Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1182
Peer-review started: June 8, 2014
First decision: July 9, 2014
Revised: July 24, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: January 28, 2015
AIM: To investigate rates of re-establishing gastroenterology care, colonoscopy, and/or initiating medical therapy after Crohn’s disease (CD) surgery at a tertiary care referral center.
METHODS: CD patients having small bowel or ileocolonic resections with a primary anastomosis between 2009-2012 were identified from a tertiary academic referral center. CD-specific features, medications, and surgical outcomes were abstracted from the medical record. The primary outcome measure was compliance rates with medical follow-up within 4 wk of hospital discharge and surveillance colonoscopy within 12 mo of surgery.
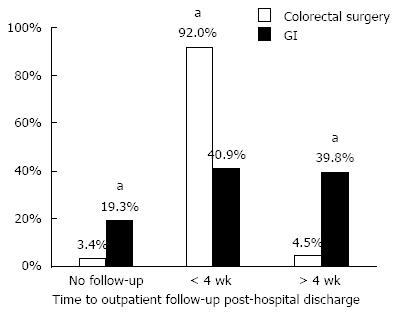
RESULTS: Eighty-eight patients met study inclusion criteria with 92% (n = 81) of patients returning for surgical follow-up compared to only 41% (n = 36) of patients with documented gastroenterology follow-up within four-weeks of hospital discharge, P < 0.05. Factors associated with more timely postoperative medical follow-up included younger age, longer length of hospitalization, postoperative biologic use and academic center patients. In the study cohort, 75.0% of patients resumed medical therapy within 12 mo, whereas only 53.4% of patients underwent a colonoscopy within 12 mo of surgery.
CONCLUSION: Our study highlights the need for coordinated CD multidisciplinary clinics and structured handoffs among providers to improve of quality of care in the postoperative setting.
Core tip: Adherence to evidence based management of patients with Crohn’s disease requires care coordination and communication between surgeons and gastroenterologists. Surgeons need to facilitate return visits after surgery to the gastroenterologists.
- Citation: Bennett JL, Ha CY, Efron JE, Gearhart SL, Lazarev MG, Wick EC. Optimizing perioperative Crohn's disease management: Role of coordinated medical and surgical care. World J Gastroenterol 2015; 21(4): 1182-1188
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1182
Most patients with Crohn’s disease will require surgical intervention during the course of their disease[1,2]. Despite the high lifetime risk of surgery, surgical resection is not curative and disease recurrence is relatively common. As a result, the decision to pursue surgical treatment for Crohn’s disease is highly personalized. Historically, prophylactic medical therapy after surgery was not routinely recommended and instead, patients were managed expectantly, with initiation of treatment at the time of symptoms recurrence. Emerging evidence suggests that early postoperative consideration of medical therapy, especially for higher-risk patients, to prevent Crohn’s disease recurrence may obviate the need for additional operations[3,4].
In Crohn’s disease, postoperative disease recurrence is the norm. Within the first year of surgery, 70%-90% of patients develop endoscopic recurrence and within three years recurrence rates increase to 80%-100%[2,5,6]. Although clinical or symptomatic recurrence occurs in up to 30% of patients with a 10% increase each additional year, subjective manifestations of Crohn’s disease may lag behind objective evidence of disease recurrence based on endoscopy[6,7]. Severity of early endoscopic lesions can predict the symptomatic course of disease after surgery. Therefore, postoperative surveillance evaluating for early endoscopic recurrence is helpful for identifying patients who will benefit from early, aggressive medical management[8]. As an example, early use of Infliximab after surgery has been demonstrated to significantly decrease the risk of endoscopic disease in patients with a history of multiple surgeries, stricturing, or penetrating disease[9]. Based on this and other emerging data on the benefits of aggressive postoperative medical management, recommendations for the postoperative management of Crohn’s disease have been updated to include timely initiation of biologics or immunomodulators[10]. As Crohn’s disease can recur starting almost immediately after surgical resection, particularly among higher-risk patients, recommendations are to initiate or resume medical therapy within the first 4 wk after ileal resection provided the postoperative recovery is unremarkable. Evaluation with repeat colonoscopy during the 6-12 mo after surgery to establish the presence of early disease recurrence is a cornerstone of this algorithm[10].
Little is known about the barriers of reestablishing care with gastroenterologists, early colonoscopy, and/or initiation of postoperative medical therapy after surgery for Crohn’s disease in an academic surgery setting with referrals from both within the health system and from the community. We hypothesized that patient, provider, and system-related factors impact a patient’s adherence to current postoperative medical management prophylaxis recommendations. Identification of these factors may guide quality improvement efforts aimed at improving the care of Crohn’s patients and support the use of coordinated multidisciplinary care in this patient population.
We performed a single-center retrospective cohort study of Crohn’s disease patients who underwent small bowel or ileocolonic resection surgery at the Johns Hopkins Hospital between January 2009 and January 2012. Patients who did not have a primary anastomosis at the time of operation were excluded, as surveillance recommendations do not apply to patients without an anastomosis. Additionally, patients with primary colon surgery (e.g., colectomies) and incomplete postoperative data for review were excluded from evaluation. The Johns Hopkins University Institutional Review Board approved this study.
Patient, procedure, and 30-d outcome information was abstracted from hospital’s National Surgical Quality Improvement Program (NSQIP) database[11]. Specific information related to the patients gastroenterology care (provider location, office and colonoscopy visit dates and reports, medical therapy, providers, and past medical history including smoking history) were abstracted from the electronic health record by two clinicians (JB and CH). Colonoscopy reports were reviewed; preoperative Inflammatory Bowel Disease phenotypic information using the Montreal classification and Rutgeerts scores for postoperative surveillance colonoscopies were assigned (CH)[12,13]. Disease recurrence was classified as a Rutgeerts score of i2 or greater.
The majority of patients received their Crohn’s disease care at two hospitals in the Johns Hopkins Health System (Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center). For these patients, complete medical records were available in the health system electronic medical records. For patients referred for surgical resection from non-Johns Hopkins gastroenterologists, written consent to review pre and postoperative gastroenterology office and colonoscopy records was obtained. Consent and medical records were obtained from 44.1% (15/34) of patients receiving care from non-Johns Hopkins gastroenterology practices. Records were reviewed from the 12 mo prior to and following the operation date.
The primary goal of the study was to determine compliance with medical follow-up within 4 wk of hospital discharge and surveillance colonoscopy within 12 mo of surgery.
Descriptive statistics were reported as percentages, means, and standard deviations of the mean. All continuous variable comparisons were unpaired, and all tests of significance were two-tailed. Continuous variables were compared using the Student t test for normally distributed variables. The Fisher’s exact test was used for comparison of categorical variable with a P-value ≤ 0.05 considered as statistically significant. Statistical analyses were performed using SPSS version 20, IBM Corp.
We identified 88 patients with Crohn’s disease who underwent resectional surgery with a primary anastomosis between January 2009 and January 2012. The average age at time of operation was 40.0 ± 14.4 years and at Crohn’s disease diagnosis was 26.2 ± 12.5 years. Twenty two patients (26%) reported smoking cigarettes at the time of surgery and 11 patients (13%) were former smokers. Surgery was performed for non-stricturing non-penetrating disease (n = 3, 3.4%), stricturing disease (n = 55, 62.5%), and penetrating disease (n = 30, 34.0%). Forty percent (n = 38) of patients had prior intestinal resections for strictures or abdominal abscesses. Most patients were receiving medical therapy at the time of surgery: 43% (n = 38) anti-tumor necrosis-alpha (anti-TNF) therapy, 22% immunomodulators (n = 19), and 40% steroids (n = 35). About a quarter (27%, n = 24) of patients were not on immunosuppression preoperatively and 17% (n = 15) were receiving total parenteral nutrition.
Almost all patients returned for surgical follow-up (n = 81, 92%) within a four-week window following hospital discharge. However, only 41% (n = 36) of patients returned to their medical provider during this interval (Figure 1). Patients who had established medical care at Johns Hopkins Hospital prior to surgery, were being treated with TNF-alpha inhibitors before surgery, were younger patients, and had a longer length of stay at the time of operation were more likely to re-establish medical care (Table 1).
| Demographics | 4 wk follow-up with GI (n = 36) | > 4 wk follow-up with GI (n = 35) | No follow-up or lost to GI follow-up (n = 17) |
| Male | 24 (67) | 18 (51) | 6 (35) |
| Receiving all care at Johns Hopkins Hospital | 22 (61)a | 22 (63) | 4 (24) |
| Age at operation | 33.6 +/- 10.8ac | 44.5 +/- 15.2 | 44.5 +/- 14.9 |
| Laparoscopic Technique | 20 (56) | 21 (60) | 5 (29) |
| Preoperative Admission within < 60 d | 11 (31) | 6 (17) | 4 (24) |
| Current smoker | 8 (22) | 13 (37) | 2 (12) |
| Disease phenotype | |||
| B1 | 1 (2.8) | 1 (2.9) | 1 (5.9) |
| B2 Stricturing | 19 (53) | 24 (69) | 12 (71) |
| B3 Penetrating | 16 (44) | 10 (29) | 4 (24) |
| Perianal disease | 4 (11) | 6 (17) | 4 (24) |
| Preoperative Therapy | |||
| Preoperative antibiotics | 16 (44) | 10 (29) | 4 (24) |
| Preoperative ASA | 19 (53) | 19 (54) | 8 (47) |
| Preoperative Steroids | 17 (47) | 13 (37) | 5 (29) |
| Preoperative IMM | 9 (25) | 5 (14) | 5 (29) |
| Preoperative anti-TNF | 20 (56)c | 11 (31) | 7 (41) |
| Preoperative TPN | 9 (25) | 3 (8.6) | 3 (18) |
| Mean Length of Hospital Stay | 8.5 +/- 5.5c | 6.5 +/- 5.5 | 9.8 +/- 4.9 |
| Postoperative Readmission within 30 d | 6 (167) | 4 (11) | 4 (24) |
| Any Postoperative Complication | 6 (17) | 8 (23) | 1 (5.9) |
| Postoperative Medical Complication | 5 (14) | 7 (20) | 1 (5.9) |
| Postoperative Surgical Complication | 2 (5.6) | 3 (8.6) | 0 (0) |
A subgroup analysis of patients with Crohn’s disease stratified by risk factors for postoperative recurrences is summarized in Table 2. All patient subgroups had lower rates of postoperative gastroenterology follow-up compared to colorectal surgery follow-up. In all patient subgroups, the rate of follow-up to colorectal surgery exceeded 90%, while rates of follow-up to gastroenterology varied among subgroups from only 34.8% to 53.3%. The subgroup with the lowest gastroenterology follow-up was current smokers. They also had the lowest rate of return to colorectal surgery.
| Postoperative follow-up at 4 wk | Postoperative 12 mo | ||||
| With GI | With colorectal surgery | P-value | Colonoscopy | Medical therapy resumption | |
| Overall (n = 88) | 36 (40.9) | 81 (92.0) | < 0.0001 | 47 (53.4) | 66 (75.0) |
| Patient subgroups: | |||||
| Less than 10 yr since diagnosis or B2 disease (n = 35) | 13 (37.1) | 33 (94.3) | < 0.0001 | 20 (57.1) | 18 (51.4) |
| 2 or more surgeries (n = 38) | 16 (42.1) | 36 (94.7) | < 0.0001 | 21 (84.0) | 25 (65.8) |
| B3 disease (n = 30) | 16 (53.3) | 28 (93.3) | 0.0009 | 15 (50.0) | 16 (53.3) |
| Current smokers (n = 23) | 8 (34.8) | 21 (91.3) | 0.0001 | 14 (60.9) | 17 (73.9) |
| Preoperative anti-TNF (n = 38) | 20 (52.6) | 35 (92.1) | 0.0002 | 20 (52.6) | 25 (65.8) |
| Preoperative Immunomodulator (n = 19) | 9 (47.4) | 19 (100.0) | 0.0004 | 10 (52.6) | 12 (63.2) |
| Preoperative steroids (n = 35) | 17 (48.6) | 33 (94.3) | < 0.0001 | 20 (57.1) | 26 (74.3) |
Overall, about half of all patients (n = 47, 53.4%) received their postoperative surveillance colonoscopy within 12 mo. The subgroup of patients with two or more prior surgeries was most likely to have a postoperative colonoscopy (n = 21, 84%) compared to the remaining subgroups. Among patients whom underwent a postoperative colonoscopy by 12 mo, 27% had evidence of endoscopic recurrence with a Rutgeerts i2 score or higher.
Even though early postoperative gastroenterology follow-up was low among our study cohort, most of patients (n = 66, 75%) resumed or initiated at least one type of Crohn’s disease medication by 12 mo: 48.9% (n = 43) on anti-TNFs, 13.6% (n = 12) on immunomodulators, and 21.6% (n = 19) on antibiotics. However, 8% (n = 7) were started on corticosteroids and 30.7% (n = 27) on 5-aminosalycilatesaminosalicylates, despite having a minimal role in postoperative Crohn’s disease management.
Crohn’s disease postoperative management is a complex interplay of coordination between patients, gastroenterologists, and surgeons. From the patient perspective, postoperative follow-up care is involved and the role of complementary providers can be confusing, particularly with respect to care coordination. In our study, we chose four-weeks as a primary endpoint for clinic follow-up based on the studies that investigated starting postoperative therapy within four-weeks of surgery[9]. The 12-mo cut-off for colonoscopy was based on the recommendation to undergo surveillance colonoscopy within 6-12 mo of surgery depending on disease behavior[14]. Our study demonstrates a substantial discrepancy in follow-up clinic visit rates to surgeons compared to gastroenterologists, driving the call to address gastroenterology follow-up for operative patients and motivating providers to redefine Crohn’s disease perioperative management. The necessity of gastroenterology follow-up may be undermined by the overall sense of wellness Crohn’s patients may feel following surgery, with substantially less disease burden following a surgically-induced “remission.” It has been well documented that adherence to therapy and patients’ perceptions regarding the need for continued therapy decreases during the maintenance of remission phase[15,16].
As we have discovered, the majority of patients returned to their surgeons for a postoperative appointment. The decision for surgical intervention is best made with the gastroenterologist and surgeon in conjunction with the patient[17]. Thereby, the preoperative integration of surgery and gastroenterology should be continued into the postoperative period[17]. The need for surgical follow-up in the postoperative period may be more apparent to patients who develop postoperative complications, as they do not return to their baseline sense of wellness as rapidly as those who do not experience complications. Patients may be unaware of any disease recurrence until it progresses further as clinical symptoms of Crohn’s disease are often absent with endoscopic recurrence[18]. The absence of symptoms underscores the importance of close gastroenterology follow-up, yet patients are less likely to accept treatment risks when their symptoms are minimal[19,20]. Patients need to be well-informed on their options for postoperative therapy as it tends to be a long-term commitment and patients dissatisfied with their medication may discontinue them or seek care from another provider[21]. Conversations and structured education prior to surgery about postoperative medical therapy is a prime opportunity to reiterate that surgery is not curative and restarting medical therapy postoperatively at some point should be an expectation.
In understanding the discrepancy in follow-up to surgeons compared to gastroenterologists in our data, we acknowledge the limitations in our ability to examine detailed reasons for the lack of gastroenterology follow-up. Patients may have been given follow-up appointments but may have cancelled or failed to appear. We also acknowledge that lack of a follow-up appointment does not inherently indicate that a patient is not receiving disease and medication counseling by their gastroenterologist as our data shows a sizable proportion of patients on postoperative medications by 12 mo. Furthermore, our study was not sufficiently powered to establish if medical follow-up/early resumption of medications impacted disease recurrence or Rutgeerts scores.
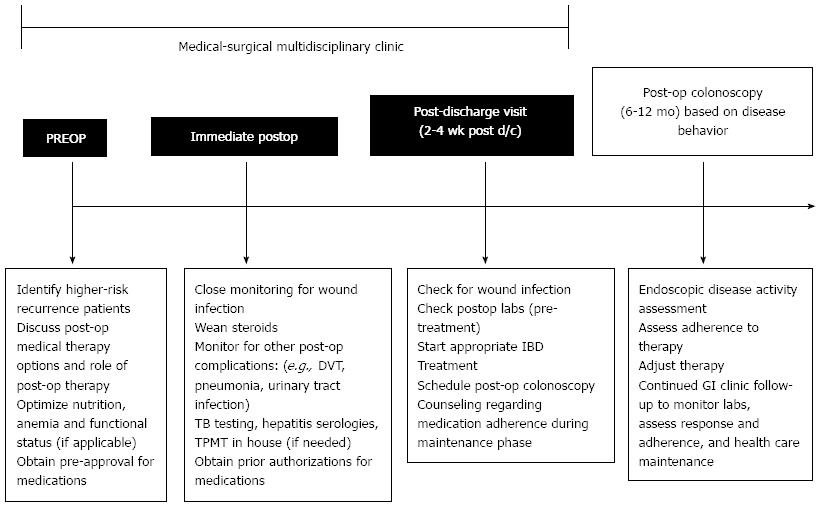
Despite our institution being a tertiary referral center, we feel our results are likely to reflect many institutions in the United States. Inefficiencies within the increasingly more complicated and disjointed healthcare system along with lack of coordination between healthcare providers has led to higher costs, errors and complications[22,23]. The Institute of Healthcare Improvement has suggested the creation of multidisciplinary teams along with effective teamwork and communication as means to decrease patient harm and mortality, improve patient satisfaction, ensure reliable evidence-based care is provided without gaps in care by race, ethnicity or language[24]. In other fields, the creation and implementation of multidisciplinary teams have improved the quality of care provided for patients and patient outcomes[22,25,26]. A Cochrane Review found evidence to support practice-based interprofessional collaborations can improve healthcare processes and outcomes[27]. The multidisciplinary clinic model should be considered as the new standard for perioperative Crohn’s disease management as outlined in Figure 2. Although the ability to create a multidisciplinary clinic may be influenced by the patient care setting and the resources available to fund a multidisciplinary clinic, the coordination of care is an important improvement for perioperative Crohn’s management. Structured handoffs between providers should be considered for patients seeking care from multiple institutions. Other groups have also endorsed the effectiveness of multidisciplinary clinics and proposed multidisciplinary clinics as the new standard of care at academic centers for inflammatory bowel disease patients[28-31].
We view our data as an introduction into the issues and barriers surrounding the postoperative management of operative Crohn’s disease patients. Effective multidisciplinary care will allow gastroenterology and surgery to take joint responsibility for the care of Crohn’s patients and ultimately improve the quality of perioperative management and maintain care continuity.
The authors thank Lucy Mitchell, RN, MA for her assistance in data acquisition through ACS NSQIP.
Emerging evidence suggests that early resumption of medical therapy after surgery for Crohn’s disease (CD) can prevent recurrence. In order for patients to resume medical therapy, their care needs to be coordinated between their surgeon’s and gastroenterologist’s office.
Guidelines suggest early colonoscopy and resumption of medical therapy can prevent CD recurrence after surgery. Multidisciplinary, team-based practices can facilitate care.
CD care is complex and without structured handoffs and communications between surgeons and gastroenterologists frequently gastroenterology followup and resumption of medical therapy is delayed.
Hospitals and practices should consider structured multidisciplinary care and handoff tools for optimal management of CD patients.
This is honest assessment of CD care at a major academic medical center. It highlights areas that need to be targeted for improvement to adhere to new treatment guidelines.
P- Reviewer: Klinge U S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 443] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Caprilli R, Gassull MA, Escher JC, Moser G, Munkholm P, Forbes A, Hommes DW, Lochs H, Angelucci E, Cocco A. European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations. Gut. 2006;55 Suppl 1:i36-i58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Moss AC. Prevention of postoperative recurrence of Crohn’s disease: what does the evidence support? Inflamm Bowel Dis. 2013;19:856-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Hashash JG, Regueiro MD. The evolving management of postoperative Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2012;6:637-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 346] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Rutgeerts PJ. From aphthous ulcer to full-blown Crohn’s disease. Dig Dis. 2011;29:211-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956-963. [PubMed] [Cited in This Article: ] |
| 8. | Nos P, Domenech E. Postoperative Crohn’s disease recurrence: a practical approach. World J Gastroenterol. 2008;14:5540-5548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, Harrison J, Plevy SE. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology. 2009;136:441-450.e1; quiz 716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 412] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 10. | Regueiro M. Management and prevention of postoperative Crohn’s disease. Inflamm Bowel Dis. 2009;15:1583-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | American Cancer Society. NSQIP. Available from: http://site.acsnsqip.org/program-specifics. [Cited in This Article: ] |
| 12. | Vermeire S, Van Assche G, Rutgeerts P. Classification of inflammatory bowel disease: the old and the new. Curr Opin Gastroenterol. 2012;28:321-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] [Cited in This Article: ] |
| 14. | Ng SC, Kamm MA. Management of postoperative Crohn’s disease. Am J Gastroenterol. 2008;103:1029-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Kane S, Dixon L. Adherence rates with infliximab therapy in Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1099-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010;105:525-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Ricci C, Lanzarotto F, Lanzini A. The multidisciplinary team for management of inflammatory bowel diseases. Dig Liver Dis. 2008;40 Suppl 2:S285-S288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 535] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Siegel CA. Shared decision making in inflammatory bowel disease: helping patients understand the tradeoffs between treatment options. Gut. 2012;61:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Siegel CA. Making therapeutic decisions in inflammatory bowel disease: the role of patients. Curr Opin Gastroenterol. 2009;25:334-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Kennedy ED, To T, Steinhart AH, Detsky A, Llewellyn-Thomas HA, McLeod RS. Do patients consider postoperative maintenance therapy for Crohn’s disease worthwhile? Inflamm Bowel Dis. 2008;14:224-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Roussel MG, Gorham N, Wilson L, Mangi AA. Improving recovery time following heart transplantation: the role of the multidisciplinary health care team. J Multidiscip Healthc. 2013;6:293-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Available from: http://www.qualityforum.org/Topics/Effective_Communication_and_Care_Coordination.aspx. [Cited in This Article: ] |
| 24. | Available from: http://app.ihi.org/imap/tool/imap.html. [Cited in This Article: ] |
| 25. | Jung H, Sinnarajah A, Enns B, Voroney JP, Murray A, Pelletier G, Wu JS. Managing brain metastases patients with and without radiotherapy: initial lessonsfrom a team-based consult service through a multidisciplinary integrated palliative oncology clinic. Support Care Cancer. 2013;21:3379-3386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Wensing M, Wollersheim H, Grol R. Organizational interventions to implement improvements in patient care: a structured review of reviews. Implement Sci. 2006;1:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Zwarenstein M, Goldman J, Reeves S. Interprofessional collaboration: effects of practice-based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;CD000072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 430] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 28. | Ghosh S. Multidisciplinary teams as standard of care in inflammatory bowel disease. Can J Gastroenterol. 2013;27:198. [PubMed] [Cited in This Article: ] |
| 29. | Mekechuk J, Dieleman LA. Are clinical outcomes in IBD improved by multidisciplinary clinics? Inflamm Bowel Dis. 2008;14 Suppl 2:S65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Windsor A, Forbes A. Is the multidisciplinary team essential for the future management of patients with inflammatory bowel disease? Colorectal Dis. 2007;9:478-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Nicholls J. The inflammatory bowel disease unit and the multidisciplinary team meeting. Colorectal Dis. 2007;9:477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |