Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10080
Peer-review started: February 8, 2015
First decision: March 10, 2015
Revised: March 27, 2015
Accepted: May 2, 2015
Article in press: May 2, 2015
Published online: September 21, 2015
AIM: To investigate whether accelerated catabolism of ganglioside and decreased ganglioside content contribute to the etiology of pro-inflammatory intestinal disease.
METHODS: Intestinal mucosa from terminal ileum or colon was obtained from patients with ulcerative colitis or inflammatory Crohn’s disease (n = 11) undergoing bowel resection and compared to control samples of normal intestine from patients with benign colon polyps (n = 6) and colorectal cancer (n = 12) in this observational case-control study. Gangliosides and phospholipids of intestinal mucosa were characterized by class and ceramide or fatty acid composition using liquid chromatography triple-quad mass spectrometry. Content and composition of ganglioside classes GM1, GM3, GD3, GD1a, GT1 and GT3 were compared among subject groups. Content and composition of phospholipid classes phosphatidylcholine (PC) and phosphatidylethanolamine were compared among subject groups. Unsaturation index of individual ganglioside and phospholipid classes was computed and compared among subject groups. Ganglioside catabolism enzymes beta-hexosaminidase A (HEXA) and sialidase-3 (NEU3) were measured in intestinal mucosa using western blot and compared among subject groups.
RESULTS: Relative GM3 ganglioside content was 2-fold higher (P < 0.05) in intestine from patients with inflammatory bowel disease (IBD) compared to control intestine. The quantity of GM3 and ratio of GM3/GD3 was also higher in IBD intestine than control tissue (P < 0.05). Control intestine exhibited 3-fold higher (P < 0.01) relative GD1a ganglioside content than IBD intestine. GD3 and GD1a species of ganglioside containing three unsaturated bonds were present in control intestine, but were not detected in IBD intestine. The relative content of PC containing more than two unsaturated bonds was 30% lower in IBD intestine than control intestine (P < 0.05). The relative content of HEXA in IBD intestine was increased 1.7-fold (P < 0.05) and NEU3 was increased 8.3-fold (P < 0.01) compared to normal intestine. Intestinal mucosa in IBD is characterized by increased GM3 content, decreased GD1a, and a reduction in polyunsaturated fatty acid constituents in GD3, GD1a and PC.
CONCLUSION: This study suggests a new paradigm by proposing that IBD occurs as a consequence of increased metabolism of specific gangliosides.
Core tip: This research suggests a new paradigm by proposing that increased metabolism of specific gangliosides is fundamental to the etiology of inflammatory bowel disease. The study demonstrates that the catabolism of gangliosides is elevated in the intestinal mucosa of patients with inflammatory bowel disease and suggests that intervention with appropriate dietary gangliosides potentially reduces intestinal permeability, improving intestinal integrity.
- Citation: Miklavcic JJ, Hart TD, Lees GM, Shoemaker GK, Schnabl KL, Larsen BM, Bathe OF, Thomson AB, Mazurak VC, Clandinin MT. Increased catabolism and decreased unsaturation of ganglioside in patients with inflammatory bowel disease. World J Gastroenterol 2015; 21(35): 10080-10090
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10080.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10080
Inflammatory bowel disease (IBD) is a global problem[1] and is particularly prevalent in the United States[2] and Canada[3]. IBD is associated with elevated standardized all-cause mortality ratio[4] and there is an alarming 38% increase in all-cause mortality for Crohn disease (CD). IBD can present with abdominal pain, gastrointestinal bleeding, increased stool frequency, diarrhea, weight loss, and malnutrition. Ulcerative colitis (UC) is localized only to the large intestine where continuous inflammation is present superficially and Th2 cells characterize disease. CD is characterized by Th1 cells and granulomatous inflammation occurs in transmural fashion anywhere in the gastrointestinal tract with segmented distribution[5]. CD can also be associated with development of joint, liver and kidney diseases and a much elevated risk of small intestinal lymphoma and colorectal cancer. Some individuals with IBD do not respond to drug treatment, while others experience serious adverse effects[6]. The cause of IBD is unknown and there is a clear need for innovative knowledge of disease mechanisms to enable novel treatment strategies.
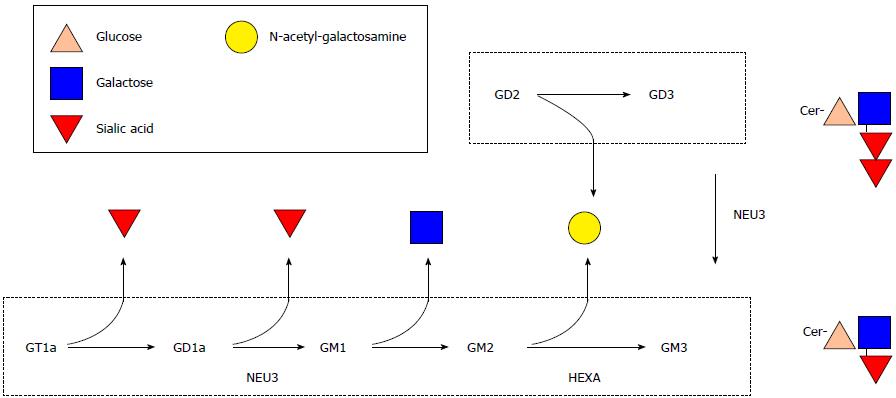
Gangliosides are structurally complex molecules of many molecular species varying in sialic acid and ceramide composition (Figure 1). Certain species of ganglioside are involved in regulation of cell signalling, apoptosis and receptor-ligand interactions. Dietary ganglioside intake is very low unless a person is consuming whole organ foods (ex. brain), whole milk or buttermilk in excessively high quantities that are not practical on a daily basis[7]. Infants normally obtain[8] gangliosides from human milk[9] for incorporation into brain[10].
Providing ganglioside in the diet increases ganglioside content in intestinal mucosa[11]. Important observations from animal studies reveal that inflamed intestinal mucosa has less GD3 ganglioside than healthy intestinal mucosa[12]. Moreover, increasing ganglioside content of intestinal cells through diet decreases pro-inflammatory cytokine signalling in intestinal mucosa[12], and prevents hypoxia-induced bowel necrosis and cell injury in cultured infant bowel[9] downregulating the same signals underlying IBD. Caco-2 cells incorporate GD3[13] when provided with ganglioside at physiological concentrations in vitro[14]. Localization of GD3 in the lateral and basolateral membrane surfaces and GM3 to the brush border membrane of enterocytes[11] suggests specific mechanisms by which ganglioside supports intestinal cell functions like barrier integrity. Collectively, these studies of animal and infant intestinal mucosa indicate that content of GD3 is decreased with inflammation and higher levels of GM3 or other ganglioside metabolites can be associated with intestinal cell injury and proinflammatory signalling[15].
The balance of n-3 and n-6 polyunsaturated fatty acids (PUFA) appears to be important in the inflamed intestine. Experimental induction of colitis in mice results in an increase of phospholipase A2 activating protein (PLAP)[16]. Increased levels of PLAP may result in increased liberation of n-6 arachidonic acid from phospholipid for conversion to pro-inflammatory mediators like prostaglandin E2 and leukotriene B4 which promote the perpetual inflammatory signaling cascade in IBD. Moreover, experimental colitis can be alleviated in mice by consumption of n-3 PUFA[17]. Difference in intestinal PUFA content of phospholipid has been demonstrated in UC[18], but detailed composition of phospholipid classes has not been thoroughly characterized in human intestinal mucosa. In addition to the class of ganglioside (i.e., GM3, GD3), the role or importance of fatty acid and ceramide constituents within gangliosides remains elusive. Thus, the present study aimed to verify the difference in intestinal PUFA from previous study[18] and to determine whether differences in unsaturation index are exclusive to intestinal phospholipid or whether gangliosides also exhibit differences in unsaturation among IBD and healthy intestine.
In this study, classes of ganglioside and phospholipid were characterized in intestinal mucosa from control subjects and compared to patients with IBD. Detailed structural characterization of ceramide and lipid species in ganglioside classes GM1, GM3, GD3, GD1a, GT1 and GT3 and phospholipid classes phosphatidylcholine (PC) and phosphatidylethanolamine (PE) was performed. Finally, the content of ganglioside catabolism enzymes beta-hexosaminidase A (HEXA) and sialidase-3 (NEU3) were measured in intestinal mucosa to explain the observed content of specific ganglioside species in the intestinal mucosa.
A first control group (n = 6) consisted of subjects undergoing bowel resection for non-neoplastic benign adenomatous polyposis (BAP). The BAP group (non-familial, non-malignant) was characterized by > 10 advanced polypoid lesions (diameter: 10-20 cm) with high-grade dysplasia localized in the descending colon. A second age- and sex-matched control group (n = 12) consisted of intestine supplied from the Canada Breast Cancer Foundation Tumour Bank (Calgary, Canada). The control tissue consisted of non-cancerous regions of large bowel from subjects with low-to-moderate grade colorectal cancer (CRC) with no known metastases, chemo- or radiotherapy treatment. Patients with IBD (n = 11) requiring surgical resection of bowel were recruited from the University of Alberta Hospital surgical program. Surgical referral was based upon usual and accepted standards of care and included patients with indications such as hemorrhage, obstruction and perforation. Diagnosis of ulcerative colitis (UC) and CD was based on established radiologic, endoscopic, and histologic criteria. Male and non-pregnant female adults (> 17 year of age) were eligible for study. Patients with inadequate liver or renal function, active infectious disease, history of alcohol/drug abuse or other serious medical conditions were excluded from study. Patients were taking a variety of medications preoperatively for their IBD, such as melamine, corticosteroids, immunosuppressants, anti-TNF biological agents or antibiotics.
Visually normal regions of intestine were excised from surgical sections of control tissue at least 10 cm from the closest polyp in BAP controls. Intestine from terminal ileum or colon was obtained from surgical sections of viable tissue in the vicinity of the most prominent ulcerative lesions from subjects with IBD. Mucosa was separated from the bowel wall by scraping with a glass slide[9]. The sample was collected in a sterile cryovial before being snap-frozen in liquid nitrogen within 20 min of devascularisation and stored at -80 °C until analysis.
Gangliosides were isolated from tissues using a modified Folch extraction[19]. The final supernatant was used for ganglioside profiling. Aqueous extracts were injected onto a Poroshell 120 EC-C18 column using an Agilent 1260 Infinity LC system. Gangliosides were separated using reverse-phase chromatography and the eluent was directed to the inlet of an Agilent 6430 Triple-Quad MS system. Electrospray ionization generated deprotonated gas-phase ions from the various ganglioside species. The mass spectrometer was operated in multiple reaction monitoring mode to provide selective and sensitive ganglioside detection by allowing only select precursor ions and characteristic gas-phase fragments to be detected. The mass spectra were screened against a library of theoretical precursor ions from over 900 gangliosides with variable ceramide and carbohydrate compositions. The relative percent of ganglioside subspecies GM1, GM3, GD3, GD1, GT1 and GT3 were determined using Mass Hunter Qualitative Analysis software. GD1b was not detected in the majority of specimens and so all GD1 was assumed to be present in the configuration of GD1a.
Western blotting was performed to analyze HEXA and NEU3 content in intestinal samples. Total protein was extracted from intestinal mucosa using T-PER Tissue Protein Extraction Reagent (#78510, Fisher Scientific, ON Canada) in the presence of protease inhibitor (No. 78410, Fisher Scientific, ON Canada). The RC DC Protein Assay Kit II (#500-0122, Bio-rad, ON Canada) small volume method was used to quantify total protein using a Molecular Devices SPECTRAmax 190 against a known concentration of bovine serum albumin (BSA) standard. The supernatant was collected and stored at -80 °C until analysis. Standard ladder (#161-0305, Bio-rad, ON Canada) and 10 μg of protein were loaded into 100 mL/L sodium dodecyl sulphate-polyacrylamide gel (#08091, Sigma-Aldrich, ON Canada) after boiling samples for 2 min. Protein electrophoresis was conducted at 200 V using the Hoefer SE260 mini-vertical electrophoresis unit.
Gel was transferred onto nitrocellulose membrane using the Hoefer TE22 Mighty Small Tank Transfer unit. Following transfer, membrane blots were blocked using 50 mL/L BSA in tris-buffered saline with 1 mL/L tween 20 (TBST) (pH 7.6). Blots were incubated for 1 h with primary antibodies diluted in 50 mL/L BSA in TBST: 1/250 rabbit polyclonal IgG anti-HEXA (#93111, Abcam, MA United States), 1:7000 rabbit polyclonal IgG anti-NEU3 (#80541, Abcam, MA United States), 1:2500 rabbit polyclonal IgG anti-glyceraldehyde 3-phosphate dehydrogenase (#9485, Abcam, MA United States). After incubation, blots were rinsed twice with TBST. Blots were incubated with 1:2000 horseradish peroxidise-conjugated goat anti-rabbit IgG secondary antibody (#7074, Cell Signalling Technology, MA United States) at room temperature for 1 h. Blots were rinsed as before then incubated with Amersham enhanced chemiluminescence prime reagent (RPN2232, Sigma-Aldrich, ON Canada) as per manufacturer instructions. The blot was imaged using a GE Typhoon 8600 Variable Imager and Scanner and the image was analyzed with ImageQuant software. Protein bands were manually selected to determine band density.
Phospholipids were isolated from tissues using a modified Folch extraction[19]. Organic extracts were frozen at -20 °C until analysis. Upon analysis, samples were thawed at room temperature, dried under nitrogen and resuspended in 750 mL/L acetonitrile, 250 mL/L water. Samples were subjected to normal phase chromatography with an Agilent Zorbax RX-Sil column (3.0 mm × 100 mm, 1.8 μm particle size) using a 1260 infinity LC system (Agilent). The mobile phase was composed of 375 mL/L acetonitrile, 125 mL/L water, methanol 500 mL/L with 5 mmol/L ammonium acetate and 0.1 mL/L acetic acid. The flow rate of LC was 0.5 μL/min (10 min). All MS measurements were obtained using an Agilent 6430 Triple-Quad MS system operating in positive ion mode. Protonated gas-phase ions of the various phospholipid species were obtained using electrospray ionization, with the electrospray needle held at 4500 V. The MS was operated in multiple reaction monitoring mode. A library of theoretical precursor ions was generated for PC and PE with various predicted fatty acid compositions. The first quadruple mass filter was set to scan for these specific precursor ions, allowing each to sequentially pass into the hexapole collision cell where ions were fragmented using collision induced dissociation (CID). PC species readily undergo head group specific fragmentation, so the second mass filter was set to monitor m/z = 184. For PE species, which fragment with the neutral loss of 141 mass units, the second mass filter monitored the pre-cursor m/z minus 141. The CID and ion source voltages for each phospholipid class were optimized using the Agilent Optimizer software. Data acquisition and analysis were carried out using the Agilent Mass Hunter software package.
Mean and pooled standard error of the mean are presented for ganglioside and phospholipid content of intestine and relative expression of HEXA and NEU3. Unsaturation index (UI) of gangliosides and phospholipids was computed as per previous study[20]. Ganglioside content, ganglioside catabolism and phospholipid content were compared among study groups using an ANOVA. Student’s t-test was used to compare individual means across subjects groups. Subjects with IBD were not stratified by drug intake as corticosteroids[21] have not been observed to influence glycosphingolipid metabolism and the small number of subjects taking immunosuppressants and biological agents stopped this therapy at least 30 d prior to surgery. Some parent drug compound or metabolite may still be present after 30 d. However, any effect of parent drug compound or metabolite on ganglioside metabolism is not believed to occur to any substantial degree as adverse neurologic effects would likely be detected due to the high abundance of ganglioside in nervous tissues. The recruitment of Crohn colitis and ileitis patients in this study is not believed to be confounded by region of intestine with respect to measures of ganglioside because previous study has shown that the ratio of GD3 to GM3 ganglioside does not differ between colon and small intestine[22]. The statistical methods of this study were reviewed by John Miklavcic from The University of Alberta.
No difference was observed in content of gangliosides between BAP and CRC control groups. Relative GM3 content was increased by 2-fold (P < 0.05) in IBD intestine compared to BAP and CRC control groups. Control intestine groups exhibited about 3-fold higher GD1a content (P < 0.01) than IBD intestine (Table 1).
| Control (BAP)(n = 6) | Control (CRC)(n = 12) | IBD(n = 11) | PooledSEM | |
| GM3 | 27.5a | 24.4a | 48.6b | 3.8 |
| GD3 | 26.3 | 39.4 | 31.4 | 2.4 |
| GD1a | 38.0c | 31.7c | 11.6d | 2.6 |
Total GM3 was 6-fold higher (P < 0.05) in IBD intestine than CRC control intestine when standardized to wet tissue weight and 4 fold-higher (P = 0.057) when standardized to protein content of tissue. Total GM3 did not differ between IBD and BAP control groups when standardized to wet tissue weight, protein content, or phospholipid content of tissue. Total GD3 was 1.8-fold higher in BAP control intestine than IBD intestine when standardized to protein content of tissue and 2.5-fold higher when standardized to phospholipid content of tissue (P > 0.05). Total GD3 did not differ among subject groups when standardized to wet tissue weight, protein content or phospholipid content. The ratio of GM3/GD3 was about 4-fold greater in IBD intestine than BAP control intestine when standardized to wet weight of tissue (P < 0.05) and protein content (P < 0.05) and 2.5-fold greater (P < 0.05) when standardized to phospholipid content of tissue. The ratio of GM3/GD3 was about 4 fold greater in IBD intestine than CRC control intestine when standardized to wet weight of tissue (P < 0.05) and protein content (P < 0.05) but did not differ when standardized to phospholipid content of tissue (Table 2).
| Control (BAP) (n = 6) | Control (CRC) (n = 12) | IBD(n = 11) | Pooled SEM | |
| GM3 | ||||
| μg/g tissue | 2.4a,b | 1.0a | 5.8b | 0.8 |
| μg/g protein | 62.3c,d | 19.2c | 77.8d | 14.1 |
| mg/mol PL | 317.7 | 379.2 | 196.9 | 67.7 |
| GD3 | ||||
| μg/g tissue | 1.4 | 1.6 | 1.4 | 0.2 |
| μg/g protein | 36.8 | 31.3 | 20.2 | 6.1 |
| mg/mol PL | 38.1 | 17.7 | 15.4 | 3.0 |
| GM3/GD3 | ||||
| Tissue | 1.7a | 1.4a | 5.6b | 0.90 |
| Protein | 1.8a | 1.5a | 5.6b | 0.90 |
| PL | 8.4a | 19.3 b | 20.1b | 2.40 |
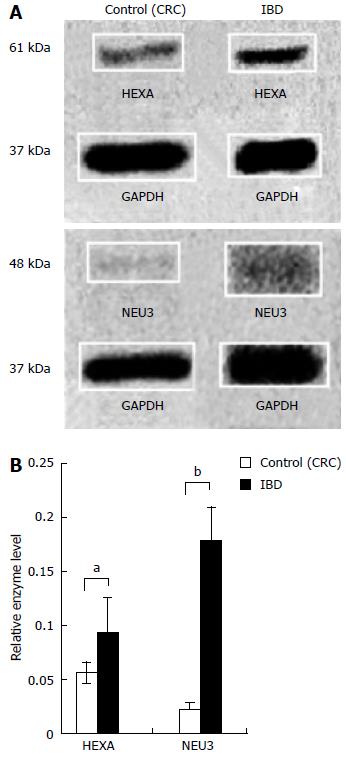
Content of ganglioside catabolic enzymes HEXA and NEU3 were measured in normal intestinal mucosa and compared to intestinal mucosa from subjects with IBD (Figure 2A). The mean relative expression of HEXA in CRC and IBD groups was 0.06 (± 0.01) and 0.09 (± 0.03), respectively. The mean relative expression of NEU3 in CRC and IBD groups was 0.02 (± 0.01) and 0.18 (± 0.03), respectively (Figure 2B). The mean relative expression of HEXA in IBD exhibited 1.7-fold increase (P < 0.05) in comparison to the control intestine. The mean relative expression of NEU3 in IBD was increased 8.3-fold (P < 0.01) vs the control group.
IBD group exhibited less GD3 and GD1a (P < 0.01) with 3 unsaturated bonds in the fatty acid ceramide component than BAP and CRC control groups. IBD intestine did not contain detectable levels of GD3 or GD1a with a ceramide constituent composed of 3 unsaturated bonds. Control subjects exhibited about 5-fold greater GD3 (P < 0.01) with 2 unsaturated bonds and about 3.5-fold greater GD1a (P < 0.01) with 2 unsaturated bonds than subjects with IBD. There was a corresponding 1.5-fold increase (P < 0.01) in relative monounsaturated GD3 and GD1a content in IBD intestine compared to control intestine (Table 3). The unsaturation indices of gangliosides GD3 and GD1a were 1.3-fold higher in control intestine as compared with inflamed intestine (P < 0.01).
| Unsaturated Bonds | Control (BAP) (n = 6) | Control (CRC) (n = 12) | IBD(n = 11) | Pooled SEM | |
| GM3 | 0 | 5.73a | 2.77b | 3.3a,b | 0.41 |
| 1 | 69.96 | 73.40 | 68.34 | 1.30 | |
| 2 | 21.86 | 21.62 | 25.24 | 1.05 | |
| 3 | 2.45 | 2.22 | 3.10 | 0.21 | |
| UI | 1.21 | 1.23 | 1.28 | 0.07 | |
| GD3 | 0 | 9.28 | 6.18 | 5.91 | 1.18 |
| 1 | 59.39c | 58.67c | 87.88d | 2.91 | |
| 2 | 28.28c | 32.98c | 6.21d | 2.45 | |
| 3 | 3.09c | 2.18c | NDd | 0.30 | |
| UI | 1.25c | 1.31c | 1.00d | 0.17 | |
| GD1a | 0 | 5.32a,b | 6.8a | 3.11b | 0.68 |
| 1 | 64.95c | 59.26c | 89.93d | 3.05 | |
| 2 | 26.21c | 30.51c | 8.06d | 2.44 | |
| 3 | 3.55c | 3.43c | NDd | 0.44 | |
| UI | 1.28c | 1.31c | 1.06d | 0.17 |
There were about 3-fold increases (P < 0.01) in monounsaturated content of GD3 and GD1a ganglioside containing 34, 38 and 40 carbons within ceramide of IBD intestine as compared to control intestine. However, monounsaturated GD3 and GD1a containing more than 40 carbons within ceramide was not detected in IBD intestine (Table 4).
| Ceramide | Control (BAP) (n = 6) | Control (CRC) (n = 12) | IBD(n = 11) | Pooled SEM | |
| GD3 | 43:0 | 8.4c | 5.2c | NDd | 0.7 |
| 34:1 | 8.9c | 8.2c | 20.2d | 1.6 | |
| 36:1 | 2.4a | 4.0a | 6.0b | 0.4 | |
| 38:1 | 2.9c | 3.9c | 10.8d | 0.8 | |
| 40:1 | 14.2c | 15.9c | 48.4d | 3.3 | |
| 42:1 | 24.8c | 22.0c | NDd | 2.3 | |
| 40:2 | 1.0c | 1.1c | 3.67d | 0.3 | |
| 42:2 | 26.8c | 31.8c | NDd | 2.9 | |
| 42:3 | 3.1c | 2.4c | NDd | 0.3 | |
| GD1a | 43:0 | 4.3c | 3.0c | NDd | 0.4 |
| 34:1 | 6.5a | 3.1a | 12.0b | 1.1 | |
| 36:1 | 16.9 | 14.8 | 24.0 | 2.3 | |
| 38:1 | 8.7c | 8.8c | 19.3d | 1.3 | |
| 40:1 | 13.1c | 13.9c | 33.9d | 2.4 | |
| 42:1 | 15.3c | 15.1c | NDd | 1.8 | |
| 40:2 | 2.0a | 1.7a | 4.3b | 0.4 | |
| 42:2 | 22.2c | 28.5c | NDd | 2.7 | |
| 42:3 | 3.5c | 3.4c | NDd | 0.4 |
There were no differences among subject group intestine in PC containing zero, one or two unsaturated bonds. Control subjects had about 1.5-fold greater amount of PC (P < 0.05) with at least three unsaturated bonds than subjects with IBD. The unsaturation index of PC was about 15% higher (P = 0.01) in control intestine as compared with inflamed intestine (Table 5). There was a trend towards greater relative amount of PE with at least three unsaturated bonds in control subjects than patients with IBD but there was no significant difference among subject groups (data).
| Unsaturated Bonds | Control (BAP)(n = 6) | Control (CRC)(n = 12) | IBD(n = 11) | Pooled SEM |
| 0 | 9.82 | 10.05 | 12.43 | 0.76 |
| 1 | 6.14 | 7.77 | 8.8 | 0.59 |
| 2 | 53.78 | 54.57 | 58.87 | 1.69 |
| > 2 | 30.26a | 27.61a | 19.9b | 1.38 |
| UI | 2.33a | 2.27b | 2.06b | 0.03 |
The significance of this study resides in novel data showing that ganglioside content and enzymatic regulation of ganglioside metabolism differ between IBD and healthy intestine. These observations suggest an innovative hypothesis that altered ganglioside metabolism is fundamental to the etiology of IBD and these differences may impact intestinal barrier and immunity functions altered in IBD. We propose that increased ganglioside metabolism to produce specific imbalance in gangliosides in intestinal mucosa is fundamental to the etiology of IBD. This new paradigm also suggests that remission could potentially be achieved if increasing intake of appropriate gangliosides restores normal ganglioside balance in diseased intestine by reducing intestinal permeability and enhancing intestinal barrier integrity.
The relative quantity of GM3 is increased and content of GD1a is decreased in inflamed intestinal mucosa from patients with IBD. HEXA is a lysosomal enzyme that catalyzes conversion of GM2 to GM3 and NEU3 is primarily located at the cell surface where it catalyzes conversion of GD3 to GM3 and GD1a to GM1 (Figure 1). As it has not previously been measured in the intestinal mucosa of patients with IBD, the functional consequence of a two-fold increase in HEXA content is unknown. The elevation in relative GM3 content and lower relative content of GD1a in IBD (Table 1) is corroborated by the increases observed in HEXA and NEU3 protein content in IBD intestine (Figure 2). The elevation in absolute GM3 content and GM3/GD3 content (Table 2) is also explained by increased NEU3 and possibly HEXA content supporting the notion that increasing dietary GD3 intake may correct the imbalance of altered ganglioside content and potentially ameliorate burdensome disease processes in IBD.
The relative and absolute abundance of GD3 was not different among study groups, however; polyunsaturated (3 unsaturated bonds) GD3 species were not detected in IBD intestine. In addition, polyunsaturated GD1a species were also not detected in IBD intestine (Table 3). Accordingly, NEU3 may preferentially cleave species of GD3 and GD1a that contain ceramide having a higher number of unsaturated constituents. Similar modes of enzyme specificity have been observed in the regulation of essential fatty acids by desaturase enzymes[23]. Within ganglioside classes GD3 and GD1a, the relative decrease in ceramide species containing 2 and 3 unsaturated bonds occurred in conjunction with a relative increase in monounsaturated gangliosides (Table 3).
An exact quantitative method for comparison of ganglioside content in tissue has not been routinely employed in lipid research. In the present study, the relative content of ganglioside and content of ganglioside standardized to wet tissue weight, protein and phospholipid is presented. Standardizing measures of ganglioside to tissue phospholipid content is limited as previous study has shown that phospholipid content of tissue differs between healthy and IBD intestine[24]. Furthermore, presence of inflammatory infiltrate may change wet tissue weight and protein content in IBD as compared with healthy intestine. Finally, standardization of ganglioside to nucleic acid content was not attempted as DNA content varies among region of intestine[25] and the present study utilized intestinal specimens from multiple regions including terminal ileum and/or colon. The study shows an increased level of GM3 in IBD intestine which is substantiated by the agreement among (1) relative abundance of GM3; (2) quantitative measure and GM3 with respect to wet tissue weight and protein; and (3) the ratio to GM3/GD3.
In probing individual ceramide species (Table 4), there was higher relative content of monounsaturated GD3 and GD1a ganglioside containing shorter ceramide chains (< 41 carbons) in IBD intestine than control intestine whereas GD3 and GD1a containing longer ceramide chains (> 40 carbons) were not detected in IBD intestine (Table 4). This might indicate that the decrease in polyunsaturated constituents in either the fatty acid or sphingosine positions of intestinal gangliosides GD3 and GD1a in IBD may decrease concurrently with long-chain monounsaturated constituents in the corresponding sphingosine or fatty acid position. The functional implications of the corresponding fatty acid species which decreases as a result of decreased polyunsaturated species in either the phospholipid or ganglioside in IBD is not yet understood, but is of interest in future investigation.
IBD intestine also exhibited decreased unsaturation index of PC compared to control intestine (Table 5). Patients with previous bowel resection were excluded from recruitment as intestinal resection has been associated with changes in phospholipid class and composition[26]. Evidence suggests that there is low dietary intake of total PUFA in patients with IBD[27]. Results from the European Prospective Investigation into Cancer and Nutrition study suggest that about 30% of UC cases could be attributed to very high intakes of n-6 PUFA[28]. Furthermore, mice harbouring a mutation that enables production of n-3 fatty acids from n-6 fatty acids have an increase in n-3 fatty acid status in all tissues assessed including colon and have longer colon length and decreased histological score compared to wildtype mice[29]. Patients with UC have been shown to have an increase in saturated fatty acids as a component of PC[24]. The corresponding decrease in relative content of n-3 PUFA status may impede production of pro-resolving mediators like resolvins and hinder subsequent resolution of inflammation[30]. This study demonstrates that the differences in unsaturation index described in previous study are not exclusive to specific phospholipid classes but also include specific classes of ganglioside. These studies suggest that patients with IBD may have higher n-6:n-3 fatty acid consumption and that modulating n-3 PUFA content of intestinal ganglioside and phospholipid may improve outcomes in IBD.
Intestinal permeability is mediated by tight junction proteins[31] which are located primarily in lipid rafts[32]. Treatment with PUFA prevents displacement of tight junction proteins from lipid rafts and attenuates histological score in a rat model of colitis[17]. Furthermore, low level of GD3 in intestinal mucosa is associated with degradation of tight junction proteins[33] and increasing GD3 content of intestine reduces degradation of tight junction proteins, thus improving integrity and reducing intestinal permeability. Membranes containing saturated ceramides form gel domains and are more ordered than membranes with the corresponding unsaturated ceramide[34]. Accordingly, gangliosides containing ceramides with higher PUFA content localize to lipid rafts and form the sealing elements of tight junctions[35]. The sum of evidence suggests that dietary ganglioside may have positive effects on intestinal permeability as ganglioside higher in polyunsaturated content locates primarily to lipid rafts where interaction with proteins protect the tight junction and support intestinal integrity which is compromised in IBD[17,31-35].
A role for the microbiome in gut health has been proposed[36] but the precise mechanisms and modes of action are largely unknown. Moreover, the influence of specific bacteria residing in the gut or bacterial enzymes on ganglioside metabolism is unknown. The microbiome is transient and differs within and between individuals on the basis of age, genetics, geography, dietary intake and medication use[37]. The microbiome is arguably as diverse among healthy individuals as it is between healthy and disease states. Whether potential intestinal dysbiosis in IBD may be responsible for aberration in ganglioside content and composition of intestinal mucosa could not be discerned in the present study and remains a challenge in future research.
As healthy colon tissue may not be readily available in sufficient quantity, the use of tissue excised from surgeries or biopsy constitute a viable alternative to serve for control comparison. Use of healthy regions of tissue from patients with CRC requires unique considerations. Inflammation may be present in malignant tumours of the colon. To properly distinguish the inflammatory phenotype of IBD from control CRC tissue, CRC control tissue assayed in this study was sampled at least 10 cm from tumour. This sampling method was also believed to be sufficient to avoid sampling gangliosides potentially altered in tumour tissue. Anterior resection cases were excluded as anterior resection of colorectal tumours is often treated with radiotherapy prior to surgery. Normal intestinal tissue from patients with familial BAP however may more closely resemble that of colon cancer than healthy colon[38,39]. Accordingly, this study assessed normal regions of excised intestine from non-familial cases of BAP. Anterior resection cases may be inappropriate for control comparison to IBD intestine for the purposes of ganglioside analysis since ganglioside content and exposure to radiation are interrelated[40]. Another possible region of control comparison consists of healthy tissue in patients with IBD as a measure of internal control. This strategy was not employed in the present study however as the level of inflammatory mediator like leukotriene B4 generated from PUFA is similar between inflamed tissue and adjacent-to-inflamed tissue[41]; thus the classes of lipids of interest in this study were not hypothesized to differ between inflamed and adjacent-to-inflamed tissue.
Future study warrants comparison of IBD intestine to other instances of intestinal inflammation (i.e., diverticulitis) to ascertain whether altered ganglioside and phospholipid content and composition occur generally in intestinal inflammation or whether these findings are exclusive to IBD. This study benefits from comparison of intestinal mucosa from patients with IBD to two groups of healthy control intestine from BAP and CRC participants and from the high correlation between control intestine groups with respect to relative ganglioside content (Table 1) and composition (Table 3) and phospholipid composition (Table 5).
The present study demonstrates an elevation in ganglioside catabolism and corresponding alteration in ganglioside content of intestinal mucosa in IBD. Moreover, ganglioside in healthy intestine is characterized by a higher proportion of PUFA species. Whereas in IBD intestine, polyunsaturated constituents of gangliosides and phospholipids are lower or absent. Collectively, the present study shows that metabolism of specific gangliosides is altered at the level of intestinal mucosa in IBD and that ganglioside may constitute a bioavailable dietary treatment having potential beneficial effects on intestinal permeability.
We kindly thank the Canada Breast Cancer Foundation Tumour Bank for providing specimens of intestine. Thanks to Justin Taylor who aided in technical aspects of image quality for western blot figure presentation.
There is significant morbidity and decreased quality of life associated with inflammatory bowel disease. Pathogenesis of ulcerative colitis and Crohn’s disease has been described, and the several risk factors for inflammatory bowel disease (IBD) have also been identified; however, the underlying etiology is still unknown. Each patient responds differently to dietary treatment. This study provides rationale for use of dietary ganglioside in future clinical investigation for potential therapeutic application in IBD.
In previous studies, the role of ganglioside in inflammation has been described in animal models and infant intestine.
This is one of the first studies to demonstrate the presence of altered ganglioside content, composition and metabolism in human intestinal mucosa in patients with IBD.
The authors hypothesize that increased catabolism of ganglioside may precede the perpetual pro-inflammatory cascade in IBD. The catabolism of ganglioside may be mitigated or overcome by intake of appropriate dietary gangliosides to induce remission of IBD. Future research spawned by the present study may inform new dietetic practice and treatment guidelines for IBD.
Gangliosides are glycosphingolipids consisting of ceramide, a fatty acid, sugar moieties and one or more sialic acids. Gangliosides are ubiquitous throughout the human body and influence a number of cellular processes: infectivity of pathogens in the gut, regulation of specific immune cells, intestinal inflammation. All of these processes are known to be altered in IBD.
This is an interesting study with well though experimental design. In this manuscript, the authors investigate the difference in ganglioside catabolism and unsaturation between patients with IBD and control patients without IBD. They also investigate the differential content and composition of phospholipids classes between the groups. This study is significant because previous studies have only addressed this issue in animal models of colitis and in infants. Detailed ganglioside and phospholipid composition has not been thoroughly characterized in human intestinal mucosa.
P- Reviewer: Keyashian K, Smolinska S S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39:690-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 643] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 3. | Bernstein CN, Wajda A, Svenson LW, MacKenzie A, Koehoorn M, Jackson M, Fedorak R, Israel D, Blanchard JF. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 422] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 4. | Bewtra M, Kaiser LM, TenHave T, Lewis JD. Crohn’s disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflamm Bowel Dis. 2013;19:599-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Yantiss RK, Odze RD. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology. 2006;48:116-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008;14:354-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 165] [Cited by in F6Publishing: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Pham PH, Duffy TL, Dmytrash AL, Lien VW, Thomson AB, Clandinin MT. Estimate of dietary ganglioside intake in a group of healthy Edmontonians based on selected foods. J Food Compos Anal. 2011;24:1032-1037. [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Carlson SE. N-acetylneuraminic acid concentrations in human milk oligosaccharides and glycoproteins during lactation. Am J Clin Nutr. 1985;41:720-726. [PubMed] [Cited in This Article: ] |
| 9. | Schnabl KL, Larsen B, Van Aerde JE, Lees G, Evans M, Belosevic M, Field C, Thomson AB, Clandinin MT. Gangliosides protect bowel in an infant model of necrotizing enterocolitis by suppressing proinflammatory signals. J Pediatr Gastroenterol Nutr. 2009;49:382-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Wang B, McVeagh P, Petocz P, Brand-Miller J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am J Clin Nutr. 2003;78:1024-1029. [PubMed] [Cited in This Article: ] |
| 11. | Park EJ, Suh M, Ramanujam K, Steiner K, Begg D, Clandinin MT. Diet-induced changes in membrane gangliosides in rat intestinal mucosa, plasma and brain. J Pediatr Gastroenterol Nutr. 2005;40:487-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Park EJ, Suh M, Thomson B, Ma DW, Ramanujam K, Thomson AB, Clandinin MT. Dietary ganglioside inhibits acute inflammatory signals in intestinal mucosa and blood induced by systemic inflammation of Escherichia coli lipopolysaccharide. Shock. 2007;28:112-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Schnabl KL, Larcelet M, Thomson AB, Clandinin MT. Uptake and fate of ganglioside GD3 in human intestinal Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G52-G59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Bode L, Beermann C, Mank M, Kohn G, Boehm G. human and bovine milk gangliosides differ in their fatty acid composition. J Nutr. 2004;134:3016-3020. [PubMed] [Cited in This Article: ] |
| 15. | Miklavcic JJ, Schnabl KL, Mazurak VC, Thomson AB, Clandinin MT. Dietary ganglioside reduces proinflammatory signaling in the intestine. J Nutr Metab. 2012;2012:280286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Peterson JW, Dickey WD, Saini SS, Gourley W, Klimpel GR, Chopra AK. Phospholipase A2 activating protein and idiopathic inflammatory bowel disease. Gut. 1996;39:698-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Li Q, Zhang Q, Zhang M, Wang C, Zhu Z, Li N, Li J. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J. 2008;275:411-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Nishida T, Miwa H, Shigematsu A, Yamamoto M, Iida M, Fujishima M. Increased arachidonic acid composition of phospholipids in colonic mucosa from patients with active ulcerative colitis. Gut. 1987;28:1002-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. [PubMed] [Cited in This Article: ] |
| 20. | Clandinin MT. Fatty acid composition changes in mitochondrial membrances induced by dietary long chain fatty acids. FEBS Lett. 1976;68:41-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Brands M, van Raalte DH, João Ferraz M, Sauerwein HP, Verhoeven AJ, Aerts JM, Diamant M, Serlie MJ. No difference in glycosphingolipid metabolism and mitochondrial function in glucocorticoid-induced insulin resistance in healthy men. J Clin Endocrinol Metab. 2013;98:1219-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Keranen A. Gangliosides of the human gastrointestinal mucosa. Biochim Biophys Acta. 1975;409:320-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 505] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 24. | Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis. 2009;15:1705-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Marway JS, Preedy VR. Contractile and non-contractile proteins and nucleic acids in the stomach, whole jejunum and seromuscular layers of the duodenum, jejunum, ileum and large intestine in response to chronic ethanol feeding. Alcohol Alcohol. 1991;26:549-557. [PubMed] [Cited in This Article: ] |
| 26. | Ruiz-Gutierrez V, Vazquez CM, Quintero FJ. Effect of intestinal resection on phospholipid class distribution and fatty acid composition of mucosal cells in the rat large intestine. J Biochem. 1994;115:32-36. [PubMed] [Cited in This Article: ] |
| 27. | Marion-Letellier R, Savoye G, Beck PL, Panaccione R, Ghosh S. Polyunsaturated fatty acids in inflammatory bowel diseases: a reappraisal of effects and therapeutic approaches. Inflamm Bowel Dis. 2013;19:650-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, Hallmans G, Palmqvist R, Sjodin H, Hagglund G, Berglund G. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58:1606-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA. 2006;103:11276-11281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 289] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 30. | Weylandt KH, Kang JX, Wiedenmann B, Baumgart DC. Lipoxins and resolvins in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:797-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Sultana R, McBain AJ, O’Neill CA. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl Environ Microbiol. 2013;79:4887-4894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Lee DB, Jamgotchian N, Allen SG, Abeles MB, Ward HJ. A lipid-protein hybrid model for tight junction. Am J Physiol Renal Physiol. 2008;295:F1601-F1612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Park EJ, Thomson AB, Clandinin MT. Protection of intestinal occludin tight junction protein by dietary gangliosides in lipopolysaccharide-induced acute inflammation. J Pediatr Gastroenterol Nutr. 2010;50:321-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Pinto SN, Silva LC, Futerman AH, Prieto M. Effect of ceramide structure on membrane biophysical properties: the role of acyl chain length and unsaturation. Biochim Biophys Acta. 2011;1808:2753-2760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 35. | Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771-1781. [PubMed] [Cited in This Article: ] |
| 36. | Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014;40:843-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 37. | Annalisa N, Alessio T, Claudette TD, Erald V, Antonino de L, Nicola DD. Gut microbioma population: an indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediators Inflamm. 2014;2014:901308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Will OC, Robinson J, Günther T, Phillips RK, Clark SK, Tomlinson I. APC mutation spectrum in ileoanal pouch polyps resembles that of colorectal polyps. Br J Surg. 2008;95:765-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Uckun FM, Erbeck D, Qazi S, Venkatachalam T, Tibbles HE, Vassilev A. Effect of targeting janus kinase 3 on the development of intestinal tumors in the adenomatous polyposis coli(min) mouse model of familial adenomatous polyposis. Arzneimittelforschung. 2007;57:320-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Paris R, Morales A, Coll O, Sánchez-Reyes A, García-Ruiz C, Fernández-Checa JC. Ganglioside GD3 sensitizes human hepatoma cells to cancer therapy. J Biol Chem. 2002;277:49870-49876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Brock TG. Expression of 5-lipoxygenase in specialized epithelial cells of nasopharyngeal-associated lymphoid tissue. J Mol Histol. 2005;36:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |