Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6296
Peer-review started: November 30, 2014
First decision: January 8, 2015
Revised: February 7, 2015
Accepted: March 30, 2015
Article in press: March 31, 2015
Published online: May 28, 2015
AIM: To evaluate the outcomes of patients with end-stage biliary disease (ESBD) who underwent liver transplantation, to define the concept of ESBD, the criteria for patient selection and the optimal operation for decision-making.
METHODS: Between June 2002 and June 2014, 43 patients with ESBD from two Chinese organ transplantation centres were evaluated for liver transplantation. The causes of liver disease were primary biliary cirrhosis (n = 8), cholelithiasis (n = 8), congenital biliary atresia (n = 2), graft-related cholangiopathy (n = 18), Caroli’s disease (n = 2), iatrogenic bile duct injury (n = 2), primary sclerosing cholangitis (n = 1), intrahepatic bile duct paucity (n = 1) and Alagille’s syndrome (n = 1). The patients with ESBD were compared with an end-stage liver disease (ESLD) case control group during the same period, and the potential prognostic values of multiple demographic and clinical variables were assessed. The examined variables included recipient age, sex, pre-transplant clinical status, pre-transplant laboratory values, operation condition and postoperative complications, as well as patient and allograft survival rates. Survival analysis was performed using Kaplan-Meier curves, and the rates were compared using log-rank tests. All variables identified by univariate analysis with P values < 0.100 were subjected to multivariate analysis. A Cox proportional hazard regression model was used to determine the effect of the study variables on outcomes in the study group.
RESULTS: Patients in the ESBD group had lower model for end-stage liver disease (MELD)/paediatric end-stage liver disease (PELD) scores and a higher frequency of previous abdominal surgery compared to patients in the ESLD group (19.2 ± 6.6 vs 22.0 ± 6.5, P = 0.023 and 1.8 ± 1.3 vs 0.1 ± 0.2, P = 0.000). Moreover, the operation time and the time spent in intensive care were significantly higher in the ESBD group than in the ESLD group (527.4 ± 98.8 vs 443.0 ± 101.0, P = 0.000, and 12.74 ± 6.6 vs 10.0 ± 7.5, P = 0.000). The patient survival rate in the ESBD group was not significantly different from that of the ESBD group at 1, 3 and 5 years (ESBD: 90.7%, 88.4%, 79.4% vs ESLD: 84.9%, 80.92%, 79.0%, χ2 = 0.194, P = 0.660). The graft-survival rates were also similar between the two groups at 1, 3 and 5 years (ESBD: 90.7%, 85.2%, 72.7% vs ESLD: 84.9%, 81.0%, 77.5%, χ2 = 0.003, P = 0.958). Univariate analysis identified MELD/PELD score (HR = 1.213, 95%CI: 1.081-1.362, P = 0.001) and bleeding volume (HR = 0.103, 95%CI: 0.020-0.538, P = 0.007) as significant factors affecting the outcomes of patients in the ESBD group. However, multivariate analysis revealed that MELD/PELD score (HR = 1.132, 95%CI: 1.005-1.275, P = 0.041) was the only negative factor that was associated with short survival time.
CONCLUSION: MELD/PELD criteria do not adequately measure the clinical characteristics and staging of ESBD. The allocation system based on MELD/PELD criteria should be re-evaluated for patients with ESBD.
Core tip: In this work, we evaluated the clinical characteristics of end-stage biliary disease (ESBD) and demonstrated that ESBD comprises a subset of disease that significantly differs from end-stage liver disease (ESLD), which is caused by hepatitis and cirrhosis. However, previous research on ESBD has been classified within the category of ESLD. The model for end-stage liver disease (MELD) does not adequately measure the clinical characteristics and stages of patients with ESBD before liver transplantation. Patients with ESBD would be less likely to receive priority for liver transplantation, and thus, the allocation system based on the MELD score is inappropriate and should be re-evaluated for patients with ESBD. In addition, the concept of ESBD and the indications for liver transplantation are established in this paper.
- Citation: Lai YH, Duan WD, Yu Q, Ye S, Xiao NJ, Zhang DX, Huang ZQ, Yang ZY, Dong JH. Outcomes of liver transplantation for end-stage biliary disease: A comparative study with end-stage liver disease. World J Gastroenterol 2015; 21(20): 6296-6303
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6296.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6296
Liver transplantation (LT) has rapidly evolved from a high-risk experimental procedure to a mainstream therapy for patients with end-stage liver disease (ESLD)[1]. Currently, LT is primarily performed in patients with benign ESLD and hepatocellular carcinoma (HCC). However, LT for biliary diseases is not rare[2]. In 1963, Starzl et al[3] completed the first clinical LT, for an indication of congenital biliary atresia. However, equitable organ allocation to patients with biliary disease awaiting LT has been controversial in the model for end-stage liver disease (MELD) era[4]. Huang et al[5] proposed the concept of end-stage biliary disease (ESBD) in 2002 to distinguish ESBD-associated diseases from ESLD caused by hepatocyte damage due to hepatitis and liver cirrhosis. Currently, the indications for LT, the optimal timing of the operation, the surgical method, and the clinical outcomes for the concept of ESBD remain ambiguous. Therefore, in this study, data of 43 patients with ESBD who underwent LT from June 2002 to June 2014 in two LT centres in China were retrospectively analyzed. This study aimed to evaluate the clinical characteristics of ESBD and the efficacy of the LT treatment modality through a retrospective analysis of a multi-institutional cohort. The outcomes of this cohort were also compared with those of a group of patients with ESLD who underwent LT.
Between June 2002 and June 2014, 43 patients with ESBD caused by primary or secondary biliary disease underwent LT at the Department of Liver Transplantation Centre of PLA General Hospital in Beijing and at Southwest Hospital in Chongqin. All operations were performed by two surgical teams under the direction of the same senior surgeon (Dong JH).
The data that were obtained from the two transplant centres were combined in a retrospective cohort study. Patients with ESBD who underwent LT were compared with the case controls with ESLD during the same time. Two controls were randomly selected and matched by sex, age (± 5 years), allograft type, and transplant year (± 5 years). Similar exclusions were used for the case-control group. The demographic distributions of the two patient groups at the two centres were similar. Patients were excluded if they had concomitant liver malignancies, had undergone multi-organ transplantation, or were lost to follow-up. Complications were analysed using the Clavien-Dindo classification[6]. Patients with complications of degree III and higher were enrolled in this study. The examined variables included recipient age, sex, pre-transplant clinical status, and pre-transplant laboratory values, as well as the survival rates of the patients and allografts.
The patients were assessed regularly by specialists at the two centre registries. The database prospectively collected pre-transplant data, transplant, and follow-up data for all individuals considered for LT at the two centres. All demographic data for the recipients, the statuses of the patients and their laboratory values, and the survival data for the patients and allografts were obtained from the database.
Continuous variables were compared as means by using Student’s t-test or the Mann-Whitney non-parametric test. Categorical variables were compared using Pearson’s χ2 test. Survival analysis was performed using Kaplan-Meier curves, and the different survival rates were compared using log-rank tests. A Cox proportional hazard regression model was used to determine the effects of the study variables on the outcomes in the study group. All variables identified through the univariate analysis with P values < 0.100 were subjected to multivariate analysis. SPSS 18.0 (SPSS Corp., Chicago, IL, United States) was used for all statistical analyses. Statistical significance was defined as a P < 0.05 for all tests.
The statistical methods of this study were reviewed by Xin-Yuan Tong from Chinese PLA General Hospital, Chinese PLA Postgraduate Medical School.
A total of 129 patients were enrolled in this study. The aetiologies for LT are presented in Table 1. Based on the study design, the following recipient variables were similar in the two groups: age, sex, allograft type, and transplant year. Patients in the ESBD group had a significantly higher rate of previous surgery than patients in ESLD group (P < 0.05). The pre-transplant laboratory values for both groups are summarised in Table 2. Patients in the ESBD group had significantly higher platelet (PLT) counts, total bilirubin (TBIL) levels, glutamyl transpeptidase (r-GT) levels, and serum albumin (Alb), whereas serum creatinine levels, MELD/Paediatric End-Stage Liver Disease (PELD) scores, and international normalised ratios (INR) were significantly lower in patients in the ESBD group compared with patients in the ESLD group (P < 0.05).
| Aetiology of liver transplant | Number |
| ESBD | 43 |
| Primary sclerosing cholangitis | 1 |
| Intrahepatic bile duct paucity | 1 |
| Alagille’s syndrome | 1 |
| Congenital biliary atresia | 2 |
| Caroli’s disease | 2 |
| Iatrogenic bile duct injury | 2 |
| Primary biliary cirrhosis | 8 |
| Cholelithiasis | 8 |
| Graft cholangiopathy | 18 |
| ESLD | 86 |
| Autoimmune cirrhosis | 3 |
| Drug-related cirrhosis | 3 |
| Hepatitis C virus-related cirrhosis | 3 |
| Wilson’s disease | 4 |
| Alcoholic cirrhosis | 6 |
| Hepatitis B virus-related cirrhosis | 67 |
| Index | ESBD group (n = 43) | ESLD group (n = 86) | P value |
| Age (yr)1 | 40.2 ± 15.5 (0.5-65) | 43.2 ± 15.3 (5-65) | 0.105 |
| Gender (M:F)2 | 28:15 (65%:35%) | 56:30 (65%:35%) | 1.000 |
| WBC (× 109/L)1 | 6.7 ± 4.3 (1.1-23.4) | 6.1 ± 4.5 (1.3-24.5) | 0.178 |
| Hb (g/L)1 | 103.3 ± 22.6 (47.2-149) | 102.3 ± 20.1 (55-154) | 0.798 |
| Plt (× 109/L)1 | 126.0 ± 59.6 (37-247) | 91.0 ± 74. 6 (11-379) | 0.000 |
| TBIL (mg/dL)1 | 17.0 ± 13.8 (1.29-47.57) | 9.4 ± 5.4 (0.96-22.16) | 0.030 |
| INR1 | 1.3 ± 0.4 (0.86-2.35) | 1.829 ± 0.648 (1.12-5.57) | 0.000 |
| Cr (mg/dL)1 | 0.9 ± 0.8 (0.15-3.92) | 1.1 ± 0.8 (0.35-5.06) | 0.042 |
| Alb (g/L)1 | 35.1 ± 4.7 (26-50.5) | 33.4 ± 3.4 (28-44.3) | 0.016 |
| ALT (U/L)1 | 122.6 ± 96.3 (28-399) | 112.9 ± 167.2 (26.5-1400) | 0.156 |
| γ-GT (U/L)1 | 515.7 ± 578.2 (17-2374) | 119.9 ± 119.6 (11.7-588) | 0.000 |
| MELD/PELD score1 | 19.2 ± 6.6 (8-39) | 22.0 ± 6.5 (11-38) | 0.023 |
| Previous surgery1 | 1.8 ± 1.3 (0-5) | 0.1 ± 0.2 (0-1) | 0.000 |
A significant difference in operation time was observed between the two groups (P < 0.05), but intra-operative blood loss was similar in the ESBD and ESLD groups (P > 0.05). The overall incidence of complications in the ESBD group was 17 (39.5%), of which 10 (23.3%) were Clavien-Dindo grade III and 7 (16.3%) were Clavien-Dindo grade IV to V. The overall incidence of complications in the ESLD group was 27 (31.4%), of which 13 (15.1%) were Clavien-Dindo grade III and 14 (16.3%) were Clavien-Dindo grade IV to V. Overall, the incidence of complications in the two groups was similar (P > 0.05). The data for complications after LT are presented in Table 3.
| Index | ESBD group | ESLD group | P value |
| Operative time (min)1 | 527.4 ± 98.8 (300-720) | 443.0 ± 101.0 (300-660) | 0.000 |
| Intraoperative blood loss (mL)1 | 3062.8 ± 2632.9 (200-12000) | 2745.3 ± 1893.1 (500-10000) | 0.751 |
| Total complications2 | 17 (39.5) | 27 (31.4) | 0.358 |
| Clavien-Dindo grade III | |||
| Hepatic artery embolisation | 2 | 0 | |
| Re-operation for bile leakage | 1 | 3 | |
| Intraabdominal bleeding | 4 | 7 | |
| Hepatic vein stenosis | 0 | 1 | |
| Bile duct stenosis | 0 | 1 | |
| Portal vein embolisation | 1 | 1 | |
| Intestinal fistula | 2 | 0 | |
| Clavien-Dindo grade IV-V | |||
| Acute kidney failure | 3 | 5 | |
| MODS | 4 | 9 | |
| Intensive care time (d)1 | 12.74 ± 6.6 (6-41) | 10.0 ± 7.5 (5-52) | 0.000 |
| Hospital stays (d)1 | 32.53 ± 7.5 (20-50) | 30.42 ± 6.9 (18-52) | 0.079 |
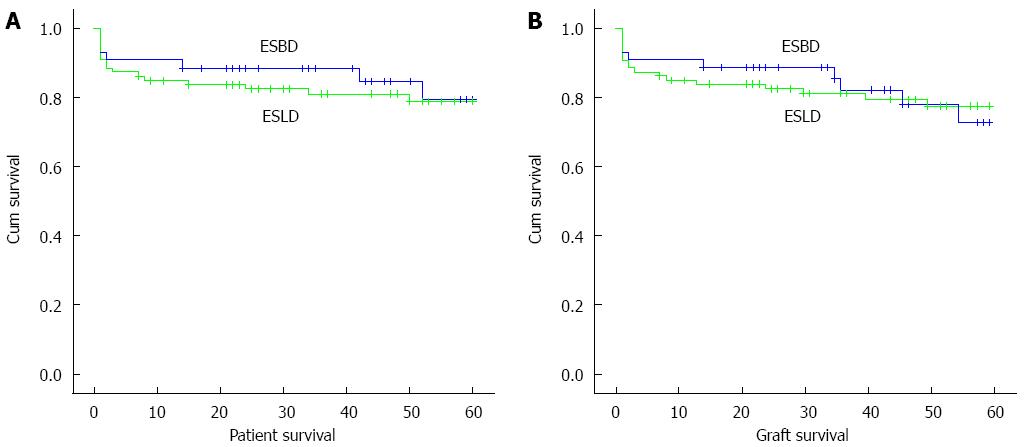
Kaplan-Meier analysis was used to estimate the overall patient survival rates with a median patient follow-up time of 43 mo (range: 1-130 mo). The 1-, 3-, and 5-year patient survival rates were 90.7%, 88.4%, and 79.4%, respectively, for the 43 patients with ESBD and 84.9%, 80.92% and 79.0%, respectively, for the matched ESLD group. The log-rank test revealed that the difference between the two groups was not statistically significant (χ2 = 0.194, P = 0.660). Kaplan-Meier estimates of allograft survival revealed 1-, 3-, and 5-year survival rates of 90.7%, 85.2%, and 72.7%, respectively, in the ESBD group and 84.9%, 81.0%, and 77.5%, respectively, in the ESLD group. The log-rank test indicated that the difference between the two groups was not statistically significant (χ2 = 0.003, P = 0.958). The graft losses were primarily due to the death of the patients. The causes of death in both groups are listed in Table 4.
| Cause of death | ESBD group | ESLD group | ||
| n | Total deaths (%) | n | Total deaths (n) | |
| Graft failure | 3 | 7.0 | 4 | 4.7 |
| Recurrent hepatitis | 0 | 0.0 | 2 | 2.3 |
| Biliary complication | 2 | 4.7 | 2 | 2.3 |
| Portal vein thrombosis | 1 | 2.3 | 0 | 0.0 |
| Multisystem organ failure | 4 | 9.3 | 10 | 11.6 |
| Cardiovascular | 0 | 0.0 | 2 | 2.4 |
| Myocardial infarction | 0 | 0.0 | 1 | 1.2 |
| Arrhythmia | 0 | 0.0 | 1 | 1.2 |
| Graft-vs-host disease | 0 | 0.0 | 1 | 1.2 |
| Upper gastrointestinal haemorrhage | 0 | 0.0 | 1 | 1.2 |
| Central nervous system | 1 | 2.3 | 2 | 2.3 |
| Epilepsy | 1 | 2.3 | 0 | 0.0 |
| Intracranial haemorrhage | 0 | 0.0 | 1 | 1.2 |
| Brain infarction | 0 | 0.0 | 1 | 1.2 |
| Lung cancer | 1 | 2.3 | 0 | 0.0 |
| Total | 9 | 20.9 | 20 | 23.3 |
Univariate analysis was performed to determine the effect of individual variables on the survival of the patients in the study group. Demographic parameters such as age, sex and blood type did not affect survival. Pre-transplant medical conditions (e.g., previous abdominal surgery, allograft type, re-transplantation, Hb, PLT, serum ALT level, γ-GT level, Alb level, and INR) had no effect on survival. Univariate analysis revealed that bleeding volume and MELD/PELD score had a significant negative effect on survival after LT. White blood cell (WBC) count exhibited a trend toward poorer survival, but this trend was not statistically significant.
The variables identified by univariate analysis with P values < 0.100 (i.e., WBC count, MELD/PELD score, and bleeding volume) were subjected to multivariate analysis. In this model, the MELD/PELD score exhibited a significant and independent effect on outcomes. These findings are summarised in Table 5; whereas the survival curves for the patients and allografts from the two groups are shown in Figure 1.
| Study variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| MELD/PELD score | 1.213 | 1.081-1.362 | 0.001 | 1.132 | 1.005-1.275 | 0.041 |
| WBC count | 0.761 | 0.553-1.046 | 0.093 | 0.823 | 0.563-1.203 | 0.314 |
| Blood loss | 0.103 | 0.020-0.538 | 0.007 | 0.171 | 0.026-1.146 | 0.069 |
LT has been studied comprehensively as a treatment for benign ESLD. Evaluation and treatment have gradually been subject to more specifications after establishing the MELD system in organ allocation. Biliary diseases are an important indication for LT. Biliary disease is a common indication for LT in European countries and has accounted for 15% of all LTs over the last decade, including primary sclerosing cholangitis (PSC) (5%), primary biliary cirrhosis (PBC) (4%), secondary biliary cirrhosis (1%), congenital biliary disease (4%), and biliary tract carcinoma (0.5%)[7]. A considerable number of patients with advanced biliary diseases have been observed in China; thus, the role of LT in biliary surgery has gained increasing attention. The present study indicated that ESBD has unique characteristics that differ from those of ESLD; however, standard LT for ESBD has not yet been established, and this concept remains ambiguous. Therefore, this study examined several aspects of ESBD.
Based on the present study, we observed that ESBD differs from ESLD. Although ESBD and ESLD share several clinical characteristics, these disease categories should not be confused with each other. Patients with ESBD occasionally also present with cirrhosis and symptoms of portal hypertension, which are typically ESLD-specific symptoms. In addition, most patients with ESBD do not exhibit complete loss of liver parenchymal function. Although MELD scores accurately predict the 3-mo mortality of patients on the LT waitlist, the scores of patients with conditions such as PSC do not[8]. In this study, the MELD/PELD score of patients with ESBD was significantly lower than that of patients in the control group (P < 0.05). Assessment of ESBD using classical evaluation criteria does not fully describe the characteristics and severity of the disease, and thus, a clear concept and position of ESBD would be clinically significant.
The concept of ESBD has not yet been defined. As previously described, we maintain that ESBD primarily refers to benign biliary tract disease. In the end-stage of these type of diseases, irreversible changes appear in the diffused liver and biliary system. Without effective treatment, patients typically die of hepatobiliary failure within a short period of time[5]. The current debate focuses on whether ESBD should include malignant biliary tumours and the diagnosis standards for ESBD. When the developmental process of the disease and the prognosis are considered, some cholangiocarcinomas caused by benign biliary diseases such as cholelithiasis or PSC are chronic, long-term, and gradually progressive. Moreover, the prognosis of malignant biliary tumours is relatively worse than that of HCC because of the difficulty in early diagnosis and the high degree of malignancy[9]. Thus, cholangiocarcinoma belongs in the end-stage disease category when considered from a prognostic perspective. Moreover, we considered ESBD as a related concept to benign ESLD; the exclusion of malignant biliary disease provides a more effective indication of the clinical characteristics of this disease. Therefore, we define ESBD as a group of benign diseases from primary or secondary causes that leads to irreversibly diffuse lesions in the bile duct tree combined with lipid metabolic disorders, presenting with persistent jaundice, recurrent cholangitis, and biliary sludge, cast or stones. This condition eventually causes liver fibrosis and liver failure.
Benign ESBD, including biliary atresia, cystic fibrosis, PBC, and graft cholangiopathy, is recognised as an appropriate indication for LT[10,11]. ESBD has a lower risk of recurrence than viral-related hepatitis and malignant liver tumours. In the present study, all patients in the ESBD group except three died during the peri-operative period, whereas the remaining patients recovered well after the surgery. The overall 5-year patient survival rate of patients in the ESBD group was 79.4%, which was similar to that of patients in the ESLD group (P > 0.05). The classification of PSC as a relapsing form of ESBD remains controversial. The current 1- and 5-year patient survival rates for PSC after LT are 83% to 97.2% and 75% to 95.4%, respectively[12]. Acute cellular rejection and recurrence are considered major risks of LT[13]. Recurrence occurs in 23.5% of patients an average of 4.6 years after LT[14]. Due to the limited sample size of patients with PSC, this finding could not be confirmed in our series.
With advances in LT techniques, surgical safety for patients with ESBD has further improved. However, LT in cases of ESBD is more challenging for surgeons than that for ESLD. We observed that patients with ESBD were more likely to have undergone abdominal surgery prior to LT than patients in the ESLD group (P < 0.05). In addition to portal hypertension and varicose veins, the surgical risk is mainly due to severe abdominal adhesions caused by repeated abdominal surgeries and biliary tract infections. These factors all increase the possibility of causing haemorrhage and damage to the surrounding organs during the removal of the liver. To reduce intra-operative bleeding, we used an innovative method known as “dry blood hepatectomy” to optimize the procedure for liver resection. First, we dissociated and blocked the blood flow of the first hepatic portal (including the portal vein), and then we dissociated and excised the diseased liver, simultaneously reducing bleeding, shortening the operation time, and improving safety[15]. Although the difficulty of surgery in patients in the ESBD group was significantly increased, no significant difference was observed between the two groups with respect to the amount of blood loss and post-operative complications (P > 0.05).
LT has been the final choice for patients with ESBD because traditional surgery has limited effectiveness. The optimal selection of the operation time for patients with ESBD is very important because of the severe shortage of liver donors, the high risks associated with LT, and the high cost of the operation. In contrast to ESBD, a standard operation time has been established for patients with ESLD. The MELD scoring system can dynamically monitor changes in patients with ESLD, and the optimal time for LT can be quantitatively evaluated. The MELD scoring system was adopted in the United States on February 28, 2002, as a liver allocation tool for patients with chronic liver disease who are candidates for LT. Patients with high MELD scores are prioritised for LT[16]. However, whether patients with ESBD should be an exception to the use of the MELD scoring system remains controversial. Several studies have considered conferring additional scores to patients with biliary disorders to fit the characteristics of recurrent infective cholangitis and intractable pruritus[17,18]. However, Goldberg et al[19] speculated that the prioritisation of patients with cholangitis for LT is not necessary because morbidity during this period is similar in patients with and without cholangitis. In the present study, we observed that the main characteristics of ESBD are recurrent cholangitis, intractable pruritus, and frequent hospital admissions. Because resolving pruritus is very difficult[20], the occurrence of intractable pruritus may be an indication for LT[21]. Moreover, the curative effect of repeated surgical treatment is very limited for patients with ESBD. However, the MELD score mainly comprises parameters that indicate the synthetic and detoxification functions of the liver, of which only bilirubin is associated with biliary diseases. Moreover, bilirubin is a low-weighted coefficient in the formula that determines the MELD score[22]. Thus, the MELD score does not reflect the main characteristics of biliary diseases. Although the MELD score is more accurate for the prediction of wait-list mortality than post-transplant survival, it is a risk factor for death after LT[23,24]. In our study, multivariate analysis indicated that the MELD/PELD score was the only independent risk factor for poor outcomes. Moreover, the MELD/PELD score of patients in the ESBD group was significantly lower than that of patients in the control group. When MELD criteria are used, patients with ESBD will be less likely to be prioritised for LT. Our study also indicated a higher risk associated with surgery in patients with ESBD, primarily during the peri-operative period. In the ESBD group, three patients died during the early post-operative period, two experienced liver failure prior to surgery and had a MELD score > 20, whereas the remaining patient developed a recurrent biliary infection that resulted in MODS. This result demonstrates that the risks associated with LT increase when ESBD develops into decompensated biliary cirrhosis or hepatic failure. Safeguards of the MELD scoring system must be developed to avoid futile transplants in recipients with high MELD scores[25]. Therefore, the MELD scoring system is inappropriate for the evaluation of patients with ESBD. An allocation system based on a “sickest patient first” policy is evidently unfair for patients with ESBD and could also contribute to a reduction in pre-LT mortality, worsen post-LT results, and an increase in organ waste. The MELD-based graft allocation system has failed to improve the efficacy of LT[26] and should be re-evaluated and modified[27-29]. According to data from the Organ Procurement and Transplantation Network, as of 2011, 12.2% of LT recipients with PSC received exception points. Since the publication of consensus recommendations that state that exception points be granted to patients with PSC and bacterial cholangitis, no systematic evaluation of their outcome[19] and standardization of exception points for patients have been established[8,30]. Thus, further studies should be performed to establish a model that precisely demonstrates the degree of severity and the clinical stages of ESBD. We proposed that LT should be performed in patients with ESBD to achieve optimal therapeutic effectiveness in the following cases: recurrence of cholangitis or intractable pruritus, when medical and surgical treatment cannot alleviate the condition, and when symptoms of presence of decompensated biliary cirrhosis are present.
In conclusion, this study analyzed the clinical characteristics of ESBD and demonstrated that ESBD is a group of diseases that are independent of ESLD. LT provides satisfactory long-term patient and graft survival rates in patients with liver-biliary failure caused by irreversible biliary disease. In this study, the MELD/PELD score was the only independent risk factor for poor outcome but this score does not adequately measure the clinical characteristics of ESBD. However, our observations have several limitations. The data obtained were retrospective in nature, and we were reliant on the accuracy of the documentation in the medical records for our data. Furthermore, data from a large, prospective, multi-centre trial are needed to confirm our findings at a national and international level. The evaluation of ESBD and the clinical standards of staging will be particularly beneficial for scientific decision-making and the improvement of therapeutic effectiveness.
The authors thank all the transplantation centres that participated in the transplantation program.
End-stage biliary disease (ESBD), a novel concept that we developed, is one of the main indications for liver transplantation (LT) in China. However, previous research on ESBD has been classified into the category of end-stage liver disease (ESLD). Moreover, the use of an organ allocation system based on the model for end-stage liver disease (MELD) criteria remains controversial for ESBD.
Studies have noted that the MELD-based graft allocation system has failed to improve the efficacy of LT, and disputes about the modification of the MELD scoring system have continued to arise. Difference among diseases must be considered when allocation systems are used.
In this work, the authors demonstrated that ESBD comprises a subset of diseases that significantly differs from ESLD caused by hepatitis and cirrhosis. However, previous research on ESBD has been classified into the category of ESLD. The MELD scoring system does not adequately measure the clinical characteristics and staging of ESBD before LT. Patients with ESBD are less likely to be prioritized for LT, and therefore an allocation system based on MELD scores is unfair and should be re-evaluated for patients with ESBD. In addition, the concept of ESBD and indications for LT are established in this paper.
The MELD scoring system does not adequately measure the clinical characteristics and stages of ESBD before LT. The authors propose that LT should be performed in patients with ESBD to achieve optimal therapeutic effectiveness in the following cases: recurrence of cholangitis or intractable pruritus, when medical and surgical treatment cannot alleviate the condition, and when symptoms of decompensated biliary cirrhosis are present. This study provides evidence for the establishment of a model that, in the future, will precisely demonstrate the degree of severity and the clinical stages of ESBD.
ESBD, a novel concept that we developed to distinguish these diseases from ESLD caused by hepatitis and cirrhosis, is a group of benign diseases from primary or secondary causes. These diseases lead to irreversible, diffuse lesions in the bile duct tree combined with persistent jaundice, recurrent cholangitis, and biliary sludge, cast or stones. This condition eventually causes liver fibrosis and failure of liver function.
In this article, the authors demonstrate that ESBD is a subset of diseases that significantly differs from ESLD caused by hepatitis and cirrhosis; however, previous research on ESBD has been classified into the category of ESLD. The concept of ESBD is defined, and the indication for LT is established. This study provides evidence supporting the modification of the MELD system.
P- Reviewer: Dueland S, Kressel A S- Editor: Yu J L- Editor: Logan S E- Editor: Ma S
| 1. | Merion RM, Sharma P, Mathur AK, Schaubel DE. Evidence-based development of liver allocation: a review. Transpl Int. 2011;24:965-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Taniguchi M. Liver transplantation in the MELD era--analysis of the OPTN/UNOS registry. Clin Transpl. 2012;41-65. [PubMed] [Cited in This Article: ] |
| 3. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] [Cited in This Article: ] |
| 4. | Goldberg D, French B, Thomasson A, Reddy KR, Halpern SD. Waitlist survival of patients with primary sclerosing cholangitis in the model for end-stage liver disease era. Liver Transpl. 2011;17:1355-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Huang ZQ, Huang XQ, Zhou NX. Treatment for End-Stage Biliary Disease in liver transplantatio n era. Digestive Surgery. 2002;1:381-392. [Cited in This Article: ] |
| 6. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] [Cited in This Article: ] |
| 7. | Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 606] [Cited by in F6Publishing: 590] [Article Influence: 49.2] [Reference Citation Analysis (1)] |
| 8. | Goldberg D, Bittermann T, Makar G. Lack of standardization in exception points for patients with primary sclerosing cholangitis and bacterial cholangitis. Am J Transplant. 2012;12:1603-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Robles R, Sánchez-Bueno F, Ramírez P, Brusadin R, Parrilla P. Liver transplantation for hilar cholangiocarcinoma. World J Gastroenterol. 2013;19:9209-9215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Milkiewicz P, Wunsch E, Elias E. Liver transplantation in chronic cholestatic conditions. Front Biosci (Landmark Ed). 2012;17:959-969. [PubMed] [Cited in This Article: ] |
| 11. | Luo Y, Ji WB, Duan WD, Ye S, Dong JH. Graft cholangiopathy: etiology, diagnosis, and therapeutic strategies. Hepatobiliary Pancreat Dis Int. 2014;13:10-17. [PubMed] [Cited in This Article: ] |
| 12. | Carbone M, Neuberger J. Liver transplantation in PBC and PSC: indications and disease recurrence. Clin Res Hepatol Gastroenterol. 2011;35:446-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Fosby B, Karlsen TH, Melum E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J Gastroenterol. 2012;18:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 103] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, Neuberger J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Dong JH, Leng JJ, Yang ZY. Atlas of Liver Transplantation. 1st ed. Shanghai: Shanghai Scientific & Technological Education Publishing House 2012; . [Cited in This Article: ] |
| 16. | Lladó L, Figueras J, Memba R, Xiol X, Baliellas C, Vázquez S, Ramos E, Torras J, Rafecas A, Fabregat J. Is MELD really the definitive score for liver allocation? Liver Transpl. 2002;8:795-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Gores GJ, Gish RG, Shrestha R, Wiesner RH. Model for end-stage liver disease (MELD) exception for bacterial cholangitis. Liver Transpl. 2006;12:S91-S92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Freeman RB, Gish RG, Harper A, Davis GL, Vierling J, Lieblein L, Klintmalm G, Blazek J, Hunter R, Punch J. Model for end-stage liver disease (MELD) exception guidelines: results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transpl. 2006;12:S128-S136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Goldberg DS, Camp A, Martinez-Camacho A, Forman L, Fortune B, Reddy KR. Risk of waitlist mortality in patients with primary sclerosing cholangitis and bacterial cholangitis. Liver Transpl. 2013;19:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Imam MH, Gossard AA, Sinakos E, Lindor KD. Pathogenesis and management of pruritus in cholestatic liver disease. J Gastroenterol Hepatol. 2012;27:1150-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Karlsen TH, Boberg KM. Update on primary sclerosing cholangitis. J Hepatol. 2013;59:571-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B, Geier A, Clavien PA. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745-753; discussion 753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 303] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 24. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 25. | Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, Holt C, Harlander-Locke M, Hong JC, Rana AR. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg. 2013;258:409-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Bouygues V, Compagnon P, Latournerie M, Bardou-Jacquet E, Camus C, Lakehal M, Meunier B, Boudjema K. MELD-based graft allocation system fails to improve liver transplantation efficacy in a single-center intent-to-treat analysis. Clin Res Hepatol Gastroenterol. 2012;36:464-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Washburn K, Pomfret E, Roberts J. Liver allocation and distribution: possible next steps. Liver Transpl. 2011;17:1005-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Galbois A, Das V, Carbonell N, Guidet B. Prognostic scores for cirrhotic patients admitted to an intensive care unit: which consequences for liver transplantation? Clin Res Hepatol Gastroenterol. 2013;37:455-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Calmus Y. Prioritization of the cirrhotic patients entering intensive care unit for liver transplantation: Do we need to change the rules? Clin Res Hepatol Gastroenterol. 2013;37:437-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Goldberg DS, Olthoff KM. Standardizing MELD Exceptions: Current Challenges and Future Directions. Curr Transplant Rep. 2014;1:232-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |