Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8615
Peer-review started: February 10, 2015
First decision: March 10, 2015
Revised: April 3, 2015
Accepted: May 20, 2015
Article in press: May 21, 2015
Published online: July 28, 2015
AIM: To investigate the roles of toll-like receptor 4 (TLR4) and nuclear factor (NF)-κB on cystathionine β synthetase (CBS) expression and visceral hypersensitivity in rats.
METHODS: This study used 1-7-wk-old male Sprague-Dawley rats. Western blot analysis was employed to measure the expression of TLR4, NF-κB and the endogenous hydrogen sulfide-producing enzyme CBS in colon dorsal root ganglia (DRG) from control and “irritable bowel syndrome” rats induced by neonatal colonic inflammation (NCI). Colon-specific DRG neurons were labeled with Dil and acutely dissociated to measure excitability with patch-clamp techniques. Immunofluorescence was employed to determine the co-expression of TLR4, NF-κB and CBS in DiI-labeled DRG neurons.
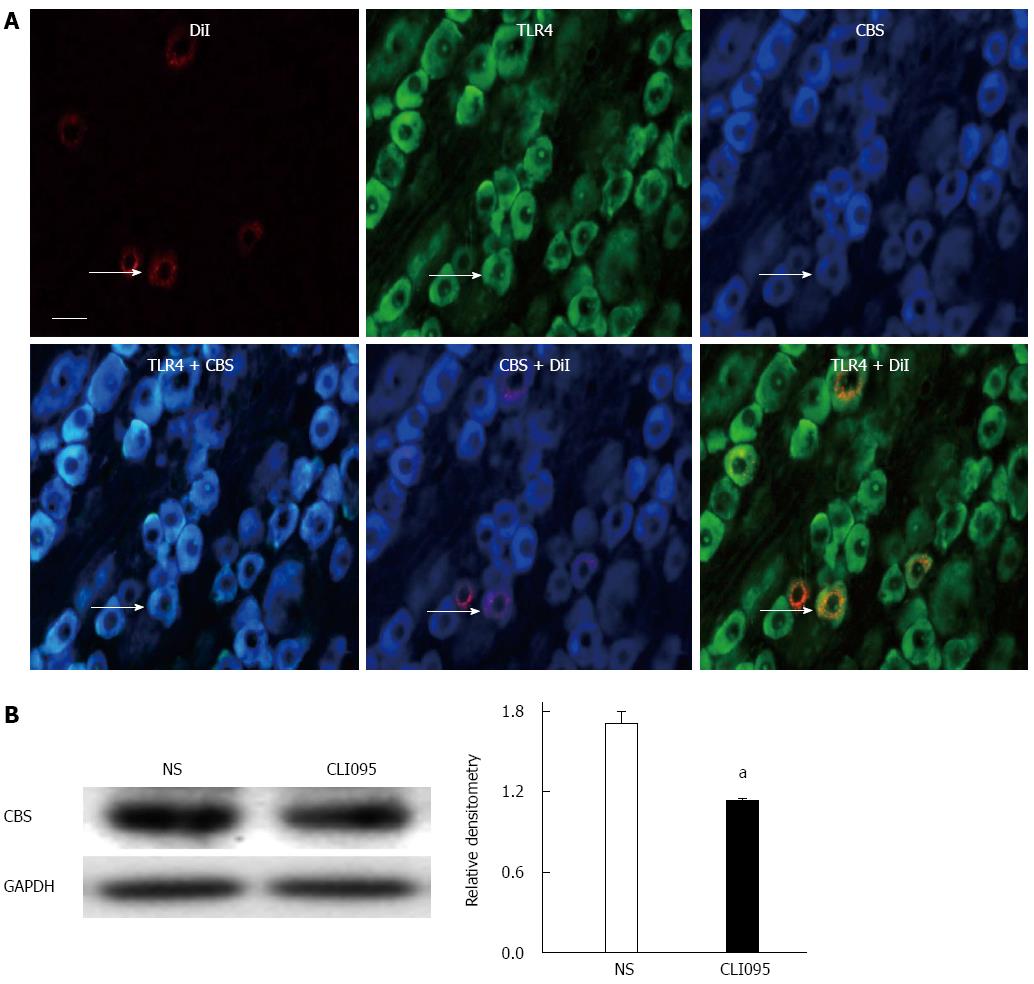
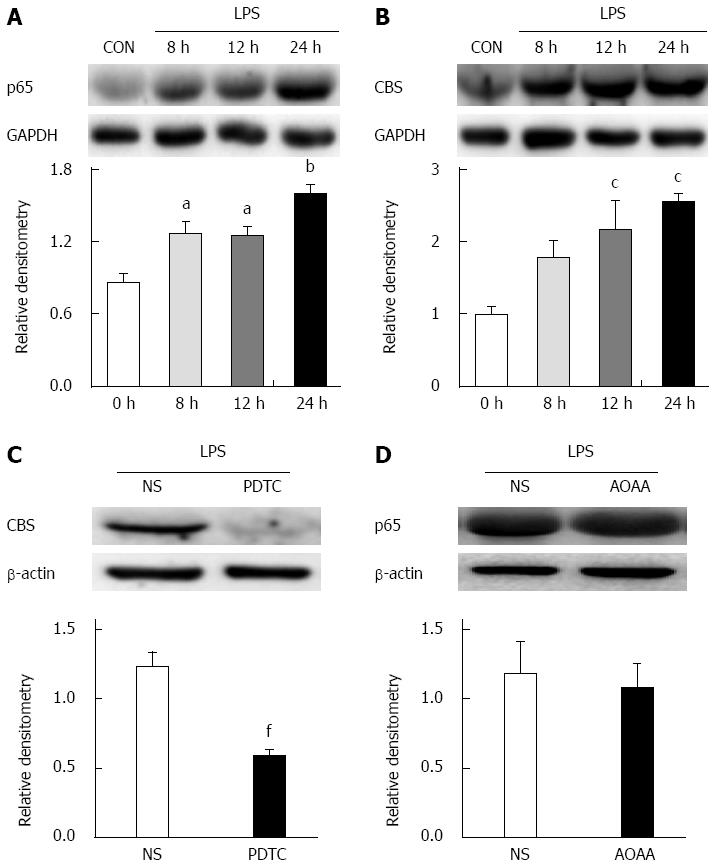
RESULTS: NCI significantly upregulated the expression of TLR4 in colon-related DRGs (0.34 ± 0.12 vs 0.72 ± 0.02 for the control and NCI groups, respectively, P < 0.05). Intrathecal administration of the TLR4-selective inhibitor CLI-095 significantly enhanced the colorectal distention threshold of NCI rats. CLI-095 treatment also markedly reversed the hyperexcitability of colon-specific DRG neurons and reduced the expression of CBS (1.7 ± 0.1 vs 1.1 ± 0.04, P < 0.05) and of the NF-κB subunit p65 (0.8 ± 0.1 vs 0.5 ± 0.1, P < 0.05). Furthermore, the NF-κB-selective inhibitor pyrrolidine dithiocarbamate (PDTC) significantly reduced the upregulation of CBS (1.0 ± 0.1 vs 0.6 ± 0.1, P < 0.05) and attenuated visceral hypersensitivity in the NCI rats. In vitro, incubation of cultured DRG neurons with the TLR4 agonist lipopolysaccharide significantly enhanced the expression of p65 (control vs 8 h: 0.9 ± 0.1 vs 1.3 ± 0.1; control vs 12 h: 0.9 ± 0.1 vs 1.3 ± 0.1, P < 0.05; control vs 24 h: 0.9 ± 0.1 vs 1.6 ± 0.1, P < 0.01) and CBS (control vs 12 h: 1.0 ± 0.1 vs 2.2 ± 0.4; control vs 24 h: 1.0 ± 0.1 vs 2.6 ± 0.1, P < 0.05), whereas the inhibition of p65 via pre-incubation with PDTC significantly reversed the upregulation of CBS expression (1.2 ± 0.1 vs 0.6 ± 0.0, P < 0.01).
CONCLUSION: Our results suggest that the activation of TLR4 by NCI upregulates CBS expression, which is mediated by the NF-κB signaling pathway, thus contributing to visceral hypersensitivity.
Core tip: This study investigated the roles of toll-like receptor 4 (TLR4) and its signaling pathway in the pathogenesis of visceral hypersensitivity in a rat model of irritable bowel syndrome (IBS). We demonstrated that activation of TLR4 upregulates the expression of the endogenous hydrogen sulfide-synthesizing enzyme cystathionine β synthetase. This upregulation was mediated by enhanced translocation of nuclear factor-κB in dorsal root ganglion cells, through which it contributed to the visceral hypersensitivity in IBS-like rats. Our results suggest TLR4 plays a major role in the development of functional visceral pain and hence suggest a novel mechanism and potential therapeutic target for the treatment of chronic visceral pain in patients with IBS.
- Citation: Yuan B, Tang WH, Lu LJ, Zhou Y, Zhu HY, Zhou YL, Zhang HH, Hu CY, Xu GY. TLR4 upregulates CBS expression through NF-κB activation in a rat model of irritable bowel syndrome with chronic visceral hypersensitivity. World J Gastroenterol 2015; 21(28): 8615-8628
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8615.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8615
Irritable bowel syndrome (IBS), a common functional gastrointestinal disorder, is defined by recurrent symptoms of abdominal pain or discomfort associated with alterations in bowel movements[1,2]. The pathogenesis of pain in IBS patients is poorly understood, and treatment remains difficult[3-5]. Our previous studies have shown that hydrogen sulfide signaling and its endogenous synthesizing enzyme cystathionine-β-synthetase (CBS) are involved in the development and maintenance of chronic visceral hypersensitivity (CVH) in rat models of IBS[6-8]. In particular, we have provided evidence that demonstrates that neonatal colonic inflammation[7,8] or neonatal maternal deprivation[6,7] significantly upregulate CBS expression in dorsal root ganglion (DRG) neurons and that inhibition of CBS by O-(carboxymethyl)-hydroxylamine hemihydrochloride (AOAA) or aminooxyacetate attenuates CVH in the adult rats. These data strongly suggest that CBS upregulation plays an important role in the development of CVH. However, the mechanism by which CBS expression is upregulated remains largely unknown.
Toll-like receptors (TLRs) are pattern recognition receptors that are key components of innate immunity[9-11]. TLR4 activation leads to a pro-inflammatory cascade[12]. The importance of TLR4 in pain has been emphasized by recent evidence showing a role of spinal TLR4 in the initiation of pathological pain states, such as inflammatory and neuropathic pain, in preclinical models[13-16]. Clinical studies have suggested an involvement of peripheral TLR4 in patients suffering from IBS[17,18]. However, there is no evidence that indicates whether TLR4 activation contributes to the upregulation of CBS expression. Furthermore, how TLR4 activation enhances CBS expression remains unknown.
As an extension of our previous work on the peripheral sensitization of neonatal colonic inflammation (NCI)-induced CVH[7,8], in this study, we investigated the roles of TLR4 in CBS expression. We further explored whether the TLR4-induced upregulation of CBS expression was mediated by nuclear factor (NF)-κB because NF-κB is considered an important transcription factor in the processing of inflammation and pain[19-21]. Through the combination of patch-clamp techniques and Western blot analysis, we showed that NCI significantly upregulated TLR4 and p65 expression in colon DRGs and that inhibiting p65 signaling reversed the upregulation of CBS expression and attenuated visceral hypersensitivity. Our findings suggest that targeting the TLR4 signaling pathway may represent a potential strategy for the treatment of chronic visceral hypersensitivity in patients with functional gastrointestinal disorders such as IBS.
Experiments were performed on male Sprague-Dawley rats. The care and handling of these animals were approved by the Institutional Animal Care and Use Committee of Soochow University and were in accordance with the guidelines of the International Association for the Study of Pain. Chronic visceral pain was induced by NCI as described previously[22,23]. In brief, 10-d-old pups received an infusion of 0.2 mL of acetic acid solution (0.5% in normal saline) into the colon, 2 cm from the anus. Control pups received an equal volume of normal saline (NS). There were no significant inflammatory responses in the colon of rats at the age of 6-7 wk after colonic injection of acetic acid (Figure 1). Experiments were performed in these rats at the age of 6-7 wk. A total of 179 rats were used in this study: 54 in the control group and 125 in the NCI group.
DRG neurons innervating the colon were labeled by the injection of 1,1’-dioleoyl-3,3,3’,3-tetramethylindocarbocyanine methanesulfonate (DiI; Invitrogen, Carlsbad, CA, United States) into the colon wall as described previously[6,23]. Briefly, 5-wk-old rats were anesthetized with chloral hydrate (0.36 g/kg; ip). After the colon was exposed, DiI (about 1 μL) was injected at 10 sites in the exposed colon wall; these sites extended approximately 6 cm in an oral direction from the level of the bladder. After the abdomen was closed, the animals were returned to their housing and given free access to drinking water and standard food pellets.
Changes in neuronal excitability were determined using whole-cell patch-clamp recording techniques as described previously[23,24]. Ten days after the Dil injection, the rats were sacrificed by cervical dislocation, followed by decapitation. DRGs (T13-L2) were bilaterally dissected out and transferred to an ice-old, oxygenated fresh dissecting solution containing (in mmol/L): 130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose and 10 HEPES, pH 7.2; (osmolarity: 305 mOsm). The ganglia were transferred to 5 mL of the dissecting solution containing collagenase D (1.8-2.0 mg/mL; Roche, Indianapolis, IN, United States) and trypsin (1.2-1.5 mg/mL; Sigma, St Louis, MO, United States) and incubated for 1.5 h at 34.5 °C. DRGs were then transferred to 2 mL of the dissecting solution containing DNase (0.5 mg/mL; Sigma). A single-cell suspension was subsequently obtained by repeated trituration through flame-polished glass pipettes. Cells were plated onto acid-cleaned glass coverslips. DiI-labeled neurons were identified by their fluorescence under a fluorescence microscope (Olympus IX71, Japan). For the patch-clamp recordings, cells were continuously superfused (1.5 mL/min) at room temperature with normal external solution containing (in mmol/L): 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, with pH adjusted to 7.2 with NaOH; 295-300 mOsm. Patch-clamp pipettes had a resistance of 4-7 MΩ when filled with the pipette solution containing (in mmol/L): 140 potassium gluconate, 10 NaCl, 10 HEPES, 10 glucose, 5 EGTA, and 1 CaCl2, pH = 7.25 adjusted with KOH; 292 mOsm. The resting membrane potential (RP) and action potentials (APs) were recorded under current-clamp configuration using an EPC10 patch-clamp amplifier (HEKA, Germany). Neurons with a resting membrane potential more depolarized than -40 mV were excluded from the data analysis.
Ten days after the DiI injection, the rats were deeply anesthetized and perfused transcardially with 150 mL PBS (Thermo, United States), followed by 400 mL ice-cold 4% paraformaldehyde (Sinopharm Chemical Reagent Co. Ltd., China) in PBS. T13, L1 and L2 DRGs were removed and postfixed for 1 h in paraformaldehyde and then cryoprotected overnight in 20% sucrose (Sinopharm Chemical Reagent Co. Ltd.) in PBS. Ten micrometer (10 μm) sections were simultaneously incubated with 2 of the following antibodies: TLR4 (1:200; Santa Cruz), NF-κB (1:200; Santa Cruz), or CBS (1:200; Abnova) and then incubated with Alexa Fluor 355 and 488 (1:500, Life Technologies Inc., Gaithersburg, MD, United States). Negative control experiments were performed by omitting the primary antibody. Sections were viewed with filter cubes appropriate for DiI (rhodamine filter), Alexa 488, and Alexa 355. Images were captured and analyzed using Metaview software.
Nuclear proteins were extracted by NE-PER nuclear and cytoplasmic extraction reagent (Thermo) according to the manufacturer’s instructions. Briefly, immediately before use, 1% (v/v) protease inhibitors (Roche) were homogenized with 200 μL of the indicated cytoplasmic extraction reagent using a glass grinder and vortexed vigorously for 15 s, then incubated on ice for 10 min. Next, 11 μL of the second regent was added to the suspension for 1 min. The suspension was centrifuged at 16000 rpm for 5 min at 4 °C. The supernatant (cytoplasmic fraction) was collected and stored at -80 °C. Sedimented nuclei were gently resuspended in 100 μL of ice-cold nuclear extraction regent and 1% (v/v) protease inhibitors, incubated on ice, and then vortexed for 15 s every 10 min for a total of 40 min. The suspension was centrifuged at 16000 rpm for 5 min at 4 °C. The supernatant (nuclear fraction) was transferred to a pre-chilled tube and stored at -80 °C until use. Protein concentration was determined using the Bradford method (Beyotime).
Rats (7-10-d-old) were euthanized by cervical dislocation, followed by decapitation. DRGs were bilaterally dissected out and then transferred to DMEM (Gibco, United States). DRGs were washed 3 times with DMEM + 20% FBS (Gibco) and then washed 3 times with extracellular buffer. After DRGs were digested with 1 mL collagenase D for 25 min and with 1 mL trypsin for 15 min, the same volume of fetal bovine serum was added to stop the digestion. Single DRG cells were obtained. These cells were mainly neuronal cells since they were stained with βIII-tubulin antibodies (Figure 2). These cells were cultured in 6-well plates in neurobasal medium (Gibco) containing penicillin (100 U/mL, Life Technologies Inc.), streptomycin (100 U/mL, Life Technologies Inc.) as well as N2 (100 ×, Life Technologies Inc.) and B27 (50 ×, Life Technologies Inc.). Four hours later, the supernatant was carefully discarded, and new neurobasal medium was added. Cells were treated with one of the following: (1) LPS for 8 h, 12 h, or 24 h at 37 °C; (2) pre-incubation with the NF-κB antagonist PDTC (25 μmol/L) for 1 h at 37 °C followed by treatment with LPS for 12 h; or (3) pre-incubation with the CBS antagonist AOAA (250 μmol/L) for 2 h at 37 °C. Cells were collected and the proteins extracted.
The expression of TLR4, TLR3, TLR7, NF-κB and CBS in DRGs (T13-L2) from adult NCI and control rats (6-wk-old) were determined using western blot analyses as described previously[24]. The primary antibodies used are listed below: mouse anti-TLR4 (1:1000; Abcam, United States), anti-NF-κB (1:200; Santa Cruz, United States), anti-CBS (1:1000; Abnova, Taiwan, China) and anti-β-actin (1:1000; Multisciences, China); and rabbit anti-TLR3 (1:200; Santa Cruz), anti-TLR7 (1:200; Santa Cruz, United States), anti-GAPDH (1:1000; Goodhere, China) and anti-H3 (1:1000, ImmunoWay, United States).
CLI095, also known as TAK-242, a selective inhibitor of TLR4, was purchased from InvivoGen and was freshly prepared in DMSO (Sigma). At the age of 5 wk, CLI095 was administered intrathecally at 50 μg/kg body weight, one time per day for 7 consecutive days. PDTC, an antagonist of NF-κB, was purchased from Sigma and was freshly prepared in 0.9% normal saline (NS). At the age of 5 wk, PDTC was administered intraperitoneally at 50 mg/kg body weight, once daily for 7 consecutive days. Tissue from these animals was used for patch-clamp recordings or for molecular expression experiments. For the behavioral tests, a single injection of different doses (15, 50 and 150 μg/kg body weight, i.t.) of CLI095 was used in the present study.
All data are expressed as the mean ± SE. Normality of all data was checked before analysis. Statistical significance was determined by the two-sample t-test, Mann-Whitney U test, Tukey’s post hoc test following one-way analysis of variance (ANOVA) or one-way repeated ANOVA, as appropriate. A P value < 0.05 was considered statistically significant.
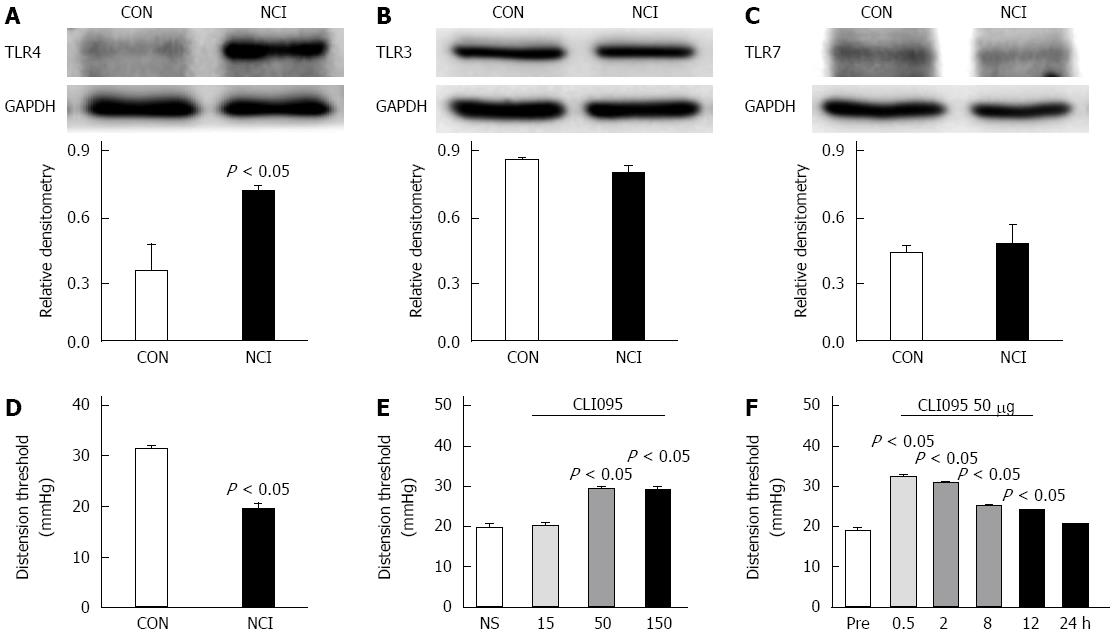
To determine whether NCI increased the expression of TLR4 in colon-related DRGs, western blotting assays were performed. The anti-TLR4 antibody labeled a protein with a molecular mass of 96 kDa. The expression level of TLR4 was significantly increased (P < 0.05, two-sample t-test, Figure 3A). To investigate whether NCI upregulated the expression of other TLRs, we examined TLR3 and TLR7 protein levels in NCI rats at the age of 6 wk. NCI did not alter TLR3 or TLR7 expression in the colon DRGs (Figure 3B and C).
We next determined whether TLR4 was involved in CVH. CVH was determined by measuring the distention threshold in response to colorectal distention at the age of 6 wk. Consistent with our previous reports[8], the distention threshold was significantly lower in NCI-treated rats than in age-matched control rats (P < 0.05, two-sample t-test, Figure 3D). More importantly, the TLR4 inhibitor CLI095 significantly increased the distention threshold in NCI rats in a dose-dependent fashion. The optimized dose of CLI095 that produced the maximal effect was 50 μg/kg body weight in this study (P < 0.05, Tukey’s post hoc test following one-way ANOVA, Figure 3E). We then determined the time course of the effects of CLI095. The effect of CLI095 at a dose of 50 μg/kg body weight lasted for approximately 12 h. Maximal inhibition occurred at 30 min (P < 0.05, Tukey’s post hoc test following one-way repeat ANOVA, Figure 3F). These data indicate that TLR4 is necessary for the NCI-induced visceral hyperalgesia.
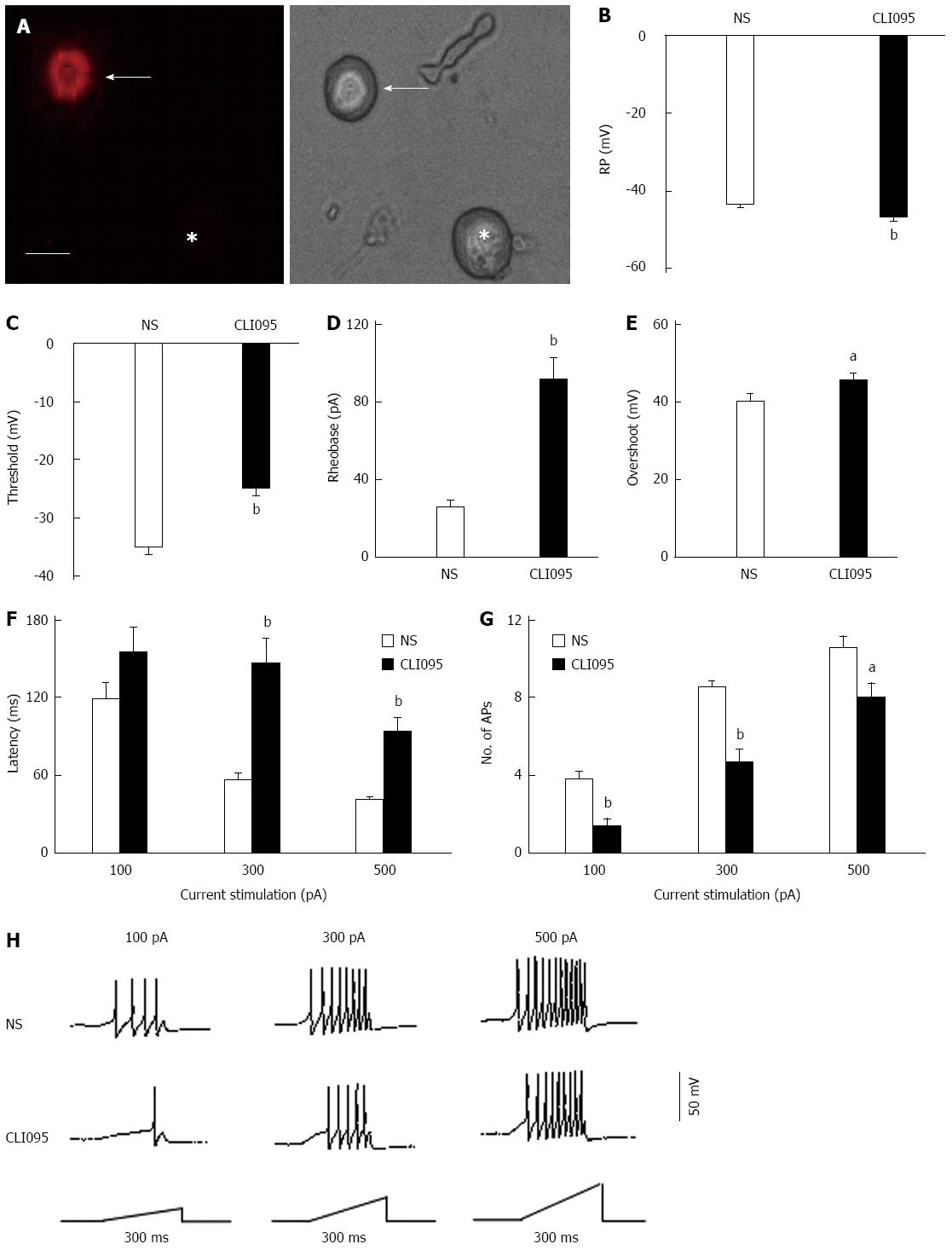
Colon-specific DRG neurons were labeled by the injection of the fluorescent dye Dil into the colon wall. Small- and medium-sized DRG neurons (Figure 4A) were used in this study because they are the primary sensory neurons responsible for pain sensation. We observed a significant hyperpolarization of the RP in DRG neurons from CLI095-treated rats (NS: -43.2 ± 0.59 mV, n = 18, CLI095: -46.7 ± 0.86 mV, n = 19, P < 0.01, two-sample t-test, Figure 4B). The AP threshold was -35.1 ± 1.54 mV (n = 18) and -24.8 ± 1.9 mV (n = 19) for NS- and CLI095-treated NCI rats, respectively. CLI095 treatment notably depolarized the AP threshold (P < 0.01, two-sample t-test, Figure 4C). Rheobase was 25.0 ± 4.9 pA (n = 18) and 91.1 ± 13.2 pA (n = 19) for colon-projecting DRG neurons isolated from NS- and CLI095-treated rats, respectively. CLI095 treatment markedly increased rheobase (P < 0.01, two-sample t-test, Figure 4D). The amplitude of the overshoot was 40.7 ± 1.7 mV (n = 18) for NS-treated rats and 45.9 ± 1.9 mV (n = 19) for CLI095-treated rats. CLI095 treatment also significantly increased the amplitude of the overshoot (P < 0.05, two-sample t-test, Figure 4E). CLI095 treatment significantly increased the response latency to stimulation with 300 and 500 pA current ramps (Figure 4F). The latency to a 300 pA current ramp was 56.4 ± 5.1 ms (n = 19) and 146.18 ± 18.9 ms (n = 18) for NS- and CLI095-treated rats, respectively. The latency to a 500 pA current ramp was 41.00 ± 3.2 ms (n = 18) and 92.9 ± 12.2 ms (n = 17) for NS- and CLI095-treated rats, respectively. CLI095 treatment greatly decreased the number of APs evoked by 100, 300 and 500 pA current ramps (Figure 4G and H). The number of APs evoked by a 100 pA current ramp was 3.8 ± 0.4 (n = 18) and 1.4 ± 0.4 (n = 19) for NS- and CLI095-treated rats, respectively. The number of APs evoked by a 300 pA current ramp was 8.5 ± 0.4 (n = 18) and 4.7 ± 0.7 (n = 19) for NS- and CLI095-treated rats, respectively. The number of APs evoked by 500 pA current ramp was 10.65 ± 0.5 (n = 18) and 7.9 ± 0.8 (n = 19) for NS- and CLI095-treated rats, respectively (100 pA, P < 0.01, two-sample t-test; 300 pA, P < 0.01, two-sample t-test; 500 pA, P < 0.05, two-sample t-test). CLI095 treatment significantly decreased the number of APs evoked by current ramp stimulation.
To determine the possible interaction between TLR4 and CBS, we first examined whether TLR4 was co-expressed in CBS-positive DRG neurons innervating the colon. Triple-labeling techniques showed that all colon-specific DRG neurons that were immunoreactive for TLR4 were also positive for CBS (Figure 5A). We then used the TLR4 inhibitor CLI095 at a dose of 50 μg/kg body weight in this study. CLI095 treatment significantly reduced the expression of CBS in colon DRGs isolated from NCI rats (P < 0.05, two-sample t-test, Figure 5B).
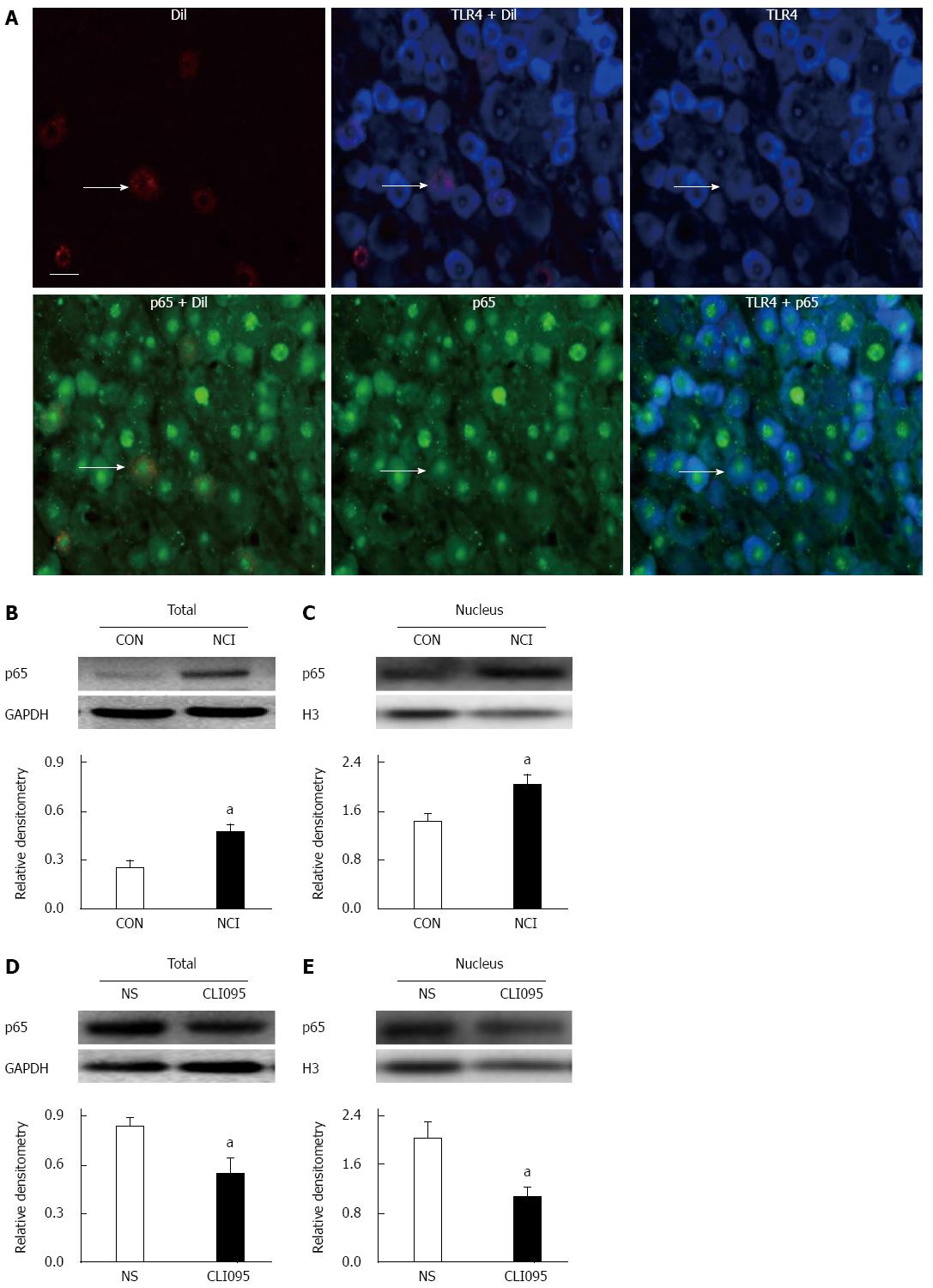
p65 is a major component in the activation of the NF-κB complex and is predominantly expressed in small- and medium-sized DRG neurons[25]. To verify the role of p65 in TLR4’s involvement in visceral pain, we first determined whether TLR4 was co-expressed in p65-positive DRG neurons innervating the colon. Triple-labeling techniques showed that all colon-specific DRG neurons that were immunoreactive for TLR4 were also positive for p65. Similarly, all colon-specific DRG neurons that were immunoreactive for p65 were also positive for TLR4 (Figure 6A).
As shown in Figure 6B, NCI dramatically increased total p65 expression in T13-L2 DRGs at 6 wk (P < 0.05, two-sample t-test, Figure 6B). Moreover, the relative densitometry of the nuclear fraction of p65 was greater in NCI rats than in controls (Figure 6C, P < 0.05, two-sample t-test). NCI increased nuclear p65 expression by 144%, indicating the translocation of p65 from the cytosol into the nucleus after NCI. We then determined whether the upregulation of p65 was mediated by TLR4. The TLR4 inhibitor CLI095 was used in this study as described above. CLI095 treatment markedly reduced the expression of p65 (P < 0.05, two-sample t-test, Figure 6D). In addition, nuclear p65 expression was also measured. CLI095 treatment markedly reduced the expression of nuclear p65 (P < 0.05, two-sample t-test, Figure 6E). Thus, CLI095 treatment reversed the upregulation of NF-κB expression in colon DRGs isolated from NCI rats.
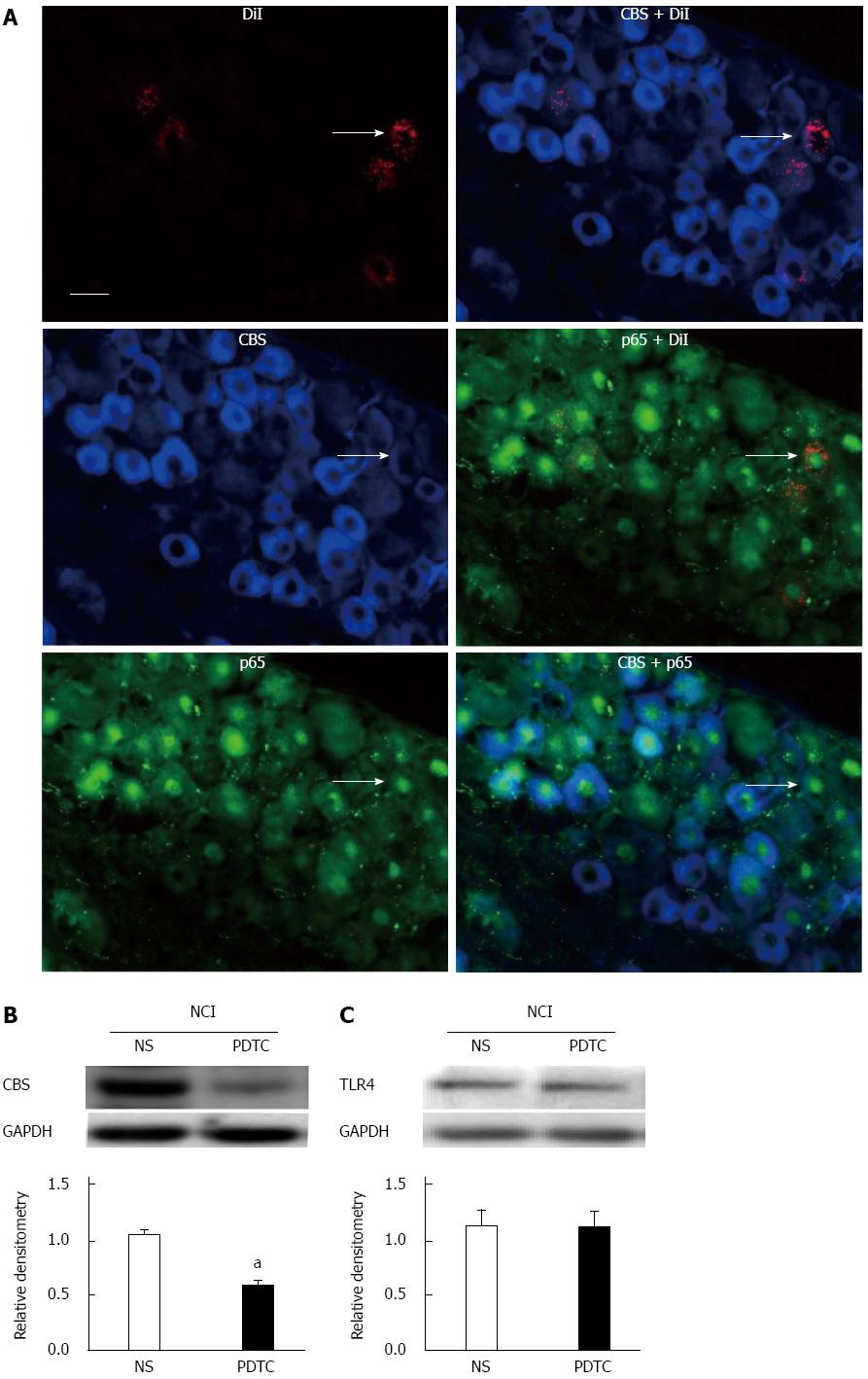
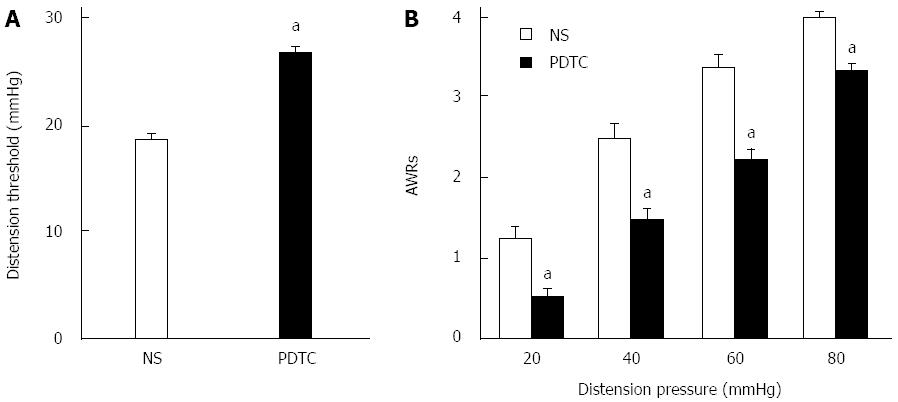
To determine the possible interaction between p65 and CBS, we first examined whether p65 was co-expressed in CBS-positive DRG neurons innervating the colon. Triple-labeling techniques showed that all colon-specific DRG neurons that were immunoreactive for p65 were also positive for CBS (Figure 7A). To further confirm the function of p65 in NCI rats, we found that PDTC treatment significantly reduced CBS expression in NCI rats (P < 0.05, two-sample t-test, Figure 7B). In contrast, PDTC treatment did not significantly alter TLR4 expression in NCI rats (P > 0.05, two-sample t-test, Figure 7C). However, PDTC treatment markedly increased the distention threshold (P < 0.05, two-sample t-test, Figure 8A) and reduced the AWR scores of NCI rats at 20, 40, 60 and 80 mmHg distention pressures (P < 0.05, Mann-Whitney U test, Figure 8B).
LPS, a TLR4 agonist, was incubated with the cultured DRG neurons for 8, 12 and 24 h. At 8, 12 and 24 h, LPS treatment significantly increased p65 expression (P < 0.05, P < 0.01, Tukey’s post hoc test following one-way ANOVA, Figure 9A). Likewise, LPS treatment markedly upregulated the expression of CBS (P < 0.05, Tukey’s post hoc test following one-way ANOVA, Figure 9B). Moreover, we also cultured primary DRG neurons treated with NS for 8, 12 and 24 h. NS did not alter p65 or CBS expression at any of these time points (data not shown). As shown in Figure 9C, PDTC pre-incubation (25 μmol/L) significantly downregulated CBS expression (P < 0.01, two-sample t-test). However, AOAA pre-incubation (250 μmol/L) did not affect p65 expression (P > 0.05, two-sample t-test, Figure 9D).
Our previous studies have shown that neonatal adverse insults produce IBS-like visceral hypersensitivity by the upregulation of CBS expression via mechanisms that are not yet known[6-8]. In the present study, we provided evidence to support our hypothesis that TLR4 and NF-κB signaling are involved in upregulated CBS expression and visceral hypersensitivity in a rat model of IBS. This conclusion was based on the following observations: First, the inhibition of TLR4 by CLI095 or the inhibition of p65 by PDTC significantly attenuated the visceral hypersensitivity observed in adult rats with NCI. Second, the inhibition of TLR4 by CLI095 reversed the hyperexcitability of DRG neurons innervating the colon. The reduction in the excitability of colon-innervating DRG neurons may underlie the reduced visceromotor responses to colorectal distention. Third, NCI significantly upregulated both TLR4 and p65 expression in rat colon DRGs. Fourth, the inhibition of TLR4 by CLI095 or the inhibition of p65 by PDTC suppressed the expression of CBS in NCI rats, indicating that TLR4 and NF-κB might be upstream regulators of CBS expression. Finally, the upregulation of CBS expression by TLR4 and p65 activation was confirmed by in vitro experiments that showed that the inhibition of either TLR4 or p65 suppressed the expression of CBS induced by LPS treatment. Collectively, these data suggest that TLR4 and NF-κB play an important role in the upregulation of CBS expression and visceral hypersensitivity in adult rats with neonatal colonic inflammation.
The findings of the present study are significant because they provide evidence to support the idea that TLR4 activation may play a vital role in functional visceral pain such as IBS, which is pain occurring in the absence of significant tissue damage or inflammation. To test this hypothesis, we used a previously validated visceral pain model of IBS. This model was induced by mild colonic irritation with diluted acetic acid at a neonatal age. This approach does not produce robust inflammation or damage to the colon; instead, it results in visceral hypersensitivity at adulthood[8,23]. TLR4 activation has been reported to participate in inflammatory and neuropathic pain states[26,27]. Here, we showed that the administration of CLI095 produced a significant analgesic effect in IBS-like rats. The dose-response and time course curves of CLI095 suggest that this antinociceptive effect is specific rather than a toxic or non-specific effect because CLI095 did not produce any effect on healthy control rats (data not shown). In addition, CLI095 treatment reversed the hyperexcitability demonstrated by a significant hyperpolarization of the resting membrane potential and action membrane threshold and by an increase in rheobase, the overshoot of the spike, and the number of APs evoked by the injection of different sizes of current ramps. The finding that NCI significantly enhanced TLR4 expression without altering the expression of TLR3 and TLR7 in rat DRGs further suggests a receptor-subtype specific upregulation of the TLR family in functional visceral pain. Therefore, our studies add TLR4 to the list of key nociceptive molecules that are involved in functional visceral hypersensitivity[28,29] and underscore the fact that such visceral hypersensitivity is accompanied by persistent plasticity of primary sensory neurons[30-34]. Future investigation is needed to explore the mechanisms by which neonatal acetic acid treatment increased TLR4 expression in DRGs.
The mechanisms by which TLR4 upregulates CBS expression remain largely unknown. In the present study, we provided evidence to demonstrate that the activation of p65 bridges the gap between TLR4 activation and CBS expression. The activation of TLR4 by NCI increased the expression and translocation of p65 into the nucleus, whereas the inhibition of TLR4 by CLI095 blocked the upregulation and nucleus translocation of p65 in the DRGs of adult rats with NCI. Once activated, p65 upregulated CBS expression. This finding is supported by the results that PDTC blocked CBS expression in NCI rats. It was further confirmed by an in vitro study that showed that PDTC blocked the upregulation of CBS expression induced by LPS treatment. These data indicate that p65 is an upstream regulator of CBS upregulation in this setting. Because PDTC reverses CBS expression but did not reverse TLR4 expression in vivo, these data further indicate that TLR4 is an upstream regulator of p65 upregulation. It is unlikely that CBS is an upstream regulator of p65 upregulation because the inhibition of CBS by AOAA did not alter the upregulation of p65 expression induced by LPS treatment in vitro. NF-κB regulates gene expression during development and inflammation[21,35,36]. It has been reported that p65 is involved in inflammatory and neuropathic pain[37,38]. Recently, we reported that p65 plays a role in gastric hypersensitivity in rats with diabetic gastroparesis[24]. Although we cannot exclude a role of other transcriptional factors such as extracellular signal-regulated protein kinase[39-41], the present study adds NF-κB to the list of key nociceptive genes that are involved in functional visceral hypersensitivity[6].
In conclusion, to the best of our knowledge, we provide the first demonstration that NCI-induced upregulation of CBS expression is mediated by TLR4 activation. This signaling pathway requires upregulation of the NF-κB p65 subunit and its translocation in DRG neurons innervating the colon, which contribute to the development of chronic visceral pain. The present results and future studies might identify a potential therapeutic target for the treatment of functional gastrointestinal disorders such as IBS.
Abdominal pain or bloating is one of the primary symptoms of patents with irritable bowel syndrome (IBS), although the underlying mechanisms are currently largely unknown. The aim of this study was to investigate the roles of toll-like receptor 4 (TLR4) and nuclear factor (NF)-κB in the upregulation of the endogenous hydrogen sulfide-producing enzyme cystathionine β-synthetase (CBS) and in visceral hypersensitivity in a rat model of IBS.
Those previous studies have shown that hydrogen sulfide signaling and its endogenous synthesizing enzyme CBS are involved in the development and maintenance of chronic visceral hypersensitivity in rat models of IBS. The importance of TLR4 in pain has been emphasized by recent evidence showing a role of TLR4 in the initiation of pathological pain states, such as inflammatory and neuropathic pain, in preclinical models.
This is the first study to indicate that the activation of TLR4 upregulates the expression of CBS, which is mediated by the enhanced translocation of NF-κB in DRG cells. These effects contribute to visceral hyperalgesia in a rat model of IBS induced by neonatal colonic injection of dilute acetic acid.
The present study demonstrated that TLR4 activation produces visceral hypersensitivity in a rat model of IBS, suggesting a novel mechanism and potential therapeutic target for the treatment of chronic visceral pain in patients with IBS.
IBS is a common functional gastrointestinal disorder characterized by chronic visceral pain or bloating in association with altered bowel movements.
This paper tried to explain the upregulation of CBS expression in IBS-like rats from the perspective of the TLR4/NF-κB signaling pathway, which is quite a novel angle to use to explore the mechanism of IBS. The paper is technically sound and the findings are interesting.
P- Reviewer: Ma XP, O’Malley D S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Ma S
| 1. | Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108-2131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 917] [Article Influence: 41.7] [Reference Citation Analysis (1)] |
| 2. | Cheung CK, Wu JC. Genetic polymorphism in pathogenesis of irritable bowel syndrome. World J Gastroenterol. 2014;20:17693-17698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 3. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [PubMed] [Cited in This Article: ] |
| 4. | Chogle A, Mintjens S, Saps M. Pediatric IBS: an overview on pathophysiology, diagnosis and treatment. Pediatr Ann. 2014;43:e76-e82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 350] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 6. | Li L, Xie R, Hu S, Wang Y, Yu T, Xiao Y, Jiang X, Gu J, Hu CY, Xu GY. Upregulation of cystathionine beta-synthetase expression by nuclear factor-kappa B activation contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Mol Pain. 2012;8:89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Hu S, Xiao Y, Zhu L, Li L, Hu CY, Jiang X, Xu GY. Neonatal maternal deprivation sensitizes voltage-gated sodium channel currents in colon-specific dorsal root ganglion neurons in rats. Am J Physiol Gastrointest Liver Physiol. 2013;304:G311-G321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Qu R, Tao J, Wang Y, Zhou Y, Wu G, Xiao Y, Hu CY, Jiang X, Xu GY. Neonatal colonic inflammation sensitizes voltage-gated Na(+) channels via upregulation of cystathionine β-synthetase expression in rat primary sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2013;304:G763-G772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Cronin JG, Turner ML, Goetze L, Bryant CE, Sheldon IM. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol Reprod. 2012;86:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lü N, Liu Q, Liu Y, Gao YJ, Liu YC. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195-2207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Kennedy CL, Najdovska M, Tye H, McLeod L, Yu L, Jarnicki A, Bhathal PS, Putoczki T, Ernst M, Jenkins BJ. Differential role of MyD88 and Mal/TIRAP in TLR2-mediated gastric tumourigenesis. Oncogene. 2014;33:2540-2546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Cappelletti C, Galbardi B, Kapetis D, Vattemi G, Guglielmi V, Tonin P, Salerno F, Morandi L, Tomelleri G, Mantegazza R. Autophagy, inflammation and innate immunity in inflammatory myopathies. PLoS One. 2014;9:e111490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 445] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856-5861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 486] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 15. | Wu FX, Bian JJ, Miao XR, Huang SD, Xu XW, Gong DJ, Sun YM, Lu ZJ, Yu WF. Intrathecal siRNA against Toll-like receptor 4 reduces nociception in a rat model of neuropathic pain. Int J Med Sci. 2010;7:251-259. [PubMed] [Cited in This Article: ] |
| 16. | Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450-15454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 349] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 17. | Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106:329-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33:1045-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Sun T, Luo J, Jia M, Li H, Li K, Fu Z. Small interfering RNA-mediated knockdown of NF-κBp65 attenuates neuropathic pain following peripheral nerve injury in rats. Eur J Pharmacol. 2012;682:79-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Sun T, Yu E, Yu L, Luo J, Li H, Fu Z. LipoxinA(4) induced antinociception and decreased expression of NF-κB and pro-inflammatory cytokines after chronic dorsal root ganglia compression in rats. Eur J Pain. 2012;16:18-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Luo JG, Zhao XL, Xu WC, Zhao XJ, Wang JN, Lin XW, Sun T, Fu ZJ. Activation of spinal NF-κB/p65 contributes to peripheral inflammation and hyperalgesia in rat adjuvant-induced arthritis. Arthritis Rheumatol. 2014;66:896-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Zhang HH, Hu J, Zhou YL, Hu S, Wang YM, Chen W, Xiao Y, Huang LY, Jiang X, Xu GY. Promoted interaction of nuclear factor-κB with demethylated cystathionine-β-synthetase gene contributes to gastric hypersensitivity in diabetic rats. J Neurosci. 2013;33:9028-9038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Berti-Mattera LN, Larkin B, Hourmouzis Z, Kern TS, Siegel RE. NF-κB subunits are differentially distributed in cells of lumbar dorsal root ganglia in naïve and diabetic rats. Neurosci Lett. 2011;490:41-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Hudson CA, Christophi GP, Cao L, Gruber RC, Massa PT. Regulation of avoidant behaviors and pain by the anti-inflammatory tyrosine phosphatase SHP-1. Neuron Glia Biol. 2006;2:235-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Liu S, Yang J, Wang L, Jiang M, Qiu Q, Ma Z, Liu L, Li C, Ren C, Zhou J. Tibia tumor-induced cancer pain involves spinal p38 mitogen-activated protein kinase activation via TLR4-dependent mechanisms. Brain Res. 2010;1346:213-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernández-Peña C, Talavera A, Kichko T. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 29. | Chen ZY, Zhang XW, Yu L, Hua R, Zhao XP, Qin X, Zhang YM. Spinal toll-like receptor 4-mediated signalling pathway contributes to visceral hypersensitivity induced by neonatal colonic irritation in rats. Eur J Pain. 2015;19:176-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Mawe GM, Strong DS, Sharkey KA. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol Motil. 2009;21:481-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Rosi S. Neuroinflammation and the plasticity-related immediate-early gene Arc. Brain Behav Immun. 2011;25 Suppl 1:S39-S49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 33. | Fallarino F, Pallotta MT, Matino D, Gargaro M, Orabona C, Vacca C, Mondanelli G, Allegrucci M, Boon L, Romani R. LPS-conditioned dendritic cells confer endotoxin tolerance contingent on tryptophan catabolism. Immunobiology. 2015;220:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Yan X, Jiang E, Weng HR. Activation of toll like receptor 4 attenuates GABA synthesis and postsynaptic GABA receptor activities in the spinal dorsal horn via releasing interleukin-1 beta. J Neuroinflammation. 2015;12:222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Dai ZK, Lin TC, Liou JC, Cheng KI, Chen JY, Chu LW, Chen IJ, Wu BN. Xanthine derivative KMUP-1 reduces inflammation and hyperalgesia in a bilateral chronic constriction injury model by suppressing MAPK and NFκB activation. Mol Pharm. 2014;11:1621-1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Qiu S, Feng Y, LeSage G, Zhang Y, Stuart C, He L, Li Y, Caudle Y, Peng Y, Yin D. Chronic morphine-induced microRNA-124 promotes microglial immunosuppression by modulating P65 and TRAF6. J Immunol. 2015;194:1021-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Li D, Huang ZZ, Ling YZ, Wei JY, Cui Y, Zhang XZ, Zhu HQ, Xin WJ. Up-regulation of CX3CL1 via Nuclear Factor-κB-dependent Histone Acetylation Is Involved in Paclitaxel-induced Peripheral Neuropathy. Anesthesiology. 2015;122:1142-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Shinozaki S, Chang K, Sakai M, Shimizu N, Yamada M, Tanaka T, Nakazawa H, Ichinose F, Yamada Y, Ishigami A. Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci Signal. 2014;7:ra106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Galan A, Cervero F, Laird JM. Extracellular signaling-regulated kinase-1 and -2 (ERK 1/2) mediate referred hyperalgesia in a murine model of visceral pain. Brain Res Mol Brain Res. 2003;116:126-134. [PubMed] [Cited in This Article: ] |
| 40. | Zhang XJ, Chen HL, Li Z, Zhang HQ, Xu HX, Sung JJ, Bian ZX. Analgesic effect of paeoniflorin in rats with neonatal maternal separation-induced visceral hyperalgesia is mediated through adenosine A(1) receptor by inhibiting the extracellular signal-regulated protein kinase (ERK) pathway. Pharmacol Biochem Behav. 2009;94:88-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Lai HH, Qiu CS, Crock LW, Morales ME, Ness TJ, Gereau RW. Activation of spinal extracellular signal-regulated kinases (ERK) 1/2 is associated with the development of visceral hyperalgesia of the bladder. Pain. 2011;152:2117-2124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |