Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1173
Peer-review started: May 2, 2014
First decision: June 10, 2014
Revised: July 23, 2014
Accepted: September 12, 2014
Article in press: September 16, 2014
Published online: January 28, 2015
AIM: To evaluate the effect of gastrectomy on diabetes mellitus (DM) and hypertension (HTN) in non-obese gastric cancer patients.
METHODS: A total of 100000 patients, diagnosed with either type 2 DM or HTN, were randomly selected from the 2004 Korean National Health Insurance System claims. Among them, 360 diabetes and 351 hypertensive patients with gastric cancer who had been regularly treated without chemotherapy from January 2005 to December 2010 were selected. They were divided into three groups according to their treatment methods: total gastrectomy (TG), subtotal gastrectomy (STG) and endoscopic resection (ER).
RESULTS: The drug discontinuation rate of anti-diabetic and anti-hypertensive agents after gastric cancer treatment was 9.7% and 11.1% respectively. DM appeared to be improved more frequently (22.8%) and earlier (mean ± SE 28.6 ± 1.8 mo) in TG group than in the two other groups [improved in 9.5% of ER group (37.4 ± 1.1 mo) and 6.4% of STG group (47.0 ± 0.8 mo)]. The proportion of patients treated with multiple drugs decreased more notably in TG group compared to others (P = 0.001 in DM, and P = 0.035 in HTN). In TG group, adjusted hazard ratio for the improvement of DM was 2.87 (95%CI: 1.15-7.17) in a multi-variate analysis and better control of DM was observed with survival analysis (P < 0.001).
CONCLUSION: TG was found to decrease the need for anti-diabetic medications which can be reflective of improved glycemic control, to a greater extent than either ER or STG in non-obese diabetic patients.
Core tip: By following the long term outcome of diabetes and hypertension after gastrectomy, we have discovered that total gastrectomy has a profound impact on the improvement of both diabetes and hypertension compared with endoscopic resection in non-obese population.
- Citation: Lee EK, Kim SY, Lee YJ, Kwak MH, Kim HJ, Choi IJ, Cho SJ, Kim YW, Lee JY, Kim CG, Yoon HM, Eom BW, Kong SY, Yoo MK, Park JH, Ryu KW. Improvement of diabetes and hypertension after gastrectomy: A nationwide cohort study. World J Gastroenterol 2015; 21(4): 1173-1181
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1173.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1173
Gastric cancer is the fourth most common cancer and the second leading cause of cancer death in the world[1] with the highest mortality rates reported in Eastern Asia. Although the worldwide incidence of gastric cancer has been declining, its prevalence still remains high in South Korea[2] with the proportion of early gastric cancer (EGC) reported to have increased[3]. The 5-year survival rate of EGC has reached 90% or higher, resulting in the increase of long-term survivors. Thus, the quality of life after cancer treatment has emerged as an important health factor for gastric cancer patients, especially for those suffering with additional chronic diseases such as diabetes mellitus (DM) and hypertension (HTN).
DM and HTN are two of the most common chronic diseases, which increase the risk of vascular complications such as stroke, myocardial infarction, and renal failure. There is a growing concern about striking increase of these two diseases. The Asian population is not exceptional[4]; the age-standardized prevalence of DM was 9.7% in China[5] and 9.1% in South Korea[6], while the age-standardized prevalence of HTN was 25.3% in Korean adults 20 years or older[7]. The prevalence of DM in patients with gastric cancer was not different from that in general population; Ogle et al[8] reported comorbid DM was 8% of patients with gastric cancer using data from National Cancer Institute Surveillance, Epidemiology, and End Results program and Sarfati et al[9] reported DM with complication occurred in 10% of gastric cancer patients using administrative data from New Zealand Cancer Registry.
Obesity is one of the most important factors commonly contributing to an increased risk of DM and HTN. Thus, obesity has become a major public health concern and incurred enormous socioeconomic costs. Fortunately, several studies suggest that bariatric surgery results in a remarkable improvement for the obesity epidemic and it has been recently recommended that surgery (preferably the Roux-en-Y gastric bypass procedure) should be considered as an appropriate treatment for obese diabetic patients[10]. However, there are only a few large population-based studies conducted on non-obese population[11]. In addition, most of the studies compared metabolic changes before and after bariatric surgery, lacking an appropriate control enough to exclude the effect of dietary-habit changes. In this work, we assessed the effect of gastrectomy on DM and HTN compared to the control group of endoscopic resection using a nationwide data.
The Korean National Health Insurance System (KNHI) program is a mandatory social insurance, covering approximately 97% of the Korean population. The database includes all information for the purpose of reimbursement, such as sex, age, residential area, diseases, prescription history and survival outcome. The KNHI claims database has been analyzed for epidemiological[12,13] and health policy studies[14].
From the KNHI claims database, we identified 1314168 type 2 DM and 2667621 HTN patients who had been treated with either condition between the dates of January 1, 2004, through December 31, 2004. The data from January 1, 2005 through December 31, 2010 were collected including patient sociodemographic information, specific target diseases (DM, HTN, and gastric cancer), surgical procedures (total gastrectomy, subtotal gastrectomy and endoscopic resection), prescriptions (anti-hypertensive, anti-diabetics and chemotherapy drugs), and deaths. Patients of both DM and HTN were analyzed separately for each disease. Patients were grouped into one of the three categories by residential area (rural, urban, and metropolitan) according to their Korean ZIP code, since regional difference could affect medical behavior.
We extensively reviewed prescription history including the duration and the types of drugs; all drugs used for treatment of DM were classified into one of the following groups: sulfonylurea, metformin, thiazolidinedione, α-glucosidase inhibitor and insulin, while drugs used for treatment of HTN were classified into the groups as “calcium channel blocker, diuretics, beta-blocker, angiotensin converting enzyme inhibitor, angiotensin receptor blocker”. This study was approved by the Institutional Review Board of the National Cancer Center, South Korea (IRB No. NCCNCS-12-563). Informed consent was waived since the study was based on routinely collected administrative data.
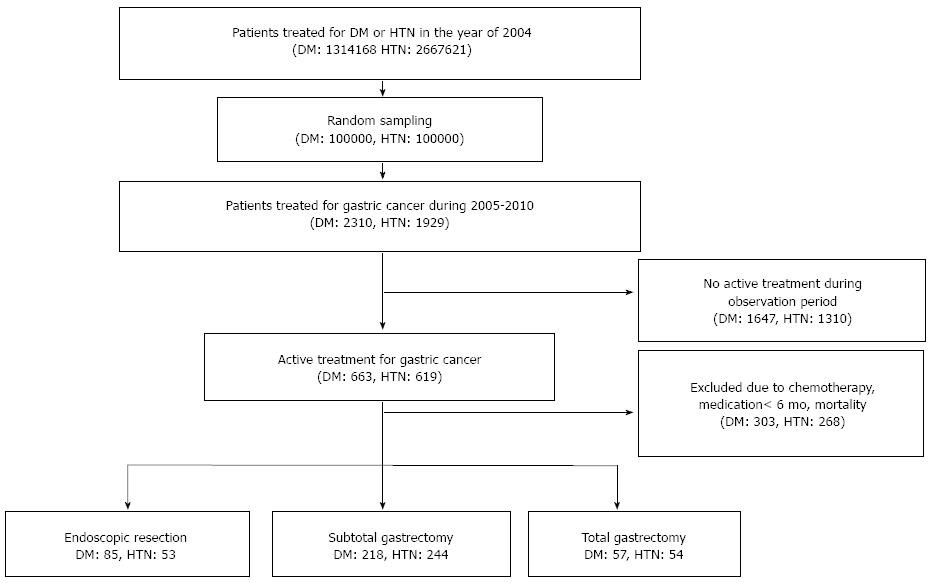
Once DM and HTN patients were identified from the KNHI claims database, we randomly sampled 100000 subjects for each disease (Figure 1). After sampling, we selected the patients with gastric cancer (code C16), according to the International Classification of Diseases, Tenth Edition, Clinical Modification (ICD-10-CM) and excluded patients who did not undergo active gastric cancer treatment during the follow-up period. In addition, to confine study population to the patients with early stage of gastric cancer and to be unable to assess nutritional status and effects of chemotherapy on DM or HTN, subjects who were treated with chemotherapeutic agents were excluded. Mortality cases in the observational periods were also excluded.
Finally, the remaining patients were divided into three groups: total gastrectomy (TG), subtotal gastrectomy (STG) and endoscopic resection (ER). The endoscopic resection procedure included both endoscopic mucosal resection and endoscopic submucosal dissection. Patients were considered to have shown an improvement of DM or HTN if anti-diabetic or anti-hypertensive drugs had not been prescribed for a minimum of 6 mo. Relapses were considered to have occurred if anti-hypertensive or anti-diabetic drugs were resumed for three months or more after remission.
Descriptive analyses were performed to identify patient demographics and procedural distributions. Continuous variables were analyzed by using Student’s unpaired two-sided t-test, whereas χ2 approximations were performed for discrete variables such as differences in patient characteristics and remission rates in each group. Categorical variables were analyzed by Fisher’s exact test. Survival analysis was used to determine the time from cancer treatment to discontinuation of drugs for DM or HTN. The cumulative probabilities of remission and the survival curves of disease relapse were obtained with Kaplan-Meier estimates. The Wilcoxon two-sample test was also used to assess whether the effect of each procedure on remission was stronger in the earlier phases of administration or became less effective over time[15].
After adjusting for age, sex and coexisting disease (DM or HTN), the effects of each procedure were evaluated using Cox’s proportional hazard regression analyses. Proportionality assumption was examined by log-log plots of the hazard estimates against follow-up time and was considered satisfactory. Schoenfeld residuals for each adjusted co-variate indicated that the proportional-hazard assumption was met (P > 0.05). The proportional changes of multiple drug medication after treatment were compared using the generalized linear mixed model. Statistical analyses were performed by SAS (version 9.2; SAS Institute Inc, Cary, North Carolina), with the predetermined upper limit of probability set at P < 0.05.
Among 100000 randomly sampled patients, 2310 diabetics and 1929 hypertensive patients who were treated for gastric cancer from January 2005 to December 2010 were identified. Patients who did not undergo active treatment during observation period (1647 diabetics and 1310 hypertensive patients) and who were dead, were ever prescribed chemotherapeutic agents, or treated with anti-diabetic or anti-hypertensive agents for less than 6 mo (303 diabetics and 268 hypertensive patients) were excluded. Finally, 360 diabetics and 351 hypertensive patients were included in this study (Figure 1). For the diabetic patients, TG was performed in 57, STG in 218, and ER in 85. In the case of HTN, TG was performed in 54 patients, STG in 244 patients, and ER in 53 patients. In comparison among three treatment groups, no difference was found for age, residential area, or the rate of coexisting DM or HTN; only the sex ratio differed between the treatment groups of the diabetic patients (Tables 1 and 2). There were also no differences in the number or class of anti-hypertensive drug at the time of gastric cancer treatment; for the diabetic patients, only the rate of patients on insulin differed between the treatment groups.
| Endoscopic resection (n = 85) | Subtotal gastrectomy (n = 218) | Total gastrectomy (n = 57) | P | |
| Male | 39 (45.9) | 142 (65.1) | 40 (70.2) | 0.0031 |
| Age at treatment (yr, mean ± SE) | 64.9 ± 7.4 | 65.1 ± 8.7 | 64.1 ± 8.5 | 0.74 |
| Comorbid HTN | 57 (67.1) | 120 (55.0) | 29 (50.9) | 0.09 |
| Diabetes medication | ||||
| Sulfonylurea (S) | 72 (84.7) | 178 (81.7) | 47 (82.5) | 0.82 |
| Metformin (M) | 42 (49.4) | 126 (57.8) | 30 (52.6) | 0.39 |
| α-glucosidase inhibitor | 20 (23.5) | 45 (20.6) | 19 (33.3) | 0.13 |
| Thiazolidinedione (T) | 8 (9.4) | 16 (7.3) | 1 (1.8) | 0.20 |
| Insulin | 54 (63.5) | 181 (83.0) | 47 (82.5) | 0.0011 |
| Combination (M, S) | 3 (3.5) | 5 (2.3) | 2 (3.5) | 0.73 |
| Combination (M, T) | 1 (1.2) | 2 (0.9) | 0 (0.0) | 1.00 |
| Others | 1 (1.2) | 9 (4.1) | 1 (1.8) | 0.48 |
| Diabetes medications (n) | ||||
| 1 | 12 (14.1) | 22 (10.1) | 6 (10.5) | 0.24 |
| 2 | 38 (44.7) | 79 (36.2) | 23 (40.4) | |
| 3 | 25 (29.4) | 85 (39.0) | 15 (26.3) | |
| ≥ 4 | 10 (11.8) | 32 (14.7) | 13 (22.8) | |
| Endoscopic resection (n = 53) | Subtotal gastrectomy (n = 244) | Total gastrectomy (n = 54) | P | |
| Male | 36 (67.9) | 153 (62.7) | 41 (75.9) | 0.17 |
| Age at treatment (yr, mean ± SE) | 65.2 ± 8.1 | 65.8 ± 8.5 | 66.3 ± 9.3 | 0.79 |
| Comorbid DM | 5 (9.4) | 46 (18.9) | 13 (24.1) | 0.13 |
| Cardiovascular medication | ||||
| Lipid lowering agent | 8 (15.1) | 52 (21.3) | 13 (24.1) | 0.49 |
| Antithrombotic agent | 22 (41.5) | 93 (38.1) | 22 (40.7) | 0.87 |
| Other | 1 (1.9) | 5 (2.0) | 2 (3.7) | 0.85 |
| Antihypertensive agent | ||||
| Calcium channel blocker (C) | 41 (77.4) | 166 (68.0) | 33 (61.1) | 0.19 |
| Diuretics (D) | 19 (35.8) | 92 (37.7) | 21 (38.9) | 0.95 |
| Beta-blocker (B) | 19 (35.8) | 76 (31.1) | 17 (31.5) | 0.80 |
| ACEi or ARB (A) | 15 (28.3) | 82 (33.6) | 18 (33.3) | 0.75 |
| Combination (A, D) | 9 (17.0) | 41 (16.8) | 5 (9.3) | 0.37 |
| Combination (B, D) | 0 (0.0) | 9 (3.7) | 1 (1.9) | 0.54 |
| Combination (A, C) | 0 (0.0) | 6 (2.5) | 3 (5.6) | 0.19 |
| Antihypertensive medications (n) | ||||
| 1 | 18 (33.3) | 73 (29.9) | 16 (30.2) | 0.70 |
| 2 | 25 (46.3) | 97 (39.8) | 22 (41.5) | |
| ≥ 3 | 11 (20.4) | 74 (30.3) | 15 (28.3) | |
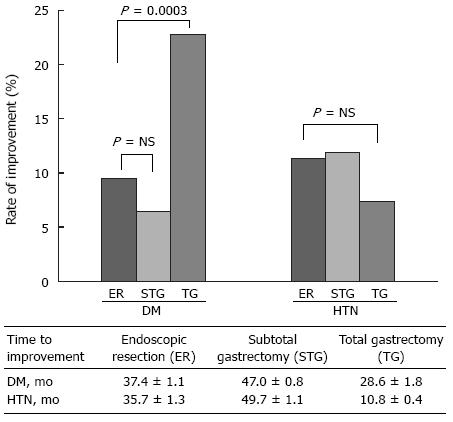
During follow-up period of median 36.7 mo in DM and 36.8 mo in HTN, approximately 10% of patients discontinued the anti-diabetics or anti-hypertensive drugs (9.7% in DM and 11.1% in HTN). Patients in TG group discontinued anti-diabetic drugs more often (ER 9.5%, STG 6.4% and TG 22.8%; P = 0.0003) and earlier [time to discontinue (means ± SE); 37.4 ± 1.1 mo in ER, 47.0 ± 0.8 mo in STG, and 28.6 ± 1.8 mo in TG] than patients in STG or ER groups (Figure 2). However, there was no difference in the ratio of patients showing improvement of HTN among the three groups (ER 11.3%, STG 11.9% and TG 7.4%).
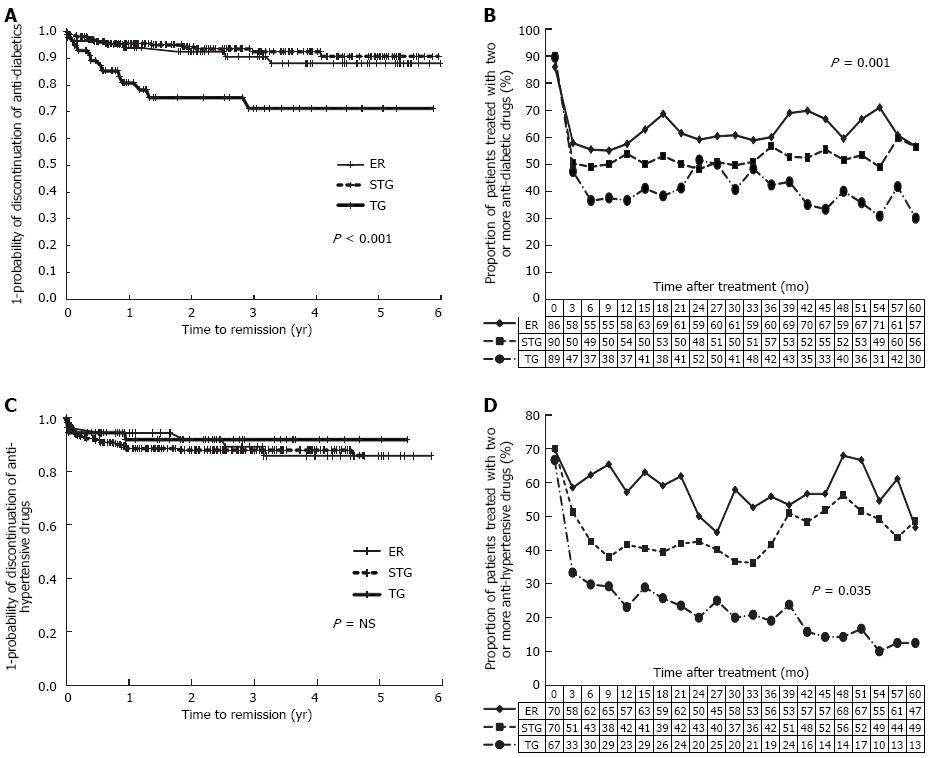
With survival analysis, the probability of improvement of DM was greater in TG group (Figure 3A) and the proportion of patients who were prescribed two or more anti-diabetic drugs was decreased from 89% to 30% after TG, from 90% to 56% after STG and from 86% to 57% after ER, which were all statistically significant (P = 0.001) (Figure 3B). The probabilities of improvement of HTN were not different among three groups with survival analysis (Figure 3C). However, the proportion of patients treated with two or more anti-hypertensive drugs was lowered in TG group than other two groups (P = 0.035) (Figure 3D).
Cox-proportional multi-variate regression analysis, adjusted for age, sex and comorbid HTN, indicated that total gastrectomy was significantly related to improvement of DM, when compared to ER group [control; adjusted hazard ratio (aHR), 2.87, 95%CI: 1.15-7.17] (Table 3). In contrast, the extent of surgical procedure was not related to improvement of HTN.
| Prescription history | Diabetic patients | Hypertensive patients | ||
| HR | 95%CI | HR | 95%CI | |
| Discontinuation | ||||
| Endoscopic resection | 1.00 | 1.00 | ||
| Subtotal gastrectomy | 0.70 | 0.29-1.71 | 1.01 | 0.41-2.46 |
| Total gastrectomy | 2.87a | 1.15-7.17 | 0.63 | 0.18-2.27 |
| Resumption | ||||
| Endoscopic resection | 1.00 | 1.00 | ||
| Subtotal gastrectomy | 5.21a | 1.64-16.60 | 2.19 | 0.65-7.39 |
| Total gastrectomy | 0.93 | 0.31-2.79 | 1.10 | 0.23-5.17 |
The resumption rate of anti-diabetic drug was significantly lower (P = 0.001) in TG group (69.2%) than in either STG (92.9%) or ER groups (75.0%). However, hypertensive patients relapsed in similar patterns among all three groups [proportion of relapse, 66.7% in ER, 75.9% in STG, and 100% in TG; and time to relapse (mean ± SE), 18.2 ± 4.5 mo in ER, 19.7 ± 3.2 mo in STG, and 26.6 ± 7.3 mo in TG]. According to Cox-proportional regression analysis, diabetic medications were re-prescribed more frequently after STG compared to ER (aHR for relapse, 5.21, 95%CI: 1.64-16.60), but the risk of relapse in HTN was not different among three treatment methods.
As indicated by the cessation of anti-diabetic drugs, this study showed that total gastrectomy has enduring effects on improving DM in gastric cancer patients. This study was the first report to evaluate the metabolic improvement of gastrectomy using a nationwide retrospective cohort study of South Korea. It has been reported that gastrectomy improves glycemic control in morbidly obese population, even independent of weight loss. However, the effect of gastrectomy in non-obese diabetic patients was controversial. In Asia including South Korea, obesity rates of general population are relatively low. The prevalence of obesity 25 kg/m2 above body mass index (BMI) was 27% in South Korea[16] or 29% in China[17], compared to 34% in the United States[18]. Thus, most Koreans (about 73%) are non-obese, which is closely related with early insulin secretary defects, a unique feature of Korean diabetes[19]. Our study population was randomly sampled from all diabetic or hypertensive patients in South Korea, which suggests the average BMI of study population would reflect that of the Korean population. Our study observed that about two times of number of patients in TG group could discontinue anti-diabetics compared to STG or ER group. This observation suggested that TG might be beneficial for glycemic control in even non-obese diabetic patients, who randomly sampled from general population.
Many investigators have investigated metabolic improvement after bariatric surgery compared to after medical intervention or efficacy of various surgical strategies such as laparoscopic gastric banding, sleeve-gastrectomy, gastric-bypass, or biliopancreatic diversion[20,21]. We compared the outcomes in three groups of TG, STG and ER to evaluate the effects depending on surgical extent. As a result, only TG was effective in the improvement of DM. One reason for the difference of two surgical methods (STG and TG) in the metabolic effect may be due to the size of the remnant stomach. Intake is larger in STG than TG, because the remnant stomach plays the role of a reservoir to store food. There were no differences found in daily food intakes from preoperative to 1 year after subtotal gastrectomy[22].
A second reason could be due to the effect of gut hormones such as ghrelin, an orexigenic and prodiabetic foregut hormone secreted from the fundus. Serum ghrelin levels were decreased by Roux-en-Y gastric bypass surgery due to the ghrelin-producing cells in the gastric fundus being unable to contact ingested nutrients, leading to profound weight loss[23-25]. Recently, one randomized prospective trial comparing Roux-en-Y gastric bypass to sleeve gastrectomy reported that the decrement of the hindgut hormones, glucagonlike peptide 1 and peptide YY after surgery, are also responsible for improved glucose homeostasis[26]. Yet another study suggested that metabolic markers show a similar improvement in both Roux-en-Y gastric bypass and sleeve gastrectomy[21]. However, it remains unclear whether the changes of gut hormones also occur in a non-obese population; therefore, a prospective study in a non-obese population is needed.
In our study, drug discontinuation rate of DM and HTN after gastric surgery were relatively lower than previous studies (9.7% in DM and 11.1% in HTN). The remission rate of DM after bariatric surgery has been reported to range between 55 to 95% in previous studies[11,20,21,27-29]. One randomized prospective trial of obese DM patients compared bariatric surgery to intensive medical therapy and found a remarkable reduction in mean glycated hemoglobin, the use of glucose lowering drugs, lipid and blood pressure level[21]. A meta-analysis including 136 observational studies and 22094 patients of morbid obesity (mean BMI of 46.85 kg/m2) showed that resolution occurred in 76.8% of DM patients and 61.7% of HTN patients after bariatric surgery[30]. Recently, the rate of glycemic improvement after bariatric surgery reported 38% in gastric-bypass group and 24% in sleeve-gastrectomy group[31], which was lower than other short-term trials. The only study including diabetic patients with mild obesity (BMI, 30-35 kg/m2) suggested that remission of DM occurred in 88% of patients[32]. Our study analyzed the effect of gastrectomy on DM in a non-obese population with gastric cancer, which was the reason for relatively lower remission rate than those of other studies. In addition, the definition of improvement was so tight (cessation of the drugs for six or more subsequent months) that the discontinuation rate might be lower than expected.
The improvement of DM after the treatment of gastric cancer has been reported in non-obese patients of mean BMI 23.4-24.9 kg/m2. The researchers evaluated the efficacy of three reconstruction methods (Roux-en-Y, Billroth I and Billroth II). However, the outcome was not compared with “non-reconstructed” control. We discovered an interesting phenomenon that remission of DM or HTN also occurred even in endoscopic treatment, a control group. We suspect this is due to a change in food intake style. After the diagnosis of gastric cancer, many Koreans tend to change their style of food intake to avoid salty food, reduce the amount of carbohydrates, and consume fresh vegetables[33]. This kind of nutritional change (low carbohydrate and salt) could temporarily improve DM and HTN. For this reason, an investigation of the metabolic effects of gastrectomy on DM or HTN should be compared to that of endoscopic resection, rather than the general population. In this study, we confined the study population in a single disease entity of gastric cancer, and compared the outcome of gastrectomy with endoscopic resection, not with medical intervention.
One of the limitations of this study is that data on the reconstruction methods used after gastrectomy were not available. However, most Korean gastric surgeons generally perform gastroduodenostomy (Billroth I) or loop gastrojejunostomy (Billroth II) after distal gastrectomy and Roux-en Y esophagojejunostomy after TG[3]. The effect on DM and HTN after TG might be caused by either gastric resection or Roux-en Y bypass but this could not reach to conclusion due to limited information. A larger population is required to obtain more detailed information.
Another limitation was that the database did not contain the patient’s body mass index, glycated hemoglobin or blood pressure information. Thus, the improvement of disease control could only be assessed from the cessation of medication (anti-diabetic or anti-hypertensive). Due to the limitation of the KNHI data, we did not adopt the concept of disease improvement, but simply discontinuation of therapeutic agents. In spite of lack of biochemical data, cessation of drugs for 6 or more months might be a powerful surrogate marker, because all prescription history have been collected in KNHI database under precise electronic medical system in South Korea. In addition, it could be supportive information that the sequential change of proportion of multiple drug prescription showed similar trends with survival curve for improvement of DM or HTN and best outcome also occurred in TG group.
In conclusion, TG showed decreased requirement of antidiabetic medications than STG or ER in the Korean general population who affected by gastric cancer. In addition, a transient improvement and reduction of the number of drugs were seen in hypertensive patients after gastrectomy.
Gastrectomy can induce improvement of diabetes or obesity, so it is recommended to treat diabetes in morbidly obese patients. But, there are no data about the long term effect of gastrectomy underwent in non-obese diabetic patients.
Gastrectomy is also a standard method for the treatment of gastric cancer and numerous operations were applied to cure gastric cancer, especially in South Korea, one of prevalent area of gastric cancer and of lower average body mass index than in other countries. In the scope of effect on public health, metabolic outcome of gastrectomy should be elucidated, but not yet. The research hotspots are how big impact of gastrectomy lied on metabolic disease such as diabetes and how long the metabolic effect of gastrectomy could be sustained.
In the previous publications, metabolic effect of gastrectomy was compared in individuals, before gastrectomy and after gastrectomy. The results can observe the degree of change of metabolic diseases; however, the effect of dietary habit change or life style modification was not excluded, which were one of important treatment modality of diabetes and hypertension. To overcome confounding effect of these factors, authors analyzed the metabolic change of patients with gastrectomy by comparing with patients with endoscopic intervention.
The study results suggest that total gastrectomy would induce the improvement of diabetes and hypertension, even in non-obese diabetic patients. Furthermore, clinicians should predict risk of hypoglycemia or symptomatic hypotension caused by metabolic improvement when they meet patients who underwent gastrectomy in clinic.
Gastrectomy is a sort of surgical method to resect part of stomach. Gastrectomy is usually performed to treat gastric cancer or gastric perforation; Endoscopic resection is an alternative method to remove small size of gastric cancer or gastric polyp and a confirmative test for pathologic diagnosis of gastric disease.
This is a good case-control study using nationwide administrative data in which the authors analyzed the beneficial effect of gastrectomy on diabetes and hypertension in non-obese patients. The results are interesting and suggest that total gastrectomy can be considered in morbidly obese gastric cancer patients with poorly controlled diabetes to expect their metabolic improvement with less number of medications.
P- Reviewer: Apikoglu-Rabus S, Saglam F, Xu XH S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 2. | Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Park EC, Lee JS. Prediction of cancer incidence and mortality in Korea, 2011. Cancer Res Treat. 2011;43:12-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129-2140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1411] [Cited by in F6Publishing: 1403] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 5. | Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090-1101. [PubMed] [Cited in This Article: ] |
| 6. | Choi YJ, Kim HC, Kim HM, Park SW, Kim J, Kim DJ. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998-2005. Diabetes Care. 2009;32:2016-2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Kim K, Cho Y, Youn T, Cho G, Chae I, Choi D, Kim C. Prevalence, awareness, treatment, and control of hypertension in Korea; Korean National Health and Nutrition Examination Survey 2007: Pp.28.103. J Hypertens. 2010;28:e480. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88:653-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 9. | Sarfati D, Gurney J, Lim BT, Bagheri N, Simpson A, Koea J, Dennett E. Identifying important comorbidity among cancer populations using administrative data: Prevalence and impact on survival. Asia Pac J Clin Oncol. 2013;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Dixon JB, Zimmet P, Alberti KG, Rubino F. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Arq Bras Endocrinol Metabol. 2011;55:367-382. [PubMed] [Cited in This Article: ] |
| 11. | Yang J, Li C, Liu H, Gu H, Chen P, Liu B. Effects of subtotal gastrectomy and Roux-en-Y gastrojejunostomy on the clinical outcome of type 2 diabetes mellitus. J Surg Res. 2010;164:e67-e71. [PubMed] [Cited in This Article: ] |
| 12. | Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, Samet JM. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779-787. [PubMed] [Cited in This Article: ] |
| 13. | Lee SY, Jung KY, Lee IK, Yi SD, Cho YW, Kim DW, Hwang SS, Kim S. Prevalence of treated epilepsy in Korea based on national health insurance data. J Korean Med Sci. 2012;27:285-290. [PubMed] [Cited in This Article: ] |
| 14. | Lee JA, Park JH, Lee EJ, Kim SY, Kim Y, Lee SI. High-quality, low-cost gastrectomy care at high-volume hospitals: results from a population-based study in South Korea. Arch Surg. 2011;146:930-936. [PubMed] [Cited in This Article: ] |
| 15. | Kleinbaum DG, Klein M. Survival analysis: a self-learning text. 2nd ed. New York, NY: Springer 2005; . [Cited in This Article: ] |
| 16. | Kim DM, Ahn CW, Nam SY. Prevalence of obesity in Korea. Obes Rev. 2005;6:117-121. [PubMed] [Cited in This Article: ] |
| 17. | Reynolds K, Gu D, Whelton PK, Wu X, Duan X, Mo J, He J. Prevalence and risk factors of overweight and obesity in China. Obesity (Silver Spring). 2007;15:10-18. [PubMed] [Cited in This Article: ] |
| 18. | Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235-241. [PubMed] [Cited in This Article: ] |
| 19. | Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of korean type 2 diabetes mellitus. Metabolism. 2001;50:590-593. [PubMed] [Cited in This Article: ] |
| 20. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585. [PubMed] [Cited in This Article: ] |
| 21. | Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567-1576. [PubMed] [Cited in This Article: ] |
| 22. | Jeon TY, Lee S, Kim HH, Kim YJ, Lee JG, Jeong DW, Kim YJ. Long-term changes in gut hormones, appetite and food intake 1 year after subtotal gastrectomy with normal body weight. Eur J Clin Nutr. 2010;64:826-831. [PubMed] [Cited in This Article: ] |
| 23. | Li F, Zhang G, Liang J, Ding X, Cheng Z, Hu S. Sleeve gastrectomy provides a better control of diabetes by decreasing ghrelin in the diabetic Goto-Kakizaki rats. J Gastrointest Surg. 2009;13:2302-2308. [PubMed] [Cited in This Article: ] |
| 24. | Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, Schindler K, Luger A, Ludvik B, Prager G. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024-1029. [PubMed] [Cited in This Article: ] |
| 25. | Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518-2525. [PubMed] [Cited in This Article: ] |
| 26. | Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740-748. [PubMed] [Cited in This Article: ] |
| 27. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [PubMed] [Cited in This Article: ] |
| 28. | Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148. [PubMed] [Cited in This Article: ] |
| 29. | Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275-287. [PubMed] [Cited in This Article: ] |
| 30. | Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420-1428. [PubMed] [Cited in This Article: ] |
| 31. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002-2013. [PubMed] [Cited in This Article: ] |
| 32. | Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012;18:49-54. [PubMed] [Cited in This Article: ] |
| 33. | Yu EJ, Kang JH, Yoon S, Chung HK. Changes in nutritional status according to biochemical assay, body weight, and nutrient intake levels in gastrectomy patients. J Korean Diet Assoc. 2012;18:16-29. [Cited in This Article: ] |