ablation and resection for resectable colorectal liver metastases. World J Gastroenterol. 2014;20:525-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Shiono S, Ishii G, Nagai K, Yoshida J, Nishimura M, Murata Y, Tsuta K, Nishiwaki Y, Kodama T, Ochiai A. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg. 2005;79:278-82; discussion 283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 577] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Sugarbaker PH, Chang D, Koslowe P. Prognostic features for peritoneal carcinomatosis in colorectal and appendiceal cancer patients when treated by cytoreductive surgery and intraperitoneal chemotherapy. Cancer Treat Res. 1996;81:89-104. [PubMed] [Cited in This Article: ] |
| 12. | Min BS, Kim JS, Kim NK, Lim JS, Lee KY, Cho CH, Sohn SK. Extended lymph node dissection for rectal cancer with radiologically diagnosed extramesenteric lymph node metastasis. Ann Surg Oncol. 2009;16:3271-3278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Min BS, Kim NK, Sohn SK, Cho CH, Lee KY, Baik SH. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. J Surg Oncol. 2008;97:136-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Pulitanò C, Bodingbauer M, Aldrighetti L, Choti MA, Castillo F, Schulick RD, Gruenberger T, Pawlik TM. Colorectal liver metastasis in the setting of lymph node metastasis: defining the benefit of surgical resection. Ann Surg Oncol. 2012;19:435-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Sato T. Color Atlas of Applied Anatomy of Lymphatics. Tokyo: Nankodo 1997; . [Cited in This Article: ] |
| 16. | Fujie Y, Ikeda M, Seshimo I, Ezumi K, Hata T, Shingai T, Yasui M, Takayama O, Fukunaga H, Ikenaga M. Complete response of highly advanced colon cancer with multiple lymph node metastases to irinotecan combined with UFT: report of a case. Surg Today. 2006;36:1133-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Ohchi T, Akagi Y, Kinugasa T, Ishibashi Y, Tanaka N, Fujino S, Kibe S, Yuge K, Sasatomi T, Mizobe T. Virchow lymph node metastatic recurrence of sigmoid colon cancer with severe lymph node metastases successfully treated using systemic chemotherapy combined with radiotherapy. Anticancer Res. 2013;33:2935-2940. [PubMed] [Cited in This Article: ] |