Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10062
Peer-review started: April 7, 2015
First decision: May 18, 2015
Revised: May 29, 2015
Accepted: August 25, 2015
Article in press: August 25, 2015
Published online: September 21, 2015
There is a discrepancy between the information from clotting tests which have routinely been used in clinical practice and evidence regarding thrombotic and bleeding events in patients with liver disease. This discrepancy leads us to rely on other variables which have been shown to be involved in haemostasis in these patients and/or to extrapolate the behaviour of these patients to other settings in order to decide the best clinical approach. The aims of the present review are as follows: (1) to present the information provided by clotting tests in cirrhotic patients; (2) to present the factors that may influence clotting in these patients; (3) to review the clinical evidence; and (4) to put forward a clinical approach based on the first 3 points.
Core tip: The lack of comprehensive tests to assess coagulopathy in liver disease ties the management of this disease to the clinical evidence. This review considers the information provided by clotting tests in cirrhotic patients, identifies clinical evidence on which to base the management of the disease, and considers possible future directions of research.
- Citation: Blasi A. Coagulopathy in liver disease: Lack of an assessment tool. World J Gastroenterol 2015; 21(35): 10062-10071
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10062.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10062
Coagulopathy in liver disease has proven to be complex as it affects pro- and anticoagulant factors as well as pro- and antifibrinolytic components. The majority of coagulation tests are aimed at assessing the patient’s procoagulant capacity in isolation and do not reveal possible compensatory effects within the system. Furthermore, patients with liver disease are usually excluded from general guidelines on clinical decisions regarding transfusion before invasive procedures or surgery, in addition to guidelines on thrombotic risk and therefore thromboprophylaxis. Correct assessment of bleeding and thrombotic risk in cirrhotic patients should take into account several aspects, some of them outside the coagulation system itself as cirrhotic patients frequently bleed for non-coagulopathic reasons. From a haemostatic point of view, it is difficult to define criteria for managing these patients efficiently and safely and most decisions are based on clinical rather than scientific evidence. The present review aims to describe a comprehensive approach to this topic and possible future directions.
Platelet count, bleeding time, platelet function analyser (PFA-100), thromboelastography, and platelet function assays are not clinically useful for stratifying bleeding risk in cirrhotic patients.
In cirrhotic patients, 76% have a platelet count below 140 × 109/L[1], 13% have moderate thrombocytopaenia (50 × 109/L-75 × 109/L), and 1% have severe thrombocytopaenia (< 50 × 109/L)[2]. The platelet count detects the risk of bleeding only at extreme levels: no more thrombin potential is obtained above 100 × 109/L platelets and 50 × 109/L-60 × 109/L platelets is sufficient to trigger the coagulation system[3]. In liver cirrhosis, high plasma levels of the principal adhesive protein [von Willebrand factor (vWF)] compensate for the reduction in platelet count, but platelet count is not useful for measuring these compensatory mechanisms. vWF factor VIII complex levels are a marker of endothelial dysfunction and are proportional to the severity of liver disease, but they are not a specific marker of bleeding alone[4,5].
Bleeding time is a global test that evaluates primary haemostasis. It is influenced by platelet number and function and by factors such as cell volume, platelet volume, blood urea concentration, and vasoreactivity[4], thus it can give false positives and false negatives. In cirrhotic patients, there is a weak correlation between bleeding time and platelet count[6], and no regular association has been found between bleeding time and bleeding risk. The administration of desmopressin shortens bleeding time, but does not reduce the incidence of variceal rebleeding in cirrhotic patients[7] or the transfusion requirements in patients undergoing hepatectomy[8]. Interestingly, a recent study showed that 1 h after administration of a dose of desmopressin, no increase in vWF was observed in cirrhosis patients[9].
This test attempts to simulate platelet function under flow conditions, measuring closure time of an aperture within a membrane coated with collagen/adenosine diphosphate or collagen/epinephrine. Its applicability in liver disease patients is currently unknown[10]. Only 2 studies have used PFA-100 in cirrhotic patients and found prolonged closure times in comparison with healthy controls[11,12].
The Thromboelastography® Platelet Mapping™ (TEG-PM) assay measures percentage platelet aggregation in the presence of adenosine diphosphate or arachidonic acid. This assay has been suggested as potentially useful for evaluating platelet function in patients with cirrhosis taking clopidogrel or aspirin[13], but little experience has been obtained as yet.
There is no universally accepted platelet function assay in cirrhosis. Most tests measuring platelet function have the handicap that they are standardized for a normal number of platelets, so the presence of thrombopenia makes them difficult to interpret. They are also expensive and require a specialized laboratory.
Aggregometry measures the in vitro capacity of platelets to aggregate in response to a given stimulus (collagen, thrombin, or adenosine diphosphate). It remains the gold standard method for assessing platelet reactivity in both clinical and research settings. However, abnormal platelet aggregation studies (demonstrating hypofunction or hyperfunction) often lack correlation with clinical complications (bleeding or thrombosis)[14,15]. This method is useful for detecting platelet function defects that are congenital or secondary to medication.
Flow cytometric quantification of membrane molecule expression measures the degree of platelet activation by assessing the presence of stimulation-dependent antigens (e.g., p-selectin) or platelet-leukocyte complexes. It assesses the risk of thrombosis, but has never been used to assess the risk of bleeding[16].
The measurement of soluble activation markers assesses the presence of products released by platelets (soluble p-selectin, PF-4, beta-TGG, soluble CD40L). It is a systematic measurement of the degree of platelet activation, but for several reasons it may lead to artifacts. It helps to predict the risk of thrombosis, but does not predict the risk of bleeding[16].
The platelet adhesion test under flow conditions simulates the in vivo platelet function using standardized reconstituted blood and measures the adhesion of platelets to the subendothelium[17]. A reduction in platelet count or defects in platelet function are compensated by high plasma levels of vWF.
Identifying the molecular mechanisms of platelet function is based on the detection of the concentrations of second messengers (calcium, cyclic adenosine monophosphate) and the release of platelet granules containing proaggregatory molecules (serotonin, adenosine triphosphate, PF4, and bTG). The most recent studies suggest an increase in platelet activation, as shown in the increase in urinary excretion of 11-dehydro-thromboxane B2, a stable metabolite of thromboxane A2[18].
Prothrombin time (PT) and activated partial thromboplastin time (APTT) were never intended to assess perioperative bleeding risk. PT and its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) were developed to monitor oral anticoagulant therapy, whereas APTT was developed to investigate inheritable single factor deficiencies such as haemophilia and to monitor heparin therapy. These tests do not reproduce in vivo coagulation as they are performed using platelet-poor plasma and thus cannot account for thrombin generation that is mediated by the presence of platelets[19]. They measure only procoagulant factors and are insensitive to plasma levels of the anticoagulants and congenital deficiencies of antithrombin, protein C, or S present with normal PT or APTT. Elevated levels of factor VIII (procoagulant) in combination with decreased protein C (anticoagulant) have been suggested as a marker of a procoagulant imbalance; whether this in vitro hypercoagulability is truly representative of what occurs in vivo remains to be established.
These tests use as the endpoint the formation of a fibrin clot, which occurs after only a small amount of the whole thrombin is generated (5%), so the overall strength and stability of the clot cannot be estimated. The INR was developed to enable comparison of PT values in patients receiving oral anticoagulation. In the other acquired coagulopathies, including liver cirrhosis, PT standardization using the INR is inadequate[20] and between-laboratory variability of INR may increase instead of decreasing[21]. The Quick assay is the methodology most widely used to measure the INR, whereas the Owren assay is used almost exclusively in northern Europe and in Japan. The Quick assay is sensitive for FVII, FX, FV, FII, and fibrinogen, whereas the Owren test is only sensitive for FVII, FX, and FII because the Owren reagent contains fibrinogen and FV; measuring the INR using the Owren test for patients not taking oral anticoagulants is especially inappropriate[22]. Many patients with liver disease have a normal APTT, despite mild baseline deficiencies of multiple procoagulant factors, possibly because of the elevated levels of factor VIII, which shorten APTT and compensate for the multiple procoagulant factor deficiencies[23]. PT/INR and APTT do not take the role of the endothelium in the haemostatic process into account. Protein C and antithrombin need to be activated to exert their full anticoagulant activity with thrombomodulin and with glycosaminoglycans[24,25], which are located on the vascular endothelium.
No studies have directly determined whether global tests such as thrombin generation and thromboelastometry are useful in predicting procedural bleeding risk in patients with liver disease. There are 2 approaches to the measurement of thrombin formation in vivo at a given moment in time. One is to quantify the activation peptide released from prothrombin upon activation by factor Xa (F1+2 fragment) and the thrombin antithrombin complex. The other is to quantify the amount of thrombin that can be generated ex vivo by the addition of a small amount of trigger (usually tissue factor) to a plasma sample: the endogenous thrombin potential[26]. The analysis of thrombin generation is usually done with platelet-poor plasma, although the method can be modified to test platelet-rich plasma. When the thrombin generation test has been modified by the addition of thrombomodulin, an endothelial receptor required for activation of the endogenous anticoagulant protein C system, patients with stable cirrhosis can generate thrombin at a normal to increased rate[27]. Similarly, acute liver failure is not associated with lower thrombin generation[28-30]. There is no correlation between the standard laboratory test and thrombin generation: the in vitro addition of normal plasma to the plasma of patients with advanced cirrhosis showed that, although the PT ratio shortened in many patients, there was no change in thrombin generation[31]. It is unclear whether the test is useful in predicting bleeding or thrombosis in cirrhotic patients. Different commercial techniques used on test samples have yielded major variations[32,33] and defining the test conditions continues to be of utmost importance. The assay is not widely available and, at present, too complex for use in routine diagnostic laboratories.
The principle of thromboelastometry-thromboelastography is based on viscoelastic changes that occur during coagulation of whole blood, giving information on the dynamics of clot formation [clotting time (CT), which provides information about coagulant and anticoagulant activity], clot strength [maximum clot firmness (MCF), which provides information about platelets and fibrinogen], and clot stability (which provides information about fibrinolysis and FXIII). Endothelial function is not directly assessed in this assay. There are 2 commercially available devices: TEG (Haemonetics Corporation, Braintree, MA, United States) and ROTEM (TEM International GmbH, Munich, Germany)[34,35]. Although TEG and ROTEM provide broadly similar information, differences in the disposable blood sampling cups and in the nature of the activators used mean that the results are not comparable[36,37]. Cirrhotic patients have a decreased whole blood clot formation capacity with an apparently unaltered resistance to clot lysis[38]. A good correlation has been found in these patients between platelet count and MCF (r = 0.691) and a reasonably good correlation between Clauss fibrinogen and MCF (r = 0.590)[39]. A small, randomized prospective study in liver transplantation patients showed a significant reduction in transfusion in the TEG monitored group, most notably in the use of fresh frozen plasma (FFP)[40]. Use of higher thromboelastogram transfusion values is not associated with greater blood loss in liver transplant surgery[41]. If the platelet contribution is inhibited by cytochalasin D or abciximab, the test has a good correlation with plasma fibrinogen levels. Although hypercoagulability can be detected by viscoelastic tests, there is no consensus on which parameters should be used to define it. Large-scale prospective outcome studies are necessary to evaluate the impact of viscoelastic tests in managing thromboprophylaxis. Table 1 outlines the available tests.
| Primary haemostasis | |
| Platelet count | Detects risks of bleeding only at extreme levels |
| Bleeding time | Generally, does not predict bleeding risk |
| Platelet function analyser | Thrombopenia makes the interpretation difficult |
| Thromboelastometry | Little experience in cirrhotic patients |
| Platelet function assay Aggregometry | Thrombopenia makes the interpretation difficult |
| Membrane molecule expression | Specialized laboratory; mostly, in research setting |
| Soluble activation markers | |
| Platelet adhesion under flow conditions | |
| Molecular mechanisms | |
| Secondary haemostasis | |
| PT and APTT | Insensitive to plasma levels of the anticoagulants |
| Thrombin generation | Too complex for use in routine diagnostic laboratories |
| Thromboelastometry | No standardization of parameters in cirrhotic patients |
| Low predictive positive value | |
| Fibrinolysis | |
| Fibrinolysis markers | No clear evidence between hyperfibrinolysis and bleeding in cirrhotic patients |
| Euglobulin clot lysis time | Not widely available |
Evidence increasingly shows that cirrhotic patients are characterized predominantly by clotting activation with secondary hyperfibrinolysis; there is no clear evidence that hyperfibrinolysis is associated with bleeding. Fibrinolysis can be investigated by measuring its individual components (pro- and antifibrinolytic drivers): tissue plasminogen activator (tPA), urokinase plasminogen activator, FXIIa, plasminogen activator inhibitor (PAI), plasmin inhibitor (alpha 2-antiplasmin), and thrombin-activatable fibrinolysis inhibitor (TAFI). Alternatively, it can be investigated through global tests designed to assess the time for clot dissolution: thromboelastography[42], diluted whole blood clot lysis assays[43], and euglobulin clot lysis time[44]. Caution should be taken in comparing results from different assays. For example, classical thromboelastography is performed using whole non-anticoagulated blood, and clot formation is initiated by (non-physiological) contact activation; variations on this technique make use of tissue factor-induced coagulation of citrated whole blood, but these variations have not yet been used to determine fibrinolytic potential in patients with liver failure. Dilute whole blood clot lysis time is determined in the absence of calcium, and a clot is formed by addition of thrombin, so this test is independent of coagulation and therefore of TAFI. Finally, as the euglobulin fraction contains no inhibitors of fibrinolysis, enhanced clot lysis times presumably only reflect elevated tPA levels[45].
These methodological differences explain why studies assessing fibrinolysis have led to controversial findings[46,47]. Hyperfibrinolysis markers have been found in 30% of cirrhotic patients and are proportional to the degree of hepatic dysfunction, pointing to activation of clotting (mediated by the FVIII/protein C imbalance), with secondary hyperfibrinolysis[48-50]. These coagulation disturbances resemble disseminated intravascular coagulation; a relatively stable platelet count and characteristically high FVIII levels distinguish this process from disseminated intravascular coagulation. The extent of fibrinolysis is likely to be overestimated in this population if the definition is based solely on indirect markers[49]. When fibrinolysis was explored with a plasma global test, the results obtained in cirrhotic patients were not different from those obtained in healthy controls[51]. An important subject of debate is whether hyperfibrinolysis plays a role in precipitating bleeding: at present, there is no clear evidence for this[52]. Thromboelastometry-thromboelastography, using the clot lysis index parameter, is the current gold standard for the diagnosis of significant hyperfibrinolysis in the clinical setting[53].
Anaemia, endotoxaemia, portal hypertension, kidney failure and underlying liver disease affect the state of haemostasis. An improvement in anaemia to at least 30% of haematocrit[54] improves platelet adhesion because more red blood cells push platelets and leucocytes from the axial centre toward the periphery, thereby enhancing cell contact with the vessel wall and formation of the primary haemostatic plug[55]. The release from red blood cells of adenosine diphosphate, a powerful inducer of platelet aggregation, and the scavenging effect on nitric oxide exerted by haemoglobin contribute to the defective primary haemostasis in the anaemic condition.
Endotoxaemia increases the tissue factor expression and the synthesis of vWF by endothelial cells[56] and has been related to the high ratio of FVIII to anticoagulant factors[57], promoting a state of low-grade disseminated intravascular coagulation[58,59]. Portal circulation has higher endotoxaemia than peripheral circulation. The administration of prophylactic antibiotics can reduce the incidence of early rebleeding after an episode of bleeding[60]. It has been suggested that endotoxaemia could be the common mechanism that links activation of clotting (portal thrombosis), hyperfibrinolysis, and gastrointestinal bleeding[61].
Portal hypertension is the main risk factor for bleeding[62] in cirrhotic patients. Patients with portal hypertension have a pooling of blood on the venous side of the circulation and arterial hypotension. Additional fluid that is introduced into the circulation is added to the venous overload. Low central venous pressure has been shown to considerably reduce perioperative blood loss during liver resection and liver transplant surgery[63-65].
Kidney failure also predicts bleeding risk as uraemia impairs platelet function[66]. Biochemical platelet alterations result in defects of aggregation and impaired platelet adhesion to injured vessel walls[67]. In addition, kidney failure is also often accompanied by anaemia.
The haemostatic balance may vary according to the degree and underlying cause of liver disease. Patients with cholestatic liver diseases are characterized by a normal or hypercoagulable state: higher PAI-1 concentrations as compared with other aetiologies. This state results in less hyperfibrinolysis in the reperfusion phase during liver transplantation and antifibrinolytic therapy is not usually administered[68]. Platelet hyperactivity has also been reported in patients with cholestatic liver disease[12,42]. A prothrombotic state has been reported in acute liver injury based on an imbalance between vWF/ADAMTS13, intact thrombin generation, and high levels of PAI-1[28,69,70]. Minimal effects on haemostasis are assessed by thromboelastography in these patients[71], despite the severe derangement shown by standard coagulation tests. Patients transplanted for familial amyloidotic polyneuropathy appear to have an increased risk of early hepatic artery thrombosis in comparison with transplant patients with cirrhosis[72]. Nonalcoholic fatty liver diseases have also been associated with hypercoagulation and thrombophilia[73]. Splanchnic vein thrombosis has been described more frequently in patients with autoimmune cirrhosis[74].
There is no correlation between coagulation tests and the incidence of bleeding complications after invasive procedures in patients with chronic liver disease. Despite the abnormalities shown in coagulation tests, these patients have a low incidence of complications during invasive procedures and, even if bleeding occurs, it is rarely related to the alteration of coagulation tests. The risk of post-procedural bleeding in patients evaluated for liver transplantation is related to platelet count, not to PT or APTT. There is no scientific evidence to indicate that the level of thrombopenia is associated with a greater risk of bleeding[49,75]. Moreover, the results of trials evaluating haemostatic agents have in general been negative or inconclusive[76]. Some of these agents are extremely expensive and are not free of side effects.
Haemostasis plays a minor role in bleeding from oesophageal varices, which accounts for 80% to 90% of bleeding episodes in cirrhotic patients. It is predominantly related to haemodynamic factors, local vascular abnormalities, and Child-Pugh score, which describes the severity of liver disease[77,78], with a minor role for haemostasis[79]. Furthermore, spontaneous bleeding is less frequent than would be expected from the abnormality shown in laboratory tests. The risk of spontaneous intracranial bleeding is equal to that of control subjects[80] and is more related to the aetiology of the underlying disease (alcohol 1.8%, hepatitis C virus 0.3%) than to the severity of the liver disease.
Liver transplantation can be performed with very low rates of transfusion when a fluid restriction policy is applied. However, it should never be performed without prophylaxis or transfusion requirements in a patient with an established (classical) coagulopathy, such as haemophilia. Transfusion requirements have dropped in liver transplantation to a median of 2-3 units of red blood cells in many centres and a substantial number of patients (up to 50%) are transplanted without the need for transfusion when a fluid restriction policy is applied[81-83]. It could be assumed that a restrictive transfusion policy would be valid for invasive procedures in this population[84]. Surprisingly, one study showed that patients with liver disease, who accounted for 7.7% of the patients receiving transfusions, utilized 32% of administered plasma and 13% of the platelets[85].
Patients with liver disease have portal vein thrombosis, peripheral vein thrombosis, and pulmonary embolism as clinical manifestations of thrombotic tendency. Northup et al[86] found that about 0.5% of cirrhotic patients had a new diagnosis of venous thromboembolism (VTE). In 2 retrospective analyses the association between cirrhosis and VTE was also evident[87,88]. Gulley et al[88] observed a VTE incidence of 2.6% in patients with moderate-severe liver failure. The largest analysis on this topic demonstrated that, compared with the general population, patients with cirrhosis have approximately double the risk of VTE and a slightly lower risk of pulmonary embolism[89]. These findings suggest that cirrhotic patients are not protected against thrombosis and, despite INR elongation, they have an increased risk of thrombosis compared with age-matched controls. Preliminary reports indicate that standard pharmacologic prophylaxis is safe[90], but its effectiveness has not been proven and the appropriate agents remain to be determined[91]. Severe cirrhosis is a major risk factor for portal vein thrombosis[92] and the risk is related in part to the severity of the liver disease[78]. Portal vein thrombosis has been encountered in 8% to 26% of patients who were candidates for liver transplantation[93]. A recent prospective randomized control study of fixed dose prophylactic low-molecular-weight heparin vs no therapy administered for 1 year in 70 patients with advanced cirrhosis[94] demonstrated that no patients in the enoxaparin group developed portal vein thrombosis, compared with 17% in the control group. In addition, the incidence of documented bacterial infections was significantly lower in the enoxaparin group (8.8% vs 33.3%). This study raises an interesting hypothesis as to whether, by improving intestinal microcirculation, anticoagulation reduces the frequency of portal thrombosis and the progression of cirrhosis.
Transfusion in an attempt to correct anomalous laboratory tests is not justified and is potentially harmful. Recent general consensus guidelines on plasma transfusion do not recommend FFP for prophylactic correction of abnormal PT or INR and studies on patients with liver disease do not support the use of FFP as a prophylactic measure[95,96]. Numerical improvement in PT requires transfusion of at least 2 to 6 units of FFP[97,98]. Zimmon et al[99] found a linear correlation between portal pressure and blood volume in cirrhotic patients: every 100 mL of blood volume expansion, in a relatively short period of time, predicted a mean increase in portal pressure of 1.03 mmHg. To reach a target INR value of 1.5 starting from INR values of 2 or 4, the expected increase in portal pressure ranges from 15.5 to 25.8 mmHg, respectively[100]. Indeed, reaching a PT target reported as safe may paradoxically trigger bleeding in liver disease patients[101].
Moreover, transfusion of FFP may be accompanied by side effects such as acute lung injury (patients with chronic liver disease have a greater individual risk of transfusion-related acute lung injury than other populations). Based on the knowledge that platelets support thrombin generation[19], platelet transfusion should be considered in severely thrombocytopenic patients. The American Association for the Study of Liver Disease guidelines recommend that platelet transfusion before liver biopsy, transcutaneously or transvenously, should be considered when platelet counts are lower than 50 × 109/L-60 × 109/L (Class 1, Level C, i.e., without evidence from randomized studies)[102]. Also, there is consensus that the platelet count should not be allowed to fall below 50 × 109/L in patients with acute bleeding[16]. Platelet transfusions have been associated with acute lung injury and decreased survival after orthotopic liver transplantation[103]. An informal survey showed the high degree of variation in practice in the assessment of bleeding risk in patients with liver disease[49]. Although the respondents acknowledged the lack of sound data, many of their opinions were influenced by legal considerations. Only very cautious use of prophylactic therapy is warranted in very high-risk procedures such as intracranial pressure monitor placement in patients with acute liver failure[30,104]. In the remaining clinical settings, there is now substantial experience supporting a wait-and-see policy and rescue therapy when bleeding occurs[105].
Fluid restriction policy, optimization of renal function and control of infection can improve haemostatic competence. The experience acquired in the field of liver transplantation suggests that a fluid restriction policy could be applied in invasive procedures. This policy should be aimed at reducing hypertension in the splanchnic area, thus avoiding unnecessary transfusion of haemoderivatives and hypervolemia. Renal status should be optimized in order to reduce the risk of bleeding. One of the main mechanisms involved is uraemia, which impairs platelet function[106]. Dialysis was the first step to improve the bleeding tendency in uraemia, although it was not entirely satisfactory. In addition, kidney failure is often accompanied by anaemia. The prophylactic administration of antibiotics to patients with cirrhosis is known to reduce mortality and improve haemostatic function, but the exact mechanism is unknown. As bacterial infection has been associated with the risk of bleeding, failure to control bleeding and mortality, the treatment of infections before invasive procedure is recommended[107,108].
Anticoagulation should not be withheld from patients with liver disease. Clinicians should be alerted to the possibility of thrombosis occurring in these patients[84]. With the current lack of definitive treatment data and a significant defined incidence of in-hospital VTE, it would seem reasonable to offer standard pharmacologic VTE prophylaxis in hospitalized cirrhotic patients unless an obvious contraindication is present, regardless of standard coagulation laboratory values. In other words, this population should be considered in the same way as any medical inpatients[3]. Treatment for new diagnoses of VTE should essentially follow anticoagulation. If a delay in treatment is expected because of high-risk eradication of oesophageal varices, a temporary inferior vena cava filter may be considered, although its use is controversial in many situations[109].
The resolution of thrombus portal circulation is becoming a critical issue that needs to be solved[110]. The effect of anticoagulants on portal vein thrombosis in cirrhosis has already been investigated in some small series[109-112]; the total number of cirrhotic patients treated with a therapeutic dose of low-molecular-weight heparin is 135. Globally, the results are positive, with a rate of recanalization of roughly 30% to 50% after a period of anticoagulation as long as approximately 6 mo[113]. The treatment seems to be safe, with little incidence of fatal or major bleedings. However, although the current tendency is to start declotting treatment, there is no consensus regarding a de novo portal thrombosis in a cirrhotic patient.
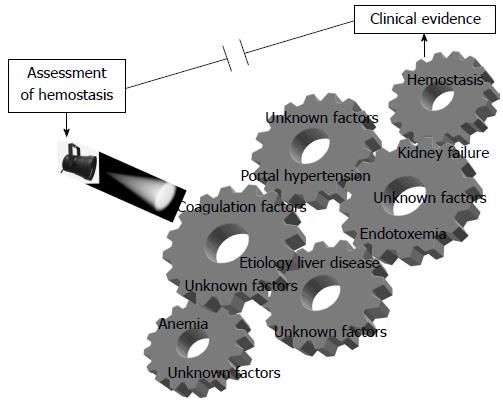
Haemostasis in liver disease is extremely complex and the main difficulty facing the physician in a patient with liver disease is the lack of a comprehensive test for clinical estimation of bleeding or clotting risk (Figure 1). From a practical point of view, each patient should be assessed individually, taking into account the underlying cause of the liver disease, other factors influencing haemostatic competence, and the clinical context.
This clinical problem can possibly be improved by (1) advancing knowledge of specific coagulation pathways, such as those related to platelet function, which have been little investigated in cirrhotic patients; and (2) developing new tests as close as possible to in vivo conditions and confronting them with a thrombotic or haemorrhagic hard clinical end point. Additionally, given that several variables affect haemostatic competence in patients with liver disease, a composite parameter including factors that have proven to be significant in large study series could be useful for assessing the risk of bleeding and thrombosis in this population.
P- Reviewer: Mendez-Sanchez N S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Wang CH
| 1. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [PubMed] [Cited in This Article: ] |
| 2. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 3. | Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 4. | Ferro D, Quintarelli C, Lattuada A, Leo R, Alessandroni M, Mannucci PM, Violi F. High plasma levels of von Willebrand factor as a marker of endothelial perturbation in cirrhosis: relationship to endotoxemia. Hepatology. 1996;23:1377-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | La Mura V, Reverter JC, Flores-Arroyo A, Raffa S, Reverter E, Seijo S, Abraldes JG, Bosch J, García-Pagán JC. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut. 2011;60:1133-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Blake JC, Sprengers D, Grech P, McCormick PA, McIntyre N, Burroughs AK. Bleeding time in patients with hepatic cirrhosis. BMJ. 1990;301:12-15. [PubMed] [Cited in This Article: ] |
| 7. | de Franchis R, Arcidiacono PG, Carpinelli L, Andreoni B, Cestari L, Brunati S, Zambelli A, Battaglia G, Mannucci PM. Randomized controlled trial of desmopressin plus terlipressin vs. terlipressin alone for the treatment of acute variceal hemorrhage in cirrhotic patients: a multicenter, double-blind study. New Italian Endoscopic Club. Hepatology. 1993;18:1102-1107. [PubMed] [Cited in This Article: ] |
| 8. | Wong AY, Irwin MG, Hui TW, Fung SK, Fan ST, Ma ES. Desmopressin does not decrease blood loss and transfusion requirements in patients undergoing hepatectomy. Can J Anaesth. 2003;50:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Arshad F, Stoof SC, Leebeek FW, Ruitenbeek K, Adelmeijer J, Blokzijl H, van den Berg AP, Porte RJ, Kruip MJ, Lisman T. Infusion of DDAVP does not improve primary hemostasis in patients with cirrhosis. Liver Int. 2015;35:1809-1815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Lisman T, Caldwell SH, Porte RJ, Leebeek FW. Consequences of abnormal hemostasis tests for clinical practice. J Thromb Haemost. 2006;4:2062-2063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Escolar G, Cases A, Viñas M, Pino M, Calls J, Cirera I, Ordinas A. Evaluation of acquired platelet dysfunctions in uremic and cirrhotic patients using the platelet function analyzer (PFA-100 ): influence of hematocrit elevation. Haematologica. 1999;84:614-619. [PubMed] [Cited in This Article: ] |
| 12. | Pihusch R, Rank A, Göhring P, Pihusch M, Hiller E, Beuers U. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol. 2002;37:548-555. [PubMed] [Cited in This Article: ] |
| 13. | James K, Bertoja E, O’Beirne J, Mallett S. Use of thromboelastography PlateletMapping to monitor antithrombotic therapy in a patient with Budd-Chiari syndrome. Liver Transpl. 2010;16:38-41. [PubMed] [Cited in This Article: ] |
| 14. | Elalamy I, Gkalea V, Gerotziafas G, Ketatni H, Hatmi M. [The usefulness of platelet function evaluation in clinical practice]. Ann Biol Clin (Paris). 2013;71:47-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 15. | Vinholt PJ, Hvas AM, Nybo M. An overview of platelet indices and methods for evaluating platelet function in thrombocytopenic patients. Eur J Haematol. 2014;92:367-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Violi F, Basili S, Raparelli V, Chowdary P, Gatt A, Burroughs AK. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J Hepatol. 2011;55:1415-1427. [PubMed] [Cited in This Article: ] |
| 17. | Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, Leebeek FW. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 18. | Davì G, Ferro D, Basili S, Iuliano L, Camastra C, Giammarresi C, Santarone S, Rocca B, Landolfi R, Ciabattoni G. Increased thromboxane metabolites excretion in liver cirrhosis. Thromb Haemost. 1998;79:747-751. [PubMed] [Cited in This Article: ] |
| 19. | Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell’Era A, Fabris F, Salerno F, Mannucci PM. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44:440-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Trotter JF, Brimhall B, Arjal R, Phillips C. Specific laboratory methodologies achieve higher model for endstage liver disease (MELD) scores for patients listed for liver transplantation. Liver Transpl. 2004;10:995-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Reverter JC. Abnormal hemostasis tests and bleeding in chronic liver disease: are they related? Yes. J Thromb Haemost. 2006;4:717-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Magnusson M, Sten-Linder M, Bergquist A, Rajani R, Kechagias S, Fischler B, Németh A, Lindahl TL. The international normalized ratio according to Owren in liver disease: interlaboratory assessment and determination of international sensitivity index. Thromb Res. 2013;132:346-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Ng VL. Prothrombin time and partial thromboplastin time assay considerations. Clin Lab Med. 2009;29:253-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Dahlbäck B. Progress in the understanding of the protein C anticoagulant pathway. Int J Hematol. 2004;79:109-116. [PubMed] [Cited in This Article: ] |
| 25. | Huntington JA. Mechanisms of glycosaminoglycan activation of the serpins in hemostasis. J Thromb Haemost. 2003;1:1535-1549. [PubMed] [Cited in This Article: ] |
| 26. | Brummel-Ziedins KE, Wolberg AS. Global assays of hemostasis. Curr Opin Hematol. 2014;21:395-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, Mannuccio Mannucci P. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 488] [Cited by in F6Publishing: 458] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 28. | Lisman T, Bakhtiari K, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost. 2012;10:1312-1319. [PubMed] [Cited in This Article: ] |
| 29. | Stravitz RT, Lisman T, Luketic VA, Sterling RK, Puri P, Fuchs M, Ibrahim A, Lee WM, Sanyal AJ. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56:129-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 30. | Munoz SJ, Stravitz RT, Gabriel DA. Coagulopathy of acute liver failure. Clin Liver Dis. 2009;13:95-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Tripodi A, Chantarangkul V, Primignani M, Clerici M, Dell’era A, Aghemo A, Mannucci PM. Thrombin generation in plasma from patients with cirrhosis supplemented with normal plasma: considerations on the efficacy of treatment with fresh-frozen plasma. Intern Emerg Med. 2012;7:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008;142:889-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Berntorp E, Salvagno GL. Standardization and clinical utility of thrombin-generation assays. Semin Thromb Hemost. 2008;34:670-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Afshari A, Wikkelsø A, Brok J, Møller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011;CD007871. [PubMed] [Cited in This Article: ] |
| 35. | Mallett SV, Chowdary P, Burroughs AK. Clinical utility of viscoelastic tests of coagulation in patients with liver disease. Liver Int. 2013;33:961-974. [PubMed] [Cited in This Article: ] |
| 36. | Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth. 2006;20:548-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 37. | Blasi A, Beltran J, Pereira A. Viscoelastic test and fibrinogen values: how much disagreement is permissible? Anesth Analg. 2014;119:1452-1453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Kleinegris MC, Bos MH, Roest M, Henskens Y, Ten Cate-Hoek A, Van Deursen C, Spronk HM, Reitsma PH, De Groot PG, Ten Cate H. Cirrhosis patients have a coagulopathy that is associated with decreased clot formation capacity. J Thromb Haemost. 2014;12:1647-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Tripodi A, Primignani M, Chantarangkul V, Viscardi Y, Dell’Era A, Fabris FM, Mannucci PM. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. 2009;124:132-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, Chan KH, Mandell S, Tsou MY. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42:2590-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 41. | Wang SC, Lin HT, Chang KY, Mandell MS, Ting CK, Chu YC, Loong CC, Chan KH, Tsou MY. Use of higher thromboelastogram transfusion values is not associated with greater blood loss in liver transplant surgery. Liver Transpl. 2012;18:1254-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Ben-Ari Z, Panagou M, Patch D, Bates S, Osman E, Pasi J, Burroughs A. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol. 1997;26:554-559. [PubMed] [Cited in This Article: ] |
| 43. | Hersch SL, Kunelis T, Francis RB. The pathogenesis of accelerated fibrinolysis in liver cirrhosis: a critical role for tissue plasminogen activator inhibitor. Blood. 1987;69:1315-1319. [PubMed] [Cited in This Article: ] |
| 44. | Comp PC, Jacocks RM, Rubenstein C, Radcliffe R. A lysine-absorbable plasminogen activator is elevated in conditions associated with increased fibrinolytic activity. J Lab Clin Med. 1981;97:637-645. [PubMed] [Cited in This Article: ] |
| 45. | Lisman T, Leebeek FW, de Groot PG. Haemostatic abnormalities in patients with liver disease. J Hepatol. 2002;37:280-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Papatheodoridis GV, Patch D, Webster GJ, Brooker J, Barnes E, Burroughs AK. Infection and hemostasis in decompensated cirrhosis: a prospective study using thrombelastography. Hepatology. 1999;29:1085-1090. [PubMed] [Cited in This Article: ] |
| 47. | Ben-Ari Z, Osman E, Hutton RA, Burroughs AK. Disseminated intravascular coagulation in liver cirrhosis: fact or fiction? Am J Gastroenterol. 1999;94:2977-2982. [PubMed] [Cited in This Article: ] |
| 48. | Violi F, Ferro D, Basili S, Quintarelli C, Musca A, Cordova C, Balsano F. Hyperfibrinolysis resulting from clotting activation in patients with different degrees of cirrhosis. The CALC Group. Coagulation Abnormalities in Liver Cirrhosis. Hepatology. 1993;17:78-83. [PubMed] [Cited in This Article: ] |
| 49. | Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, Tripodi A, Sanyal AJ. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 50. | Joist JH. AICF and DIC in liver cirrhosis: expressions of a hypercoagulable state. Am J Gastroenterol. 1999;94:2801-2803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Lisman T, Leebeek FW, Mosnier LO, Bouma BN, Meijers JC, Janssen HL, Nieuwenhuis HK, De Groot PG. Thrombin-activatable fibrinolysis inhibitor deficiency in cirrhosis is not associated with increased plasma fibrinolysis. Gastroenterology. 2001;121:131-139. [PubMed] [Cited in This Article: ] |
| 52. | Violi F, Ferro D. Clotting activation and hyperfibrinolysis in cirrhosis: implication for bleeding and thrombosis. Semin Thromb Hemost. 2013;39:426-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012;26:1-13. [PubMed] [Cited in This Article: ] |
| 54. | Livio M, Gotti E, Marchesi D, Mecca G, Remuzzi G, de Gaetano G. Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet. 1982;2:1013-1015. [PubMed] [Cited in This Article: ] |
| 55. | Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207:541-543. [PubMed] [Cited in This Article: ] |
| 56. | Moore KL, Andreoli SP, Esmon NL, Esmon CT, Bang NU. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987;79:124-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 342] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 57. | Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 963] [Cited by in F6Publishing: 877] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 58. | Thalheimer U, Triantos C, Samonakis D, Patch D, Burroughs AK, Riddell A, Perry D. Endogenous heparinoids in acute variceal bleeding. Gut. 2005;54:310-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Violi F, Ferro D, Basili S, Saliola M, Quintarelli C, Alessandri C, Cordova C. Association between low-grade disseminated intravascular coagulation and endotoxemia in patients with liver cirrhosis. Gastroenterology. 1995;109:531-539. [PubMed] [Cited in This Article: ] |
| 60. | Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Soares-Weiser K, Uribe M. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2010;CD002907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 61. | Violi F. How to concile bleeding and thrombotic tendency in liver cirrhosis? J Thromb Haemost. 2006;4:2065-2066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Tripodi A. The validity of the INR system for patients with liver disease. J Thromb Thrombolysis. 2011;31:209-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 63. | Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 287] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 64. | Feng ZY, Xu X, Zhu SM, Bein B, Zheng SS. Effects of low central venous pressure during preanhepatic phase on blood loss and liver and renal function in liver transplantation. World J Surg. 2010;34:1864-1873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Massicotte L, Lenis S, Thibeault L, Sassine MP, Seal RF, Roy A. Effect of low central venous pressure and phlebotomy on blood product transfusion requirements during liver transplantations. Liver Transpl. 2006;12:117-123. [PubMed] [Cited in This Article: ] |
| 66. | Pavord S, Myers B. Bleeding and thrombotic complications of kidney disease. Blood Rev. 2011;25:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 68. | Segal H, Cottam S, Potter D, Hunt BJ. Coagulation and fibrinolysis in primary biliary cirrhosis compared with other liver disease and during orthotopic liver transplantation. Hepatology. 1997;25:683-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Hugenholtz GC, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT, Lisman T. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology. 2013;58:752-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 70. | Gatt A, Chowdary P. Does balanced haemostasis equate to normal coagulation in patients with acute liver failure? Liver Int. 2014;34:652-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Habib M, Roberts LN, Patel RK, Wendon J, Bernal W, Arya R. Evidence of rebalanced coagulation in acute liver injury and acute liver failure as measured by thrombin generation. Liver Int. 2014;34:672-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Bispo M, Marcelino P, Marques HP, Martins A, Perdigoto R, Aguiar MJ, Mourão L, Barroso E. Domino versus deceased donor liver transplantation: association with early graft function and perioperative bleeding. Liver Transpl. 2011;17:270-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Northup PG, Argo CK, Shah N, Caldwell SH. Hypercoagulation and thrombophilia in nonalcoholic fatty liver disease: mechanisms, human evidence, therapeutic implications, and preventive implications. Semin Liver Dis. 2012;32:39-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Manzanet G, Sanjuán F, Orbis P, López R, Moya A, Juan M, Vila J, Asensi J, Sendra P, Ruíz J. Liver transplantation in patients with portal vein thrombosis. Liver Transpl. 2001;7:125-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 75. | Tripodi A, Primignani M, Mannucci PM. Abnormalities of hemostasis and bleeding in chronic liver disease: the paradigm is challenged. Intern Emerg Med. 2010;5:7-12. [PubMed] [Cited in This Article: ] |
| 76. | Bosch J, Thabut D, Bendtsen F, D’Amico G, Albillos A, González Abraldes J, Fabricius S, Erhardtsen E, de Franchis R. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123-1130. [PubMed] [Cited in This Article: ] |
| 77. | Bellest L, Eschwège V, Poupon R, Chazouillères O, Robert A. A modified international normalized ratio as an effective way of prothrombin time standardization in hepatology. Hepatology. 2007;46:528-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 78. | Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol. 2012;57:203-212. [PubMed] [Cited in This Article: ] |
| 79. | Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. N Engl J Med. 2001;345:669-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 298] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 80. | Lai CH, Cheng PY, Chen YY. Liver cirrhosis and risk of intracerebral hemorrhage: a 9-year follow-up study. Stroke. 2011;42:2615-2617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Massicotte L, Denault AY, Beaulieu D, Thibeault L, Hevesi Z, Nozza A, Lapointe R, Roy A. Transfusion rate for 500 consecutive liver transplantations: experience of one liver transplantation center. Transplantation. 2012;93:1276-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | de Boer MT, Molenaar IQ, Hendriks HG, Slooff MJ, Porte RJ. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22:265-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | Jabbour N, Gagandeep S, Mateo R, Sher L, Strum E, Donovan J, Kahn J, Peyre CG, Henderson R, Fong TL. Live donor liver transplantation without blood products: strategies developed for Jehovah’s Witnesses offer broad application. Ann Surg. 2004;240:350-357. [PubMed] [Cited in This Article: ] |
| 84. | Weeder PD, Porte RJ, Lisman T. Hemostasis in liver disease: implications of new concepts for perioperative management. Transfus Med Rev. 2014;28:107-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Shah NL, Northup PG, Caldwell SH. A clinical survey of bleeding, thrombosis, and blood product use in decompensated cirrhosis patients. Ann Hepatol. 2012;11:686-690. [PubMed] [Cited in This Article: ] |
| 86. | Northup PG, McMahon MM, Ruhl AP, Altschuler SE, Volk-Bednarz A, Caldwell SH, Berg CL. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101:1524-1528; quiz 1680. [PubMed] [Cited in This Article: ] |
| 87. | García-Fuster MJ, Abdilla N, Fabiá MJ, Fernández C, Oliver V. [Venous thromboembolism and liver cirrhosis]. Rev Esp Enferm Dig. 2008;100:259-262. [PubMed] [Cited in This Article: ] |
| 88. | Gulley D, Teal E, Suvannasankha A, Chalasani N, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53:3012-3017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 89. | Søgaard KK, Horváth-Puhó E, Grønbaek H, Jepsen P, Vilstrup H, Sørensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104:96-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 288] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 90. | Northup PG, Intagliata NM. Anticoagulation in cirrhosis patients: what don’t we know? Liver Int. 2011;31:4-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Bechmann LP, Sichau M, Wichert M, Gerken G, Kröger K, Hilgard P. Low-molecular-weight heparin in patients with advanced cirrhosis. Liver Int. 2011;31:75-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 92. | Plessier A, Rautou PE, Valla DC. Management of hepatic vascular diseases. J Hepatol. 2012;56 Suppl 1:S25-S38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 93. | Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31:366-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 94. | Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253-1260.e1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 95. | Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 471] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 96. | O’Shaughnessy DF, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S, Williamson LM. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11-28. [PubMed] [Cited in This Article: ] |
| 97. | Youssef WI, Salazar F, Dasarathy S, Beddow T, Mullen KD. Role of fresh frozen plasma infusion in correction of coagulopathy of chronic liver disease: a dual phase study. Am J Gastroenterol. 2003;98:1391-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 98. | Tripodi A, Primignani M, Chantarangkul V, Lemma L, Jovani M, Rebulla P, Mannucci PM. Global hemostasis tests in patients with cirrhosis before and after prophylactic platelet transfusion. Liver Int. 2013;33:362-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 99. | Zimmon DS, Kessler RE. The portal pressure-blood volume relationship in cirrhosis. Gut. 1974;15:99-101. [PubMed] [Cited in This Article: ] |
| 100. | Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006;126:133-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Giannini EG, Stravitz RT, Caldwell SH. Correction of hemostatic abnormalities and portal pressure variations in patients with cirrhosis. Hepatology. 2014;60:1442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1449] [Cited by in F6Publishing: 1419] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 103. | Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108:1083-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 104. | Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL, Blei AT, Fontana RJ, McGuire BM, Rossaro L. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498-2508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 105. | Lisman T, Caldwell SH, Leebeek FW, Porte RJ. Is chronic liver disease associated with a bleeding diathesis? J Thromb Haemost. 2006;4:2059-2060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Noris M, Remuzzi G. Uremic bleeding: closing the circle after 30 years of controversies? Blood. 1999;94:2569-2574. [PubMed] [Cited in This Article: ] |
| 107. | Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 245] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 108. | Vivas S, Rodriguez M, Palacio MA, Linares A, Alonso JL, Rodrigo L. Presence of bacterial infection in bleeding cirrhotic patients is independently associated with early mortality and failure to control bleeding. Dig Dis Sci. 2001;46:2752-2757. [PubMed] [Cited in This Article: ] |
| 109. | Delgado MG, Seijo S, Yepes I, Achécar L, Catalina MV, García-Criado A, Abraldes JG, de la Peña J, Bañares R, Albillos A. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 110. | Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, Denninger MH, Sauvanet A, Valla D, Durand F. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691-697. [PubMed] [Cited in This Article: ] |
| 111. | Amitrano L, Guardascione MA, Menchise A, Martino R, Scaglione M, Giovine S, Romano L, Balzano A. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol. 2010;44:448-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 112. | Senzolo M, M Sartori T, Rossetto V, Burra P, Cillo U, Boccagni P, Gasparini D, Miotto D, Simioni P, Tsochatzis E. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32:919-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 113. | Rodriguez-Castro KI, Simioni P, Burra P, Senzolo M. Anticoagulation for the treatment of thrombotic complications in patients with cirrhosis. Liver Int. 2012;32:1465-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |