Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6261
Peer-review started: November 14, 2014
First decision: December 26, 2014
Revised: January 27, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: May 28, 2015
AIM: To evaluate the efficacy of cap-assisted colonoscopy (CAC) for detection of colorectal polyps and adenomas according to the lesion location and endoscopist training level.
METHODS: Patients 20 years or older, who underwent their first screening colonoscopy in a single tertiary center from May 2011 to December 2012 were enrolled in this study. All patients underwent either CAC or standard colonoscopy (SC), and all of the procedures were performed by 11 endoscopists (8 trainees and 3 experts). All procedures were performed with high-definition colonoscopes and narrow band imaging. The eight trainees had experiences of performing 150 to 500 colonoscopies, and the three experts had experiences of performing more than 3000 colonoscopies. A 4-mm-long transparent cap was attached to the end of a colonoscope in the CAC group. We retrospectively evaluated the number of polyps and adenomas, polyp detection rate (PDR), and the number of adenomas and adenoma detection rate (ADR) according to the lesion location and endoscopist training level between CAC and SC. We also evaluated the number of polyps and adenomas according to their size between CAC and SC.
RESULTS: Overall, PDR and ADR using CAC were significantly higher than those using SC for both whole colon (48.5% vs 40.7%, P = 0.012; 35.7% vs 28.3%, P = 0.012) and right-side colon (35.3% vs 26.6%, P = 0.002; 27.0% vs 16.9%, P < 0.001). The number of polyps and adenomas per patient using CAC was significantly higher than that using SC for both the whole colon (1.07 ± 1.59 vs 0.82 ± 1.31, P = 0.008; 0.72 ± 1.32 vs 0.50 ± 1.01, P = 0.003) and right-side colon (0.66 ± 1.18 vs 0.41 ± 0.83, P < 0.001; 0.46 ± 0.97 vs 0.25 ± 0.67, P < 0.001). In the trainee group, the PDR and ADR using CAC were significantly higher than those using SC for both the whole colon (46.7% vs 39.7%, P = 0.040; 33.9% vs 26.0%, P =0.012) and right-side colon (34.2% vs 26.5%, P = 0.015; 25.3% vs 15.9%, P = 0.001). In the expert group, the PDR and ADR using CAC were significantly higher than those using SC only for the right-side colon (42.1% vs 27.0%, P =0.035; 36.8% vs 21.0%, P = 0.020).
CONCLUSION: CAC is more effective than SC for detection of colorectal polyps and adenomas, especially when performed by trainees and when the lesions are located in the right-side colon.
Core tip: Missed lesions are the main cause of interval colon cancer. Cap-assisted colonoscopy (CAC) is one of the procedures which can reduce the incidence of missed lesion. Few studies have evaluated the efficacy of CAC based on location and size of lesions or training level of endoscopist. We evaluated the efficacy of CAC, according to the location and size of lesions and the training level of the endoscopists. We suggest that CAC can improve the detection of lesions for trainees in the whole colon and right-side colon, and even for experts in the right-side colon.
- Citation: Kim DJ, Kim HW, Park SB, Kang DH, Choi CW, Hong JB, Ji BH, Lee CS. Efficacy of cap-assisted colonoscopy according to lesion location and endoscopist training level. World J Gastroenterol 2015; 21(20): 6261-6270
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6261
Colonoscopy is an effective procedure for prevention of colorectal cancer (CRC) because it allows for the detection and removal of polyps and adenomas[1]. However, colonoscopy has certain limitations with respect to prevention of CRC, because CRC may be subsequently diagnosed even after negative colonoscopy results have been obtained[2,3]. CRC that has been diagnosed within 6 to 36 mo after colonoscopy is termed interval cancer[4]. Many potential causes of interval cancer have been considered. Among these, missed lesions are considered to be the main cause[5]. A recent systemic review of tandem colonoscopy studies reported that the rate of missed polyps was 19% to 26%[6]. Right-sided lesions, flat lesions, and variable rates of adenoma detection among endoscopists were considered to be the main causes of missed lesions and interval cancer[7]. Additionally, the adenoma detection rate (ADR) is reportedly an independent predictor of the risk of interval CRC[8]. Therefore, several new technologies such as chromoendoscopy, narrow band imaging (NBI), high-definition (HD) colonoscopy, wide-angle colonoscopy, retrograde-viewing device, and cap-assisted colonoscopy (CAC) have been developed to improve polyp and adenoma detection[9]. Among these technologies, CAC is particularly useful because the cap can depress the semilunar folds, allowing the endoscopist to inspect the blind mucosal area[10]. Although CAC can reduce the blind mucosal area, and was originally expected to increase the rates of polyp and adenoma detection, many studies have produced conflicting results, raising doubt regarding the effectiveness of CAC for polyp and adenoma detection. Several studies have reported that CAC did not improve the ADR[11-14]. Furthermore, one study even showed that CAC was associated with a lower ADR[15]. In contrast, other studies have reported that CAC improved the ADR[10,16-22]. Therefore, we aimed to investigate the efficacy of CAC for polyp and adenoma detection according to the size and location of the polyps and adenomas, and the training level of the endoscopists.
This single-center, retrospective case-control study was conducted at Pusan National University Yangsan Hospital (PNUYH). The study protocol was approved by the Institutional Review Board of PNUYH (IRB No. 05-2014-050).
A total of 1134 patients underwent their first colonoscopy at PNUYH from May 2011 to December 2012. Of these patients, 1023 were enrolled in the present study. Standard colonoscopy (SC) was performed in 508 patients from May 2011 to December 2011, and CAC was performed in 515 patients from May 2012 to December 2012. The inclusion criteria were an age of ≥ 20 years and screening or evaluation of mild symptoms as the reason for examination. The exclusion criteria were a history of abdominal surgery (excluding appendectomy), a history of colonoscopy, active gastrointestinal bleeding, severe enterocolitis, and a history of inflammatory bowel disease. Bowel preparation was evaluated and graded by the Aronchick scale[23]. Patients with a poor or inadequate rating on the Aronchick scale were also excluded to eliminate the influence of bowel preparation on the ability to detect polyps and adenomas.
All patients underwent either CAC or SC, and all colonoscopies were performed by 11 endoscopists (8 trainees and 3 experts). The eight trainees had experience performing 150 to 500 colonoscopies, and three experts had experience performing more than 3000 colonoscopies. All trainees were endoscopists who had performed more than 150 colonoscopies, because technical competence in screening and diagnostic colonoscopy generally requires experience performing more than 150 procedures[24]. HD colonoscopes and NBI were used in all examinations (CF-H260AI; Olympus Optical Co., Ltd., Tokyo, Japan). Moderate sedation was induced with a combination of intravenous midazolam and meperidine. Cecal intubation was attempted in all cases. If the trainees failed to accomplish cecal intubation within 10 min or the patients complained of intolerable pain, the experts attempted the intubation instead of the trainees. When the experts successfully reached the cecum with the colonoscope, the trainees operated the colonoscope during the withdrawal phase. As soon as the colonoscope reached the cecum, the withdrawal time was measured with a stopwatch. All endoscopists were aware that the withdrawal time was recorded during the procedures. The withdrawal time included not only the time for inspection of the mucosa, but also time for fluid suction, colonic mucosa cleansing, and polyp removal. If possible, a retroflexion technique was implemented in the rectum. The retroflexion technique was not performed in the ascending colon.
In the CAC group, a 4-mm-long transparent cap (D-201-14304; Olympus Optical Co., Ltd., Tokyo, Japan) was attached to the end of a colonoscope.
Polyps that were detected during the examination were evaluated with respect to size, morphology, and location. Their sizes were estimated by the thickness of a forcep. The polyps were removed by cold forcep biopsy or hot snare polypectomy and pathologically evaluated. Diminutive polyps were defined as ≤ 5-mm polyps, and all such polyps were removed by cold forcep biopsy. As recommended in another study, multiple diminutive serrated-appearing lesions of the sigmoid colon and rectum were not removed[25]. All > 5-mm polyps were removed by hot snare polypectomy.
The primary endpoint of this study was comparison of the polyp detection rate (PDR) and ADR in the whole colon and right-side colon between the CAC and SC groups. In this study, the right-side colon included the cecum, ascending colon, hepatic flexure, and transverse colon. The PDR and ADR constituted the proportion of patients with at least one polyp and at least one adenoma, respectively. The secondary endpoint was comparison of the ADR and PDR in the whole colon and right-side colon using the two examination methods (CAC and SC) between experts and trainees.
Continuous variables are presented as mean ± SD. The Student’s t-test was used to compare the means of continuous variables between the CAC and SC groups. The χ2 test was used to compare categorical variables between the two groups. The Mann-Whitney U test was used to compare ordered categorical variables, such as bowel preparation between the two groups. The Mann-Whitney U test was also used to compare continuous variables with non-normal distribution, such as number of detected lesions of each colon segments in age ≥ 76 years. P values of < 0.05 were considered to indicate a statistically significant difference. All statistical analyses were performed using PASW Statistics 18.0 for Windows (SPSS Inc., Chicago, IL, United States).
The statistical methods of this study were reviewed by Junhee Han from Research and Statistical Support, Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital.
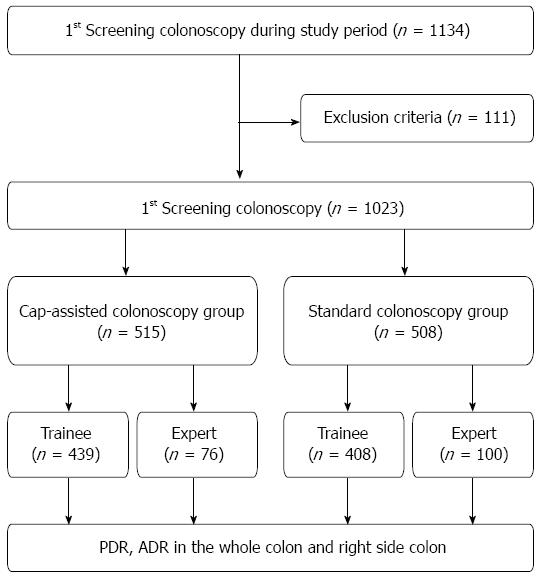
A total of 1134 patients underwent their first colonoscopy at PNUYH from May 2011 to August 2012. Of these patients, 111 were excluded owing to poor or inadequate bowel preparation (n = 96), active gastrointestinal bleeding (n = 5), history of abdominal surgery (n = 6) or diagnosis of inflammatory bowel disease (n = 4). Therefore, 1023 patients were enrolled in this study. CAC was performed in 515 patients, and SC was performed in the remaining 508 patients. In the CAC group, 76 patients underwent CAC by experts and 439 patients underwent CAC by trainees. In the SC group, 100 patients underwent SC by experts and 408 patients underwent SC by trainees (Figure 1). Evaluation of the baseline characteristics of the patients showed no significant differences in age, sex, or bowel preparation between the two groups. The combined withdrawal time of both therapeutic and non therapeutic (no biopsy or polypectomy) colonoscopies was significantly longer in the CAC group than in the SC group (14.67 ± 7.70 min vs 12.97 ± 7.20 min, P < 0.001). However, the withdrawal time of only non therapeutic colonoscopies in the CAC was not significantly different than that in the SC (10.68 ± 3.09 min vs 10.33 ± 4.24 min, P = 0.272) (Table 1).
| Characteristics | Total | CAC | SC | P value |
| Patients | 1023 | 515 | 508 | |
| mean age ± SD | 54.75 ± 10.52 | 55.06 ± 10.29 | 54.44 ± 10.74 | 0.342 |
| Gender | 0.650 | |||
| Male | 549 (53.7) | 280 (54.4) | 269 (53.0) | |
| Female | 474 (46.3) | 235 (45.6) | 239 (47.0) | |
| Withdrawal time of total colonoscopies (min) | 13.83 ± 7.50 | 14.67 ± 7.70 | 12.97 ± 7.20 | < 0.001 |
| No. of patients of non therapeutic colonoscopies | 566 | 265 | 301 | |
| Withdrawal time of non therapeutic colonoscopies (min) | 10.50 ± 3.75 | 10.68 ± 3.09 | 10.33 ± 4.24 | 0.272 |
| Bowel preparation(Aronchick scale) | 0.244 | |||
| Excellent | 38 (3.7) | 19 (3.7) | 19 (3.7) | |
| Good | 646 (63.1) | 316 (61.4) | 330 (65.0) | |
| Fair | 339 (33.1) | 180 (35.0) | 159 (31.3) |
In total, 967 polyps were detected in the whole colon and 547 polyps were detected in the right-side colon. The total number of polyps and the PDR in the whole colon were significantly higher in the CAC group than in the SC group (549 vs 418, P = 0.008 and 48.5% vs 40.7%, P = 0.012). The number of polyps per patient in the whole colon was significantly higher in the CAC group than in the SC group (1.07 ± 1.59 vs 0.82 ± 1.31, P = 0.008). The total number of polyps and the PDR in the right-side colon were also significantly higher in the CAC group than in the SC group (339 vs 208, P < 0.001 and 35.3% vs 26.6%, P = 0.002). The number of polyps per patient in the right-side colon was significantly higher in the CAC group than in the SC group (0.66 ± 1.18 vs 0.41 ± 0.83, P < 0.001). When the polyps were classified by location, the numbers of polyps in the ascending colon, hepatic flexure, and splenic flexure were significantly higher in the CAC group than in the SC group (179 vs 87, P < 0.001; 56 vs 24, P = 0.001; and 24 vs 6, P = 0.001) (Table 2).
| Whole colon | Total (n = 1023) | CAC (n = 515) | SC (n = 508) | P value |
| Total polyps | 967 | 549 | 418 | 0.008 |
| Polyps per patient | 0.95 ± 1.46 | 1.07 ± 1.59 | 0.82 ± 1.31 | 0.008 |
| PDR | 457 (44.7) | 250 (48.5) | 207 (40.7) | 0.012 |
| Right-side colon | Total (n = 1023) | CAC (n = 515) | SC (n = 508) | P value |
| Total polyps | 547 | 339 | 208 | < 0.001 |
| Polyps per patient | 0.53 ± 1.03 | 0.66 ± 1.18 | 0.41 ± 0.83 | < 0.001 |
| PDR | 317 (31.0) | 182 (35.3) | 135 (26.6) | 0.002 |
| Polyps of segment | Total (n = 1023) | CAC (n = 515) | SC (n = 508) | P value |
| Cecum | 85 | 42 | 43 | 0.888 |
| Ascending colon | 266 | 179 | 87 | < 0.001 |
| Hepatic flexure | 80 | 56 | 24 | 0.001 |
| Transverse colon | 116 | 62 | 54 | 0.560 |
| Splenic flexure | 30 | 24 | 6 | 0.001 |
| Descending colon | 65 | 31 | 34 | 0.683 |
| Sigmoid colon | 223 | 105 | 118 | 0.406 |
| Rectum | 102 | 50 | 52 | 0.804 |
In total, 623 adenomas were detected in the whole colon and 365 adenomas were detected in the right-side colon. The total number of adenomas and the ADR in the whole colon were significantly higher in the CAC group than in the SC group (370 vs 253, P = 0.003 and 35.7% vs 28.3%, P = 0.011). The number of adenomas per patient in the whole colon was significantly higher in the CAC group than in the SC group (0.72 ± 1.32 vs 0.50 ± 1.01, P = 0.003). The total number of adenomas and the ADR in the right-side colon were also significantly higher in the CAC group than in the SC group (236 vs 129, P < 0.001 and 27.0% vs 16.9%, P < 0.001). The number of adenomas per patient in the right-side colon was significantly higher in the CAC group than in the SC group (0.46 ± 0.97 vs 0.25 ± 0.67, P < 0.001). When the adenomas were classified by location, the numbers of adenomas in the ascending colon, hepatic flexure, and splenic flexure were significantly higher in the CAC group than in the SC group (129 vs 50, P < 0.001; 44 vs 18, P = 0.002 and 22 vs 6, P = 0.003) (Table 3).
| Whole colon | Total (n = 1023) | CAC (n = 515) | SC (n = 508) | P value |
| Total adenomas | 623 | 370 | 253 | 0.003 |
| Adenomas per patient | 0.61 ± 1.18 | 0.72 ± 1.32 | 0.50 ± 1.01 | 0.003 |
| ADR | 328 (32.1) | 184 (35.7) | 144 (28.3) | 0.011 |
| Right-side colon | Total (n = 1023) | CAC (n = 515) | SC (n = 508) | P value |
| Total adenomas | 365 | 236 | 129 | < 0.001 |
| Adenomas per patient | 0.36 ± 0.84 | 0.46 ± 0.97 | 0.25 ± 0.67 | < 0.001 |
| ADR | 225 (22.0) | 139 (27.0) | 86 (16.9) | < 0.001 |
| Adenomas of segment | Total (n = 1023) | CAC (n = 515) | SC (n = 508) | P value |
| Cecum | 45 | 20 | 25 | 0.490 |
| Ascending colon | 179 | 129 | 50 | < 0.001 |
| Hepatic flexure | 62 | 44 | 18 | 0.002 |
| Transverse colon | 79 | 43 | 36 | 0.529 |
| Splenic flexure | 28 | 22 | 6 | 0.003 |
| Descending colon | 46 | 23 | 23 | 0.966 |
| Sigmoid colon | 130 | 61 | 69 | 0.490 |
| Rectum | 54 | 28 | 26 | 0.829 |
The total number of polyps and adenomas in the CAC and SC groups were evaluated according to size (Table 4). Overall, 698 diminutive polyps were detected. The total number of diminutive polyps in the CAC group was significantly higher than that in the SC group (398 vs 300, P = 0.011). The total number of diminutive adenomas in the CAC group was also significantly higher than that in the SC group (253 vs 165, P = 0.003). On the other hand, the total numbers of larger polyps and adenomas in the CAC groups were not significantly higher than those in the SC group (151 vs 118, P = 0.168 and 117 vs 88, P = 0.178).
| Total number | Total (n = 1023) | CAC (n = 515) | SC (n = 508) | P value |
| Polyps < 5 mm, n | 698 | 398 | 300 | 0.011 |
| Per patient | 0.68 ± 1.15 | 0.77 ± 1.27 | 0.59 ± 1.00 | 0.011 |
| Adenomas < 5 mm, n | 418 | 253 | 165 | 0.003 |
| per Patient | 0.41 ± 0.89 | 0.49 ± 1.03 | 0.32 ± 0.71 | 0.003 |
| Polyps ≥ 5 mm, n | 269 | 151 | 118 | 0.168 |
| per Patient | 0.26 ± 0.70 | 0.29 ± 0.76 | 0.23 ± 0.63 | 0.168 |
| Adenomas ≥ 5 mm, n | 205 | 117 | 88 | 0.178 |
| Per patient | 0.20 ± 0.64 | 0.23 ± 0.70 | 0.17 ± 0.56 | 0.178 |
We evaluated the PDR, ADR, and the number of polyps and adenomas per patient of whole colon (Table 5) and right-side colon (Table 6) based on age (20-49 years, 50-65 years, 66-75 years, > 76 years). In summary, the number of polyps and adenomas per patient of right-side colon in the CAC group was significantly higher than that in the SC group, on all ages except ≥ 75 years. On the age ≥ 75 years, none of PDR, ADR and the number of polyps and adenomas per patient in the CAC group was significantly higher than those in the SC group, perhaps because the sample size of the age ≥ 75 years was too small. When we analyzed the number of polyps and adenomas per patient based on each colon segment, which of ascending colon in the CAC group was significantly higher than that in the SC group on the all ages except ≥ 75. The number of polyps and adenomas per patient of transverse and descending colon in the CAC was not significantly different than that in the SC group on the all ages.
| Whole colon | ||||
| Age (yr) | Total | CAC | SC | P value |
| 20-49 | 308 | 147 | 161 | |
| Polyps per patient | 0.51 ± 1.07 | 0.63 ± 1.32 | 0.41 ± 0.77 | 0.078 |
| PDR | 94 (30.5) | 51 (34.7) | 43 (26.7) | 0.128 |
| Adenomas per patient | 0.26 ± 0.77 | 0.37 ± 1.02 | 0.16 ± 0.41 | 0.013 |
| ADR, | 57 (18.5) | 35 (23.8) | 22 (13.7) | 0.022 |
| 50-65 | 544 | 293 | 251 | |
| Polyps per patient | 1.01 ± 1.47 | 1.09 ± 1.57 | 0.92 ± 1.34 | 0.194 |
| PDR | 260 (47.8) | 146 (49.8) | 114 (45.4) | 0.305 |
| Adenomas per patient | 0.67 ± 1.22 | 0.76 ± 1.34 | 0.57 ± 1.04 | 0.068 |
| ADR | 190 (34.9) | 107 (36.5) | 83 (33.1) | 0.400 |
| 66-75 | 154 | 68 | 86 | |
| Polyps per patient | 1.55 ± 1.84 | 1.93 ± 1.91 | 1.26 ± 1.74 | 0.024 |
| PDR | 91 (59.1) | 47 (69.1) | 44 (51.2) | 0.024 |
| Adenomas per patient | 1.04 ± 1.53 | 1.28 ± 1.62 | 0.85 ± 1.43 | 0.084 |
| ADR | 71 (46.1) | 38 (55.9) | 33 (38.4) | 0.030 |
| ≥ 76 | 17 | 7 | 10 | |
| Polyps per patient | 1.24 ± 1.20 | 1.14 ± 0.90 | 1.30 ± 1.41 | 0.959 |
| PDR | 12 (70.6) | 6 (85.7) | 6 (60.0) | 0.338 |
| Adenomas per patient | 1.00 ± 1.11 | 0.71 ± 0.75 | 1.20 ± 1.31 | 0.502 |
| ADR | 10 (58.8) | 4 (57.1) | 6 (60.0) | 1.000 |
| Right-side colon | ||||
| Age (yr) | Total | CAC | SC | P value |
| 20-49 | 308 | 147 | 161 | |
| Polyps per patient | 0.25 ± 0.82 | 0.35 ± 1.09 | 0.15 ± 0.45 | 0.030 |
| PDR | 49 (15.9) | 30 (20.4) | 19 (11.8) | 0.039 |
| Adenomas per patient | 0.13 ± 0.61 | 0.20 ± 0.83 | 0.06 ± 0.25 | 0.033 |
| ADR | 28 (9.1) | 20 (13.6) | 8 (5.0) | 0.008 |
| 50-65 | 544 | 293 | 251 | |
| Polyps per patient | 0.59 ± 1.00 | 0.68 ± 1.13 | 0.49 ± 0.82 | 0.025 |
| PDR | 194 (35.7) | 110 (37.5) | 84 (33.5) | 0.322 |
| Adenomas per patient | 0.41 ± 0.86 | 0.50 ± 1.00 | 0.31 ± 0.66 | 0.009 |
| ADR | 138 (25.4) | 82 (28.0) | 56 (22.3) | 0.129 |
| 66-75 | 154 | 68 | 86 | |
| Polyps per patient | 0.91 ± 1.31 | 1.21 ± 1.38 | 0.67 ± 1.21 | 0.012 |
| PDR | 86 (55.8) | 38 (55.9) | 30 (34.9) | 0.009 |
| Adenomas per patient | 0.62 ± 1.04 | 0.81 ± 0.99 | 0.47 ± 1.07 | 0.043 |
| ADR | 54 (35.1) | 34 (50.0) | 20 (23.3) | 0.001 |
| ≥ 76 | 17 | 7 | 10 | |
| Polyps per patient | 0.59 ± 1.00 | 0.86 ± 1.06 | 0.40 ± 0.96 | 0.167 |
| PDR | 6 (35.3) | 4 (57.1) | 2 (20.0) | 0.162 |
| Adenomas per patient | 0.41 ± 0.71 | 0.57 ± 0.78 | 0.30 ± 0.67 | 0.362 |
| ADR | 5 (29.4) | 3 (42.9) | 2 (20.0) | 0.593 |
We evaluated the PDR, ADR, and the number of polyps and adenomas per patient of whole colon and right-side colon based on gender (Table 7). The number of adenomas per patient and ADR of right-side colon in the CAC group were significantly higher than those in the SC group, based on the both genders. When we analyzed the number of polyps and adenomas per patient based on each colon segment, which of ascending colon in the CAC group was significantly higher than that in the SC group on the both genders. The number of polyps and adenomas per patient of transverse and descending colon in the CAC was not significantly different than that in the SC group on both genders.
| Gender | Total | CAC | SC | P value |
| Whole colon | ||||
| Male, n | 549 | 280 | 269 | |
| Polyps per patient | 1.25 ± 1.67 | 1.37 ± 1.84 | 1.12 ± 1.47 | 0.082 |
| PDR | 300 (54.6) | 158 (56.4) | 142 (52.8) | 0.392 |
| Adenomas per patient | 0.81 ± 1.38 | 0.90 ± 1.53 | 0.71 ± 1.20 | 0.109 |
| ADR | 219 (39.9) | 114 (40.7) | 105 (39.0) | 0.688 |
| Female | 474 | 235 | 239 | |
| Polyps per patient | 0.60 ± 1.08 | 0.71 ± 1.14 | 0.49 ± 1.00 | 0.029 |
| PDR | 157 (33.1) | 92 (39.1) | 65 (27.2) | 0.006 |
| Adenomas per patient | 0.38 ± 0.84 | 0.50 ± 0.98 | 0.26 ± 0.67 | 0.002 |
| ADR | 109 (23.0) | 70 (29.8) | 39 (16.3) | < 0.001 |
| Right-side colon | ||||
| Male | 549 | 280 | 269 | |
| Polyps per patient | 0.70 ± 1.20 | 0.87 ± 1.39 | 0.52 ± 0.94 | 0.001 |
| PDR | 205 (37.3) | 118 (42.1) | 87 (32.3) | 0.018 |
| Adenomas per patient | 0.49 ± 1.00 | 0.60 ± 1.14 | 0.37 ± 0.82 | 0.007 |
| ADR | 153 (27.9) | 90 (32.1) | 63 (23.4) | 0.023 |
| Female | 474 | 235 | 239 | |
| Polyps per patient | 0.35 ± 0.73 | 0.41 ± 0.78 | 0.28 ± 0.67 | 0.066 |
| PDR | 112 (23.6) | 64 (27.2) | 48 (20.1) | 0.067 |
| Adenomas per patient | 0.21 ± 0.56 | 0.29 ± 0.67 | 0.13 ± 0.42 | 0.002 |
| ADR | 72 (15.2) | 49 (20.9) | 23 (9.6) | 0.001 |
The PDR and ADR were evaluated according to the endoscopists’ training level. When the procedures were performed by the trainees, the PDR and ADR of the whole colon in the CAC group were significantly higher than those in the SC groups (46.7% vs 39.7%, P = 0.040 and 33.9% vs 26.0%, P = 0.012). The PDR and ADR of the right-side colon in the CAC group were significantly higher than those in the SC group (34.2% vs 26.5%, P =0.015 and 25.3% vs 15.9%, P = 0.001) (Table 8). When the procedures were performed by the experts, the PDR and ADR of the whole colon in the CAC group were not significantly different from those in the SC group (59.2% vs 45.0%, P = 0.062 and 46.1% vs 38.1%, P = 0.283). However, the PDR and ADR of the right-side colon in the CAC group were significantly higher than those in the SC group (42.1% vs 27.0%, P = 0.035 and 36.8% vs 21.0%, P = 0.020) (Table 9).
| Trainees | Total (n = 847) | CAC (n = 439) | SC (n = 408) | P value |
| Whole colon | ||||
| PDR | 367 (43.3) | 205 (46.7) | 162 (39.7) | 0.040 |
| ADR | 255 (30.1) | 149 (33.9) | 106 (26.0) | 0.012 |
| Right-side colon | ||||
| PDR | 258 (30.5) | 150 (34.2) | 108 (26.5) | 0.015 |
| ADR | 176 (20.8) | 111 (25.3) | 65 (15.9) | 0.001 |
| Experts | Total (n = 176) | CAC (n = 76) | SC (n = 100) | P value |
| Whole colon | ||||
| PDR | 90 (51.1) | 45 (59.2) | 45 (45.0) | 0.062 |
| ADR | 73 (41.5) | 35 (46.1) | 38 (38.0) | 0.283 |
| Right-side colon | ||||
| PDR | 59 (33.5) | 32 (42.1) | 27 (27.0) | 0.035 |
| ADR | 49 (27.8) | 28 (36.8) | 21 (21.0) | 0.020 |
No significant complications such as perforation or massive bleeding occurred in either group during the study period.
Colonoscopy is one of the most effective procedures for prevention of CRC. However, colonoscopy has certain limitations. According to two previous population-based studies, interval cancer may develop after colonoscopy[2,3]. New-onset CRC, incomplete polyp resection, and missed lesions are considered to be among the causes of interval cancer development; of these, missed lesions are considered to be the main cause[5]. Additionally, interval colon cancers are considered to be associated with localization in the right-side colon, microsatellite instability, and CpG island methylator phenotype-high[26,27]. Moreover, Rex et al[25] suggested that it is important to reduce the rate of both missed serrated lesions and missed adenomas to prevent right-side colon cancer. Therefore, increases in both the PDR and ADR are thought to be critical for the prevention of interval cancer.
Several new technologies have been developed to reduce the incidence of missed lesions and improve the PDR and ADR, including chromoendoscopy, NBI, HD colonoscopy, wide-angle colonoscopy, retrograde-viewing device, and CAC[9]. Most of these techniques are associated with increased procedure times and higher costs. In contrast, CAC can be easily implemented by simply attaching a transparent rubber cap to the tip of the colonoscope, can reduce the cecal intubation time, and is not associated with a high cost[10,12,14,15]. Additionally, the cap maintains an appropriate distance between the colonic mucosa and lens of the colonoscope; this helps to prevent red-out (the duration of time during which the lens of colonoscope is obscured by contact with the mucosa), improve the orientation of the lumen, and allows the endoscopist to advance the colonoscope with less air insufflation. All of these factors contribute to shortening of the cecal intubation time[28].
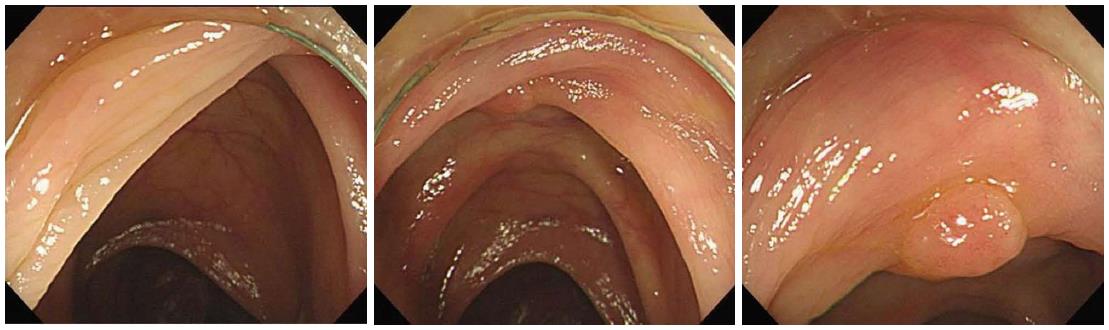
The cap not only keeps the tip of the colonoscope an adequate distance away from the colonic mucosa, but also separates and depresses the semilunar folds. Thus, the cap allows the endoscopist to maintain a continuous visual field around the colonic bends and to thoroughly inspect the blind mucosa (Figure 2), such as the proximal aspect of ileocecal valve, flexures, haustral folds, and rectal valves[10,18,28]. These advantages of CAC allow for improvement in the ADR and PDR in the proximal colon, flexures, and whole colon. The cap can also stretch or splay the colonic mucosa, further contributing to improved detection rates[19].
Eight previous studies reported that CAC was better able to detect polyps or adenomas than was SC[10,16-22]. However, our study differed from these previous studies in several aspects. Three of these previous studies did not evaluate bowel preparation between the CAC and SC groups[10,16,17]. None of the remaining five studies evaluated the efficacy of CAC according to the training level of the endoscopists. In the present study, we evaluated the grade of bowel preparation in both the CAC and SC groups. We then confirmed that there were no significant differences in bowel preparation between the CAC and SC groups, and we excluded patients with poor or inadequate bowel preparation. We evaluated the location and size of the detected polyps and adenomas, and we compared the PDR and ADR obtained by both experienced and inexperienced endoscopists.
Despite the benefits of CAC, there are conflicting results regarding its PDR and ADR. Several studies have reported that CAC did not improve polyp and adenoma detection[11-14]. However, no studies have evaluated polyp and adenoma detection according to the training level of the endoscopists with a large sample size while eliminating the influence of bowel preparation and patient characteristics. Harada et al[14] did not evaluate the bowel preparation in the CAC or SC group, and Tee et al[13] included patients with poor bowel preparation and evaluated a small sample. Although Dai et al[11] reported that CAC conducted by trainees did not improve the PDR, their study evaluated a small sample and patients in the SC group were older than those in the CAC group, especially in the trainee group. Finally, de Wijkerslooth et al[12] did not evaluate the PDR and ADR associated with CAC performed by trainees.
One study reported that CAC may decrease the PDR and ADR. Lee et al[15] reported that the ADR in the CAC group was lower than that in the SC group. However, the bowel preparation was significantly less satisfactory in the CAC group, the withdrawal time was shorter in the CAC group, and the procedure was performed with a mucosectomy cap. Mucosectomy caps are longer at 10 mm in length; this increased length may make them difficult to clean, and they may impair the endoscopist’s vision because fecal matter can more easily adhere to the longer cap. For these reasons, their study showed a lower ADR in the CAC group.
In contrast to previous studies, many of which reported conflicting results, we excluded the influence of bowel preparation and patients’ baseline characteristics and evaluated the efficacy of CAC according to lesion size and location and endoscopists’ training level. Our sample was also sufficiently large. We found that CAC can improve the PDR and ADR in the ascending colon, hepatic flexure, splenic flexure, and whole colorectum (Tables 2 and 3). The results of our study provide evidence that CAC allows endoscopists to inspect the blind mucosal surfaces of the flexures and haustral folds. Additionally, the detection rates of diminutive polyps and adenomas were higher in the CAC group than in the SC group (Table 4). We found that CAC performed by trainees was associated with a higher PDR and ADR in the whole colorectum and right-side colon, although CAC performed by experts was associated with a higher PDR and ADR only in the right-side colon (Tables 5 and 6). We assume that experts can inspect the blind mucosa without the cap to some extent, while trainees have difficulty performing this inspection without the cap. Furthermore, even when experts perform the colonoscopy, we believe that the cap would help to detect polyps and adenomas in the right-side colon.
Rex et al[29] suggested that ADR must be at least 25% for male and at least 15% for female, and ADR is known to vary widely among providers in both academic and community settings[30]. Our study reported higher PDR (40.7%) and high ADR (28.3%) in SC group than previous studies. However, recent studies reported the high PDR and ADR with long withdrawal times, self-recording of withdrawal time, HD colonoscope, fair bowel preparation and an academic setting of improving ADR[31-35]. In our study, the minimal withdrawal time of most procedures was at least 7 min, all endoscopists were aware of self-recording of withdrawal time and all procedures were performed with HD colonoscopies and NBI, and optimal or fair bowel preparation. Furthermore, quality improvement program of colonoscopy was performed at PNUYH every month. These are the reason that our PDR and ADR in SC group were higher than those of previous studies.
This study has several limitations. First, this study was a single-center, retrospective, case-controlled study. Second, four of the trainees performed the SC from May 2011 to December 2011, and the other four trainees performed CAC from May 2012 to December 2012. Thus, there is a potential for selection bias, and the sample performed by experts was relatively small. Third, the combined withdrawal time of both therapeutic and non therapeutic (no biopsy or polypectomy) colonoscopies in the CAC group was significantly longer than that in the SC group, indicating that the withdrawal time is associated with increase in PDR and ADR. However, although the combined withdrawal time of both colonoscopies in the CAC group was significantly longer than that in the SC group, the withdrawal time of only non therapeutic colonoscopies was not significantly different between the CAC group and SC group. Moreover, more polyps and adenomas were detected in CAC than SC. Therefore, we concluded that the withdrawal time of CAC was longer than that of SC because more lesions were detected in CAC than SC, and more lesion removal time, such as cold forcep biopsy or hot snare polypectomy, was needed in CAC than SC. We believe that the inspection time of CAC was not longer than that of SC, and the higher ADR of CAC was not associated with the longer withdrawal time of CAC.
In conclusion, we believe that CAC can be very helpful for trainees to detect lesions in the whole colon and even for experts to detect lesions in the right-side colon. Additionally, CAC can be very useful to prevent interval colon cancer, especially when performed by inexperienced endoscopists in patients with satisfactory bowel preparation. We recommend the routine use of CAC for screening colonoscopy.
Colonoscopy is one of the most effective procedure for prevention of colorectal cancer. However, colorectal cancer can be subsequently diagnosed after negative colonoscopy, and it is called the interval cancer. The interval cancer is known to be associated with missed lesions and right-side colon. Therefore, to reduce the incidence of missed lesion and improve polyp detection rate (PDR) and adenoma detection rate (ADR) are important to prevent the interval cancer, especially in the right-side colon. Several new technologies have been developed to improve the PDR and ADR, and cap-assisted colonoscopy (CAC) is an inexpensive and simple method among these technologies. The authors aimed to evaluate the efficacy of CAC based on location of lesions and training level of endoscopists.
This study did not only aim to evaluate the efficacy of CAC in the whole colon, but also based on location and size of lesions, and training level of endoscopists.
According to this study, CAC is more effective for experts than standard colonoscopy for detection of lesions in the right-side colon. And CAC is also more effective for trainees in the whole colon and right-side colon.
CAC is an effective procedure to reduce the incidence of missed lesion and improve the detection rate of lesions for the trainees, and even for the experts.
CAC: Cap-assisted colonoscopy. A 4-mm-long transparent cap is attached to the end of a colonoscope, and the cap is able to separate and depress the semilunar folds. Therefore, CAC allows the endoscopists to inspect the blind mucosa of colon. SC: Standard colonoscopy. PDR: Polyp detection rate. PDR is usually defined as the proportion of patients in whom at least one polyp was identified. ADR: adenoma detection rate. ADR is usually defined as the proportion of patients in whom at least one adenoma was identified. ADR is known as a quality indicator of colonoscopy.
This study aimed to evaluate the efficacy of CAC based on location of lesions and training level of endoscopists. CAC can be helpful to improve the detection rate of lesions for trainees and even experts.
P- Reviewer: Brill JV, Guimaraes DP S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3107] [Cited by in F6Publishing: 3009] [Article Influence: 97.1] [Reference Citation Analysis (1)] |
| 2. | Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 3. | Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588-2596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012;118:3044-3052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Haug U, Regula J. Interval cancer: nightmare of colonoscopists. Gut. 2014;63:865-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 878] [Cited by in F6Publishing: 868] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 7. | Kim HH. Can cap-assisted colonoscopy be a savior for right side interval cancer? Dig Dis Sci. 2013;58:289-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1351] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 9. | Rex D. Detection of neoplasia at colonoscopy: what next? Endoscopy. 2008;40:333-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Kondo S, Yamaji Y, Watabe H, Yamada A, Sugimoto T, Ohta M, Ogura K, Okamoto M, Yoshida H, Kawabe T. A randomized controlled trial evaluating the usefulness of a transparent hood attached to the tip of the colonoscope. Am J Gastroenterol. 2007;102:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Dai J, Feng N, Lu H, Li XB, Yang CH, Ge ZZ. Transparent cap improves patients’ tolerance of colonoscopy and shortens examination time by inexperienced endoscopists. J Dig Dis. 2010;11:364-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | de Wijkerslooth TR, Stoop EM, Bossuyt PM, Mathus-Vliegen EM, Dees J, Tytgat KM, van Leerdam ME, Fockens P, Kuipers EJ, Dekker E. Adenoma detection with cap-assisted colonoscopy versus regular colonoscopy: a randomised controlled trial. Gut. 2012;61:1426-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Tee HP, Corte C, Al-Ghamdi H, Prakoso E, Darke J, Chettiar R, Rahman W, Davison S, Griffin SP, Selby WS. Prospective randomized controlled trial evaluating cap-assisted colonoscopy vs standard colonoscopy. World J Gastroenterol. 2010;16:3905-3910. [PubMed] [Cited in This Article: ] |
| 14. | Harada Y, Hirasawa D, Fujita N, Noda Y, Kobayashi G, Ishida K, Yonechi M, Ito K, Suzuki T, Sugawara T. Impact of a transparent hood on the performance of total colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2009;69:637-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Lee YT, Lai LH, Hui AJ, Wong VW, Ching JY, Wong GL, Wu JC, Chan HL, Leung WK, Lau JY. Efficacy of cap-assisted colonoscopy in comparison with regular colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2009;104:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Matsushita M, Hajiro K, Okazaki K, Takakuwa H, Tominaga M. Efficacy of total colonoscopy with a transparent cap in comparison with colonoscopy without the cap. Endoscopy. 1998;30:444-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Tada M, Inoue H, Yabata E, Okabe S, Endo M. Feasibility of the transparent cap-fitted colonoscope for screening and mucosal resection. Dis Colon Rectum. 1997;40:618-621. [PubMed] [Cited in This Article: ] |
| 18. | Hewett DG, Rex DK. Cap-fitted colonoscopy: a randomized, tandem colonoscopy study of adenoma miss rates. Gastrointest Endosc. 2010;72:775-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Rastogi A, Bansal A, Rao DS, Gupta N, Wani SB, Shipe T, Gaddam S, Singh V, Sharma P. Higher adenoma detection rates with cap-assisted colonoscopy: a randomised controlled trial. Gut. 2012;61:402-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Horiuchi A, Nakayama Y, Kajiyama M, Kato N, Ichise Y, Tanaka N. Benefits and limitations of cap-fitted colonoscopy in screening colonoscopy. Dig Dis Sci. 2013;58:534-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Horiuchi A, Nakayama Y. Improved colorectal adenoma detection with a transparent retractable extension device. Am J Gastroenterol. 2008;103:341-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Horiuchi A, Nakayama Y, Kato N, Ichise Y, Kajiyama M, Tanaka N. Hood-assisted colonoscopy is more effective in detection of colorectal adenomas than narrow-band imaging. Clin Gastroenterol Hepatol. 2010;8:379-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Pontone S, Angelini R, Standoli M, Patrizi G, Culasso F, Pontone P, Redler A. Low-volume plus ascorbic acid vs high-volume plus simethicone bowel preparation before colonoscopy. World J Gastroenterol. 2011;17:4689-4695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Lee SH, Chung IK, Kim SJ, Kim JO, Ko BM, Hwangbo Y, Kim WH, Park DH, Lee SK, Park CH. An adequate level of training for technical competence in screening and diagnostic colonoscopy: a prospective multicenter evaluation of the learning curve. Gastrointest Endosc. 2008;67:683-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-129; quiz 1314, 1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 742] [Cited by in F6Publishing: 792] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 26. | Sawhney MS, Farrar WD, Gudiseva S, Nelson DB, Lederle FA, Rector TS, Bond JH. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700-1705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Arain MA, Sawhney M, Sheikh S, Anway R, Thyagarajan B, Bond JH, Shaukat A. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 28. | Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 532] [Cited by in F6Publishing: 548] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 30. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1371] [Article Influence: 137.1] [Reference Citation Analysis (0)] |
| 31. | Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 32. | Taber A, Romagnuolo J. Effect of simply recording colonoscopy withdrawal time on polyp and adenoma detection rates. Gastrointest Endosc. 2010;71:782-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Anderson JC, Butterly LF, Robinson CM, Goodrich M, Weiss JE. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: data from the New Hampshire colonoscopy registry by using a standardized preparation-quality rating. Gastrointest Endosc. 2014;80:463-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Ussui V, Coe S, Rizk C, Crook JE, Diehl NN, Wallace MB. Stability of Increased Adenoma Detection at Colonoscopy. Follow-Up of an Endoscopic Quality Improvement Program-EQUIP-II. Am J Gastroenterol. 2015;110:489-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |