Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6236
Peer-review started: December 21, 2014
First decision: January 22, 2015
Revised: February 9, 2015
Accepted: March 12, 2015
Article in press: March 12, 2015
Published online: May 28, 2015
AIM: To describe our experience using a low-accelerating-dose regimen (LADR) with pegylated interferon alpha-2a and ribavirin in treatment of hepatitis C virus (HCV) recurrence.
METHODS: From 2003, a protocolized LADR strategy was employed to treat liver transplant (LT) recipients with recurrent HCV at our institution. Medical records of 182 adult patients with recurrent HCV treated with LADR between 1/2003 and 1/2011 were reviewed. Histopathology from all post-LT liver biopsies were reviewed in a blinded fashion. Paired recipient and donor IL28B status were assessed. A novel technique was employed to ascertain recipient and donor IL28B (rs12979860) Gt data using DNA extracted from archival FFPE tissue from explanted native livers and donor gallbladders respectively. The primary endpoint was SVR; secondary endpoints examined include (1) patient and graft survival; (2) effect of anti-viral therapy on liver histology (fibrosis and inflammation); (3) incidence of on-treatment development of ACR, CDR, or PCH; (4) association of recipient and donor IL28B genotype with SVR; and (5) incidence of anti-viral therapy-associated adverse events (anemia, leukopenia, thrombocytopenia, depression) and hepatic decompensation.
RESULTS: The overall SVR rate was 38% (29% Gt1, 67% Gt2, 86% Gt3 and 58% Gt4). HCV Gt (P < 0.0001), donor age (P = 0.003), cytomegalovirus mismatch (P = 0.001), baseline serum bilirubin (P = 0.002), and baseline viral load (P = 0.04) were independent predictors for SVR. SVR rates were significantly higher in the recipient-CC/donor-non CC pairs (P = 0.007). Neither baseline fibrosis nor change in fibrosis stage after anti-viral therapy were associated with SVR. Fibrosis progressed in 72% of patients despite SVR. Median graft survival was 91 mo. Five-year patient survival was superior in patients who achieved SVR (97% vs 82%, P = 0.001). Pre-treatment ALP ≥ 150 U/L (P = 0.01), total bilirubin ≥ 1.5 mg/dL (P = 0.001) and creatinine ≥ 2 mg/dL (P = 0.001) were independently associated with patient survival. Only 13% of patients achieving SVR died during the follow-up period. Treatment discontinuation and treatment-related mortality occurred in 35% and 2.2% of patients, respectively. EPO, G-CSF and blood transfusion were needed in 89%, 40% and 23% of patients, respectively. Overall hospitalization rate for treatment-related serious adverse events was 21%. Forty-six (25%) of the patients were deceased; among those who died, 25 (54%) were due to liver-related complications, and 4 deaths (9%) occurred while receiving therapy (2 patients experienced hepatic decompensation and 2 sepsis).
CONCLUSION: LADR strategy remains relevant in managing post-LT recurrent HCV where access to DAAs is limited. SVR is associated with improved survival, but fibrosis progression still occurs.
Core tip: This study represents the largest single center experience in treating recurrent hepatitis C virus (HCV) in LT recipients utilizing a low-accelerating-dose regimen (LADR) protocol of PEG alpha-2a and RBV; achieving 38% SVR and superior five-year patient survival in patients with SVR (97% vs 82%, P = 0.001). A novel technique was used to ascertain recipient and donor IL28B (rs12979860) Gt data using archival FFPE tissue from explanted native livers and donor gallbladders. A comprehensive blinded review of available liver histology was performed. LADR strategy remains relevant in managing recurrent HCV where access to DAAs is limited. SVR is associated with improved survival, but fibrosis progression still occurs.
- Citation: Lim KB, Sima HR, Fiel MI, Khaitova V, Doucette JT, Chernyiak M, Ahmad J, Bach N, Chang C, Grewal P, Kim-Schluger L, Liu L, Odin J, Perumalswami P, Florman SS, Schiano TD. Utility of the low-accelerating-dose regimen in 182 liver recipients with recurrent hepatitis C virus. World J Gastroenterol 2015; 21(20): 6236-6245
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6236.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6236
Post-liver transplant (LT) treatment of recurrent hepatitis C virus (HCV) with pegylated (PEG)-based regimens is challenging, labor intensive, and carries sub-optimal SVR rates, and poor patient tolerability[1,2]. The optimal time to commence anti-viral therapy is controversial and adds extra complexity to this cohort of difficult-to-treat patients. Data on donor and recipient IL28B genotypes have shed some light on their impact on HCV recurrence and treatment response, with increasing awareness and relevance of donor IL28B status[3-6]. Although the advent of DAA has increased the success rate of post-LT antiviral therapy, improved SVR rates must be balanced against drug-drug interactions with immunosuppressive agents and increased adverse events resulting in treatment discontinuation[7,8]. Emerging data using interferon-free regimens have shown initial promise with improved response rates and tolerability[9,10]. However, interferon-containing regimens may still be needed in post-LT patients who do not respond to newer regimens, or when access to DAAs are limited due to financial and resource constraints[11,12].
To date, published studies on post-LT HCV therapy have typically been single center series with heterogeneous treatment strategies[13-16]. LADR is well described in cirrhotic HCV patients in the pre-LT setting[17,18], but experience of low-accelerating-dose regimen (LADR) in the post-transplant setting is limited. We herein report our experience in treating 182 LT recipients with HCV recurrence over a 9-year period using a standardized LADR protocol of PEG α-2a and RBV. To our knowledge, this represents the largest single-center treatment study of post-LT HCV using a uniform strategy. The data presented will be of utility in guiding therapy in interferon-containing regimens when the newer oral agents are ineffective, not available or are restricted for financial reasons.
Starting in 2003, a protocolized LADR strategy was employed to treat all LT recipients with recurrent HCV at our institution. The decision to commence anti-viral therapy was based on clinical need as determined by the primary hepatologist. Patients were started on 90 mcg PEG α-2a weekly and RBV 7 mg/kg daily in 2 divided doses (50% of optimal dose). Doses were increased at week 4 to PEG α-2a 135 mcg weekly and RBV 10 mg/kg daily thereafter doses were PEG alpha-2a 180 mcg weekly and RBV 14 mg/kg daily from week 8 onwards if tolerated, for a total of 48 wk regardless of HCV Gt. EPO 40000 IU once weekly was started when the hemoglobin fell below 10 g/dL. RBV dose reduction occurred if EPO was unsuccessful at maintaining hemoglobin above 10 g/dL. Patients received blood transfusions if they experienced symptomatic anemia despite EPO supplementation. G-CSF 300 mcg once weekly (up to maximum dose of 300 mcg three times weekly) was commenced when the WBC was < 1500/mm and/or ANC was < 750/mm. Dose reduction of PEG α-2a by 50% occurred when the platelet count < 50000/mm; PEG α-2a was discontinued when platelet count was less than 25000/mm.
The medical records of LT recipients age > 18 years with recurrent HCV, treated with LADR between 1/2003 and 1/2011 were reviewed. The following were excluded from the analysis: LT recipients treated with non-pegylated interferon, HCV patients co-infected with HBV or HIV, and patients enrolled in other HCV study protocols. Patients with uncontrolled psychiatric illness, poorly controlled diabetes, symptomatic cardiopulmonary disease, concomitant autoimmune disease, moderate to advanced chronic kidney disease (> CKD stage 2) and SLKT recipients were typically not treated with antiviral therapy. We defined recurrent HCV in LT recipients as the presence of typical histological features of HCV and contemporaneously detectable HCV RNA in the serum, after excluding concurrent other etiologies. LT recipients with fibrosing cholestatic hepatitis (FCH) were included in our analysis. The diagnosis of FCH was determined histologically by the presence of cholestasis, ductular reaction, mild portal inflammation, portal fibrosis and delicate fibroconnective tissue accompanying and surrounding proliferating bile ductules, as well as periportal fibrosis and exclusion of large duct biliary obstruction[19].
Clinical data including recipient and donor demographics, review of hospitalizations occurring during therapy, HCV Gt, serious adverse events, use of growth factors (EPO and G-CSF) and anti-depressants were recorded. Quantitative HCV RNA by polymerase chain reaction (Cobas TaqMan, Roche Laboratories), liver chemistries and complete blood count, at specific time points during HCV therapy, were collected. All transplanted patients received intra-operative methylprednisolone 500 mg. Patients with serum creatinine > 2 mg/dL or requiring dialysis prior to LT received basiliximab induction therapy on the day of LT and post-operative day-4. LT recipients were maintained on tacrolimus-based immunosuppression and MMF in the setting of renal dysfunction. Oral steroid therapy was tapered and discontinued during the 6 mo after LT. Severe ACR was treated with pulse methylprednisolone 500 mg daily over 3 d; mild and moderate ACR were treated by optimizing tacrolimus levels and MMF dosing.
The primary endpoint was SVR, defined as absence of HCV RNA at 6 mo after completion of anti-viral therapy. Secondary endpoints were (1) patient and graft survival; (2) effect of anti-viral therapy on liver histology (fibrosis and inflammation); (3) incidence of on-treatment development of ACR, CDR, or PCH; (4) association of recipient and donor IL28B genotype with SVR; and (5) incidence of anti-viral therapy-associated adverse events (anemia, leukopenia, thrombocytopenia, depression) and hepatic decompensation. On-treatment responses were analyzed at weeks 4 (RVR), 8, 12 (EVR), 24 and 48 (ETR). This study was reviewed and approved by the Icahn School of Medicine at Mount Sinai, NY Institutional Review Board.
Archival (FFPE) liver tissue from all post-LT biopsies was obtained and independently reviewed. Liver biopsy specimens were stained with hematoxylin and eosin and Masson trichrome. The latest liver biopsy before starting anti-viral therapy, any liver biopsy performed while receiving therapy, and the first biopsy after anti-viral therapy was concluded, were considered as the pre-, on- and post-treatment biopsies, respectively. All biopsies were reviewed by a single hepatopathologist (MIF) blinded to patient identity and timing of the liver biopsy. Inflammation and fibrosis were graded and staged using the Scheuer classification[20]. Biopsies having ACR, CR, PCH, FCH and steatosis were identified and graded utilizing standard histological classification systems[21-24].
Surviving liver recipients were tested for IL28B (rs12979860) genotype using peripheral venous blood samples. If recipient serum was not available, DNA extracted from FFPE tissue from explanted native livers was used to determine the IL28B genotype. In addition, because donor sera were not available for testing, archival FFPE tissue from the donor gallbladder was used in order to obtain adequate tissue for DNA extraction. Approximately 3 to 5 five-micron thick sections were cut from the FFPE blocks. After standardization of DNA isolation, amplification using qPCR was performed. When testing the DNA using TaqMan assay, approximately 100-200 ng DNA per reaction was required. Allelic discrimination for the “C” and “T” was analyzed using the DNA sample extracted[25-27]. The results obtained from this novel method were cross-referenced with IL28B results obtained from recipient serum in a subset of cases and showed 100% concordance, providing validation of this technique.
Statistical methods and analysis were performed by an experienced biostatistician (John T Doucette) from Icahn School of Medicine at Mount Sinai. Descriptive statistics were produced for all study variables to examine their univariate distributions. Bivariate associations with SVR were assessed using Pearson’s χ2 (or Fisher’s exact test, when appropriate) for categorical factors, and t-tests for continuous variables. All significance tests were two-sided with a level of α = 0.05. Logistic regression models were fitted to identify independent predictors of SVR. To examine predictors of survival, log-rank tests were used for bivariate associations and Cox proportional hazards models were fitted to identify independent predictors. For both the logistic regression and Cox models, a modified stepwise procedure was employed with a significance level of α = 0.05 for both entry and removal. Candidates for the stepwise procedure were all baseline characteristics that had a bivariate association with each outcome at the α = 0.20 level. Because some candidate variables had missing data, the models selected by the stepwise procedure were then re-fitted with each unselected candidate added one at a time to reassess significance while allowing inclusion of the maximum number of observations. Although patients who achieved SVR were compared to those who did not with respect to survival in bivariate analysis, SVR was not a candidate predictor for the survival models because it is not a baseline characteristic.
Twelve hundred forty-one patients underwent LT for HCV during the study period, and 158 LT recipients were treated with non-LADR protocols, either as part of study protocols, or using non-pegylated interferon with or without RBV prior to 2003. One hundred eight-two patients with recurrent HCV were treated using the LADR protocol. Patients were predominantly male (80%), Caucasian (50%), with a median age of 52 years. One hundred forty one (77%) were infected with HCV Gt1. HCV Gt sub-types were available in 131 patients with Gt1; Gt1a was more common than Gt1b in our cohort (57% vs 36%). Seventy-four (75%) had pre-treatment baseline HCV RNA > 1000000 IU/mL. Other baseline characteristics of these 182 patients are summarized in Table 1. The median time from LT to commencing anti-viral therapy was 20.5 mo; median age when therapy started was 56 years; 119 (65%) patients completed 48 wk of anti-viral therapy. One hundred thirty-five (74%) patients tolerated peak PEG α-2a dose of 180 mcg/wk, and 34 (19%) achieved RBV doses > 1000 mg/d. Median peak weekly PEG α-2a dose was 180 mcg; median daily RBV dose was 800 mg.
| Recipient characteristics | n | |
| Median age (yr) | 182 | 52 ± 8.2 |
| Male gender | 182 | 145 (80) |
| Ethnicity | 182 | |
| Caucasian | 91 (50) | |
| Black | 18 (10) | |
| Hispanic | 52 (29) | |
| Asian | 14 (8) | |
| Arabic | 6 (3) | |
| Others | 1 (1) | |
| IL28B | 122 | |
| CC | 40 (33) | |
| CT | 51 (42) | |
| TT | 31 (25) | |
| HCC (pre-LT) | 182 | 75 (41) |
| Diabetes mellitus | 182 | 71 (39) |
| CMV positivity | 181 | 126 (70) |
| Duration from LT to anti-viral therapy (mo) | 182 | 20.5 ± 43.5 |
| Median peak PEG dose (mcg/wk) | 181 | 180 ± 31.6 |
| Median peak RBV daily dose (mg) | 181 | 800 ± 294 |
| Median treatment duration (wk) | 182 | 48 ± 21.5 |
| HCV genotype | 182 | |
| 1 | 141 (77) | |
| 1a | 80/141 (57) | |
| 1b | 51/141 (36) | |
| Subtype not available | 10/141 (7) | |
| 2 | 15 (8) | |
| 3 | 14 (8) | |
| 4 | 12 (7) | |
| Median baseline lab values | ||
| ALT (IU) | 181 | 84 ± 128.3 |
| AST (IU) | 181 | 80 ± 112.5 |
| ALP (IU) | 180 | 131 ± 144.3 |
| Total bilirubin (mg) | 181 | 0.9 ± 3.1 |
| HCV RNA (IU/mL) | 181 | 3870000 ± 23602918 |
| Hemoglobin (g/dL) | 180 | 13 ± 1.7 |
| White cell count | 180 | 4.3 ± 1.9 |
| Platelets | 181 | 123 ± 73 |
| Creatinine | 181 | 1.2 ± 1.3 |
| Immunosuppression at start of LADR1 | 182 | |
| Tacrolimus | 146 (80)1 | |
| Cyclosporine | 25 (14) | |
| Sirolimus | 4 (2) | |
| Mycophenolate mofetil | 66 (36) | |
| Prednisone | 9 (5) | |
| Donor characteristics | ||
| Male gender | 175 | 99 (57) |
| Median age | 177 | 48 ± 18 |
| CMV positivity | 178 | 117 (66) |
| Ethnicity | 182 | |
| White | 118 (65) | |
| Black | 26 (14) | |
| Hispanic | 12 (7) | |
| Asian | 6 (3) | |
| American indian/alaskan native | 3 (2) | |
| Other/unknown | 17 (9) | |
| Donor IL28B | 122 | |
| CC | 66 (54) | |
| CT | 35 (29) | |
| TT | 21 (17) |
The overall SVR rate was 38% (70/182 patients). SVR stratified by HCV Gt 1, 2, 3, 4 was 29%, 67%, 86%, and 58%, respectively. Gt4 patients showed superior SVR (58%) compared to Gt1 patients (29%) (P = 0.05). No difference in SVR was observed between Gt1a (24%) and Gt1b (28%) (P = 0.6), nor between Gt2 (67%) and Gt 3 (86%) (P = 0.5).
Despite receiving lower doses from the outset, 25 (14%) patients still achieved RVR; 53 (29%) patients were HCV RNA negative at 8 wk of anti-viral therapy; 75 (41%) patients achieved EVR. Thirty-one percent who achieved RVR went on to attain SVR, while 69% of those without RVR still went on to achieve SVR (P = 0.0003). Seventy-two percent who achieved EVR had SVR; 11% of those who did not achieve EVR went on to SVR (P < 0.0001). Among patients who were HCV negative at week 8, 72% went on to achieve SVR, while only 20% who were HCV positive at week 8 achieved SVR (P < 0.0001) (Table 2). Forty-eight percent of patients failed to achieve a 2-log decline in serum HCV RNA between baseline and week 12 of anti-viral treatment (classified as non-responders); relapse and non-response rates were 20% and 41%, respectively. SVR rates were 40%, 29%, 42% for recipient IL28B genotype CC, CT and TT respectively, and 36%, 37%, 33% for donor IL28B genotype CC, CT and TT, respectively. Significantly different SVR rates were observed between the various pairs of donor and recipient IL28B. SVR rates were significantly higher in the recipient-CC/donor-non CC (72%) vs other recipient and donor combinations (41%, 43%, 24% for recipient-CC/donor-CC, recipient-non CC/donor-CC and recipient-non CC/donor-non CC respectively) (P = 0.007).
| Percentage | Percentage who went on to achieve SVR | P value | |
| RVR | 0.0003 | ||
| Yes | 14 | 31 | |
| No | 86 | 69 | |
| Week 8 negativity | < 0.0001 | ||
| Yes | 29 | 72 | |
| No | 71 | 20 | |
| EVR | < 0.0001 | ||
| Yes | 41 | 72 | |
| No | 59 | 11 |
Factors associated with SVR and survival on univariate analysis are summarized in Table 3. Multivariate analysis found HCV Gt (P < 0.0001), donor age (P = 0.003), cytomegalovirus (CMV) mismatch (P = 0.001), baseline serum bilirubin (P = 0.002), and baseline viral load (P = 0.04) to be independent predictors for SVR. Pre-treatment ALP ≥150 U/L (P = 0.01), total bilirubin ≥ 1.5 (P = 0.001) and creatinine ≥ 2 mg (P = 0.001) were independently associated with patient survival (Table 4).
| Factors associated with SVR | SVR (%) | P value |
| HCV genotype 1 vs non-1 | 29 vs 71 | < 0.0001 |
| HCV viral load < 1 million vs≥ 1 million IU/mL | 54 vs 33 | 0.009 |
| Recipient IL28B-CC vs non-CC | 55 vs 34 | 0.03 |
| Paired IL28b recipient CC and donor non-CC vs other recipient and donor combinations | 72 vs 36 | 0.007 |
| Pre-treatment total bilirubin < 1.5 mg vs≥ 1.5 mg | 44 vs 25 | 0.02 |
| Pre-treatment ALP < 150 vs≥ 150 | 44 vs 29 | 0.04 |
| Treatment duration ≥ 48 wk vs < 48 wk | 45 vs 27 | 0.02 |
| Peak RBV dose ≥ 800 mg vs < 800 mg | 44 vs 25 | 0.02 |
| Administration of MMF vs No MMF | 30 vs 43 | 0.09 |
| RVR vs no RVR | 34 vs 66 | 0.0006 |
| EVR vs no EVR | 85 vs 15 | < 0.0001 |
| Week 8 HCV RNA undetectable vs detectable | 72 vs 20 | < 0.0001 |
| Donor age ≤ 40 vs > 40 | 58 vs 29 | 0.0002 |
| Matched recipient and donor ethnicity vs unmatched | 46 vs 33 | 0.09 |
| Mismatched CMV status (D+/R-) vs other combinations | 54 vs 35 | 0.04 |
| Pre-treatment fibrosis stage 0-2 vs 3-4 | 43 vs 26 | 0.08 |
| Factors associated with patient survival | 10-year survival (%) | |
| Week 8 HCV RNA undetectable vs detectable | 71 vs 44 | 0.003 |
| Week 12 HCV RNA undetectable vs detectable | 73 vs 44 | < 0.001 |
| Pre-treatment fibrosis stage 0-2 vs 3-4 | 70 vs 31 | 0.004 |
| Pre-treatment ALP < 150 vs≥ 150 | 67 vs 42 | < 0.001 |
| Pre-treatment total bilirubin < 1.5 mg vs≥ 1.5 mg | 62 vs 46 | < 0.001 |
| Pre-treatment creatinine < 2 vs≥ 2 | 61 vs 0 | < 0.001 |
| Factors associated with SVR | Comparison | Adjusted OR for SVR | 95%CI | P value | |
| Lower | Upper | ||||
| HCV genotype | 2 vs 1 | 11.40 | 2.8 | 47.3 | < 0.0001 |
| 3 vs 1 | 43.10 | 6.5 | 286.3 | ||
| 4 vs 1 | 10.70 | 2.4 | 48.8 | ||
| Pre-treatment total bilirubin | ≥ 1.5 vs < 1.5 | 0.21 | 0.08 | 0.57 | 0.002 |
| Donor age | Each 10 yr | 0.69 | 0.54 | 0.88 | 0.003 |
| CMV mismatch | Donor +/recipient - | 4.80 | 1.8 | 12.6 | 0.001 |
| vs all others | |||||
| HCV viral load (baseline) | Each 1 million | 0.97 | 0.94 | 0.999 | 0.040 |
| Adjusted HR | |||||
| Pre-treatment ALP | ≥ 150 vs < 150 | 2.01 | 1.18 | 3.43 | 0.010 |
| Pre-treatment total bilirubin | ≥ 1.5 vs < 1.5 | 2.49 | 1.47 | 4.21 | 0.001 |
| Pre-treatment creatinine | ≥ 2 vs < 2 | 5.88 | 2.68 | 12.92 | < 0.001 |
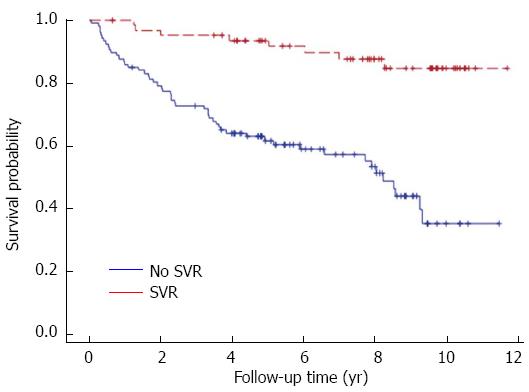
One hundred thirty-six (75%) patients were alive at time of data censure; 24 had progressed to cirrhosis or graft failure requiring re-LT. Among these 24 patients, 58% were non-responders, 29% relapsed and 13% achieved SVR. Median graft survival was 91 mo. Five-year patient survival was superior in patients who achieved SVR (97% vs 82%, P = 0.001) (Figure 1). Forty-six (25%) of the patients were deceased. Among those who died, 25 (54%) were due to liver-related complications, and 4 deaths (9%) occurred while receiving therapy (2 patients experienced hepatic decompensation and 2 sepsis).
Sixty (35%) patients discontinued therapy before 48 wk. On-treatment anemia, defined as a fall in Hb > 2 g/dL from baseline or Hb < 10 g/dL, occurred in 123 (69%). Leukopenia, defined as WBC < 1500, occurred in 18 (10%). EPO, G-CSF and blood transfusion were needed in 89%, 40% and 23% of patients, respectively. Development of anemia and leukopenia were not significantly associated with SVR or patient survival. Thirty-one percent were given anti-depressants due to clinically significant PEG α-2a-induced depression. The overall hospitalization rate for treatment-related serious adverse events was 21%. Fifteen percent of patients required two or more hospitalizations. The three most common reasons for hospitalization were anemia, pulmonary complications and infection.
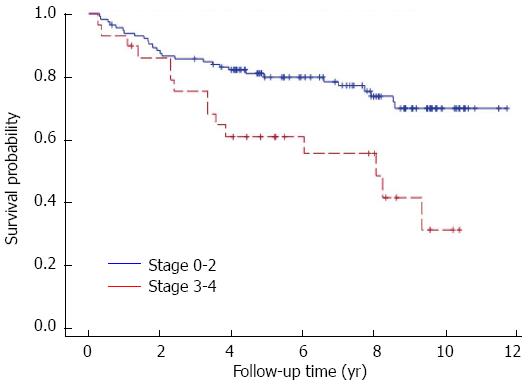
One hundred fifty-three (84%) patients had a liver biopsy prior to HCV therapy. Among the 182 patients included in this study, 153 pre-treatment, 90 on-treatment and 89 post-treatment liver specimens were available for review. Seventy-six paired pre- and post-treatment biopsies were available for analysis. Univariate analysis showed baseline fibrosis stage was significantly associated with survival (P = 0.004) (Figure 2); a trend to significance was observed between baseline fibrosis and SVR (P = 0.08). Baseline fibrosis was not significantly associated with SVR and survival on multivariate analysis. Fibrosis was observed to progress despite anti-viral therapy, while no change was seen in necroinflammatory grade, steatosis grade and stage (Table 5). Change in fibrosis stage after anti-viral therapy was not associated with SVR (P = 0.08) (Table 6). Fifty-nine patients had histology proven rejection in pre-, on-, and post-treatment biopsies (Table 7). PCH occurred in 30 patients. No association was found between paired donor and recipient IL28B status on development of on-treatment ACR, inflammation grade (HAI score), and fibrosis stage in the pre-treatment liver biopsies. Seven patients had FCH prior to anti-viral therapy. Among these difficult-to-treat FCH patients, three achieved SVR, one relapsed and three were non-responders. Three FCH patients died but four were still alive at time of data censure. All three FCH patients who achieved SVR were still alive.
| Pre-anti viral treatment | Post-anti viral treatment | P value | |
| Fibrosis stage (n = 76)(based on the Scheuer scheme) | |||
| Stage 0-2 | 65 (86) | 41 (54) | < 0.001 |
| Stage 3-4 | 11 (14) | 35 (46) | |
| Grade (n = 76) | |||
| Grade 0-2 | 51 (67) | 46 (61) | 0.33 |
| Grade 3-4 | 25 (33) | 30 (39) | |
| Degree of steatosis (brunt classification) (n = 78) | |||
| Score 0-1 | 69 (88) | 73 (94) | 0.29 |
| Score 2-3 | 9 (12) | 5 (6) | |
| Steatohepatitis grade (n = 75) | |||
| Grade 0 | 69 (92) | 71 (95) | 0.73 |
| Grade 1-2 | 6 (8) | 4 (5) | |
| Steatohepatitis stage (n = 75) | |||
| Stage 0-1 | 72 (96) | 73 (97) | 1.00 |
| Stage 2-4 | 3 (4) | 2 (3) |
| Fibrosis | SVR (n =32) P = 0.08 | Survived (n =60) P = 0.03 | |
| Progressed | |||
| one stage | 32 (42) | 17 (53) | 27 (45) |
| two stages | 10 (13) | 5 (16) | 9 (15) |
| ≥ three stages | 5 (7) | 1 (3) | 2 (4) |
| Unchanged | 23 (30) | 5 (16) | 17 (28) |
| Improved | 6 (8) | 4 (12) | 5 (8) |
| Pre-anti viral treatmentn = 153 | On-anti viral treatmentn =90 | Post-anti viral treatmentn = 89 | |
| Rejection | 39 (25) | 12 (13)1 | 8 (9) |
| ACR | 39 (25) | 10 (11) | 7 (8) |
| CDR | 0 | 5 (6) | 1 (1) |
| Plasma cell hepatitis | 7 (5) | 11 (12) | 12 (13) |
| Fibrosing cholestatic hepatitis | 7 (5) | 5 (6) | 1 (1) |
Treatment of recurrent HCV in LT recipients remains challenging. SVR rates from published studies using interferon and RBV based therapies range from 8% to 48%[15-18]. These studies are limited by small sample size, and significant heterogeneity in study design, time from LT to start of antiviral treatment and degree of fibrosis at baseline. Our study represents the largest single center experience to date in the treatment of recurrent HCV in LT recipients utilizing a uniform LADR protocol of PEG α-2a and RBV. The LADR approach achieved 38% SVR. We included patients with FCH in our analysis, which may account for the lower SVR compared to other studies which excluded these difficult-to-treat patients[28]. Five-year patient survival was superior in patients who achieved SVR (97% vs 82%, P = 0.001. We found HCV RNA negativity at week 8 more predictive than RVR for achieving SVR. One explanation for this observation is the lower starting doses of PEG α-2a and RBV utilized in the LADR approach which resulted in a longer time to achieve HCV RNA negativity. RVR and EVR data from the current study may help guide therapy for other PEG α-2a and RBV-containing regimens used in the post-LT setting. Despite the LADR approach, treatment discontinuation occurred in 35%, and anemia remained a significant problem (69%) with up to 89% of patients requiring EPO and 23% requiring blood transfusion. Our data suggest that initiating antiviral therapy in patients with less fibrosis, as well as in the setting of a creatinine < 2.0 appears warranted. Thus, even with the new all oral antiviral regimens, starting HCV treatment earlier before more advanced fibrosis or renal dysfunction develops may be beneficial. It also appears reasonable to initiate HCV therapy earlier in patients receiving an older donor liver, as both their survival and chance of SVR were appreciably lower in the current study.
The advent of potent DAAs has added further complexity to the management of this post-LT population. Recently published multi-center studies[7,8,29,30] involving treatment of recurrent HCV with telaprevir or boceprevir achieved an overall SVR of 50%-63%. The improved SVR however was counterbalanced against reduced patient tolerability, increased rates of treatment discontinuation and potential adverse events including risk of death, significant drug-drug interactions[31-33], cost, and access to therapy. Coilly et al[8] observed a 43% treatment discontinuation rate, anemia in 92% of patients with 35% requiring red blood cell transfusions, and treatment-related mortality of 8%. Burton et al[29] found that 57% of patients required blood transfusions during the first 16 wk of therapy, 27% required hospitalization and there was 9% mortality. These observations may have implications on future therapies, underscoring the fact that adopting a LADR approach in combination with DAA therapy in LT recipients may be a useful strategy to mitigate anti-viral therapy-associated adverse events and reduce early treatment discontinuation. Whether a 12%-25% increase in SVR with DAA triple therapy compared to conventional therapy with PEG and RBV in post-LT patients justifies the cost, adverse events and potential life threatening consequences is debatable. The lower treatment-related mortality rate associated with LADR may influence some transplant physicians to pursue this treatment strategy until more effective, safer and shorter duration therapies emerge.
Although early data from interferon-free regimens in the post-LT setting appear promising, longer-term relapse rates and the incidence of viral resistance remain unknown. With the current and projected financial burden of approved and evolving DAA, these interferon-free regimens may remain out of reach to individuals in some geographic areas. The administration of first generation DAA in conjunction with PEG and RBV may be the best available therapy in some patients, and the use of PEG α-2a and RBV with newer DAA may still be warranted in patients failing all-oral therapy. Anemia remains an issue in the treated patients, mostly ascribable to RBV-its dosing could not be optimized in the majority of our patients. Use of a LADR protocol may be more appropriate when using the first generation DAA because of the anemia, rather than starting with standard doses of PEG α-2a and RBV. Based on the current study’s data, a LADR protocol could also be considered in conjunction with newer DAAs, possibly for harder to treat Gt3 patients.
To date, few studies on recurrent HCV have distinguished Gt 1a and 1b frequency and outcomes. We observed a greater proportion of subtype 1a compared to 1b (61% vs 39%), with similar SVR rates (24% vs 28%). Our analysis demonstrated 71% SVR in liver recipients with HCV Gt 2-4, and 86% SVR Gt 3. The better-than-expected SVR in LT recipients with Gt3 warrants further study to confirm if longer courses of therapy are indeed needed and if PEG alpha-2a will remain necessary in the treatment regimen for Gt3 recurrent HCV with the availability of all-oral regimens. There is a paucity of data on antiviral therapy in Gt4 liver recipients; the current study shows appreciable therapeutic efficacy in this group.
A unique strength of this study was the employment of a novel technique to ascertain both donor and recipient IL28B (rs12979860) Gt data using archival FFPE tissue from donor gallbladders and explanted native livers. Adequate amounts of DNA were obtained in all patients. This enabled us to obtain 122-paired donor and recipient IL28B Gt data for analysis. Previously published studies show inconsistent associations between recipient, donor and paired donor/recipient IL28B genotype with HCV recurrence and SVR in transplant recipients[34-37]. We observed the highest SVR rates (72%) in the recipient CC/donor-non CC pairs; while donor CC was associated with 41%-43% SVR and recipient-non CC/donor-non CC pairs were associated with the poorest SVR rates of 24%. The significance of donor and recipient IL28B needs to be re-evaluated with the advent of DAA and interferon-free regimens.
This study undertook a comprehensive review of liver biopsies performed prospectively in a blinded fashion by a single hepatopathologist in pre-, on- and post-treatment liver biopsies. Our analyses of paired pre- and post-treatment liver histology showed no significant change in necroinflammatory scores but did show progression of fibrosis. Liver fibrosis progressed in 72% of patients despite achieving SVR. We found that baseline fibrosis was not significantly associated with SVR but was with overall survival. This is in contrast to a previous study of two non-contemporaneous cohorts which showed that baseline fibrosis was significantly associated with SVR[28]. All patients in our study who were cirrhotic at baseline did not achieve SVR. This is an important reminder to LT physicians in order to still aggressively treat other potential causes of liver fibrosis-namely NASH. The search for potent anti-fibrotic agents is still warranted and represents a clinical need in the post-LT setting. The number of patients with plasma cell hepatitis (PCH) doubled during and after therapy; longer term follow up of these patients is necessary in order to ascertain if their ultimate survival was negatively impacted and whether they had fibrosis progression[38]. Only 13% of patients achieving SVR died during the follow up period, underscoring the benefits of successful antiviral therapy in this population.
The retrospective nature of this study resulted in some unavailable data at selected time points. We were not able to accurately ascertain the number of patients who required dose reductions of PEG α-2a and RBV. Some pre- and post-treatment liver biopsies were unavailable to assess post-treatment necroinflammatory and fibrosis scores. The decision to treat recurrent HCV was based on the hepatologist’s discretion and not fibrosis stage; however this treatment algorithm is employed in large transplant programs in the US and thus is reflective of current practice.
In conclusion, this single-center study is the largest to date that examines the utility of a LADR protocol in the treatment of recurrent HCV in liver transplant recipients. We have shown that utilizing a LADR strategy achieved an overall SVR of 38%. Patients with GT 2, 3, and 4 had excellent SVR rates, suggesting that this regimen may still be an option for such patients failing all-oral antiviral therapy, especially when using the newer DAA as part of a LADR strategy. Major strengths of this study were the extensive review of pre-treatment and post-treatment liver histology and the utility of a novel method of obtaining IL28B genotype data from donor gallbladder. Our analysis suggests that baseline fibrosis was not associated with improved SVR, although patients having more advanced fibrosis had decreased overall survival. This makes the case for treatment of recurrent HCV at all stages of fibrosis, but especially earlier after LT before fibrosis has progressed and appreciable renal dysfunction (often with accompanying anemia) have developed. Healthcare reimbursement systems in some countries may limit the availability of DAAs. Until interferon-free HCV regimens are more extensively studied in liver recipients and become more affordable and widely available, PEG α-2a and RBV will continue to have a role in the armamentarium used to treat recurrent HCV, especially in resource-limited regions around the world.
Post-LT treatment of recurrent hepatitis C virus (HCV) with PEG-based regimens is challenging, labor intensive, and carries sub-optimal SVR rates, and poor patient tolerability. Emerging data using interferon-free DAA regimens have shown improved response rates and tolerability. However, interferon-containing regimens may still be needed in post-LT patients when access to DAAs is limited due to financial and resource constraints.
The low-accelerating-dose regimen has proven to be an effective therapeutic strategy in treatment of HCV in cirrhotic patients. The utility of low-accelerating-dose regimen in the largest post-LT cohort in a single center is described herein.
Comprehensive review of pre-, on- and post-treatment liver histology was undertaken and provides insights in treatment-related changes in liver histology. Novel techniques were employed to ascertain recipient and donor IL28B genotype using archival FFPE tissue from explanted native livers and donor gallbladders.
The study results suggest that the low-accelerating-dose regimen can be successfully employed as a treatment strategy for liver transplant recipients with recurrent HCV.
The low-accelerating-dose regimen involves commencing patients on 90 mcg PEG α-2a weekly and RBV 7 mg/kg daily in 2 divided doses (50% of optimal dose). Doses were increased at week 4 to PEG α-2a 135 mcg weekly and RBV 10 mg/kg daily thereafter doses were PEG α-2a 180 mcg weekly and RBV 14 mg/kg daily from week 8 onwards if tolerated, for a total of 48 wk regardless of HCV genotype.
This retrospective study highlights the utility of the low-accelerating-dose regimen of pegylated alpha-interferon α-2-a and RBV in patients with recurrent HCV infection following liver transplantation. This is the largest reported single-center experience to date. This is an important paper highlighting that interferon-based regimens remain relevant in the era of DAA regimens where access to these costly drugs is limited.
P- Reviewer: Malnick SDH, Tovikkai C S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Kugelmas M, Osgood MJ, Trotter JF, Bak T, Wachs M, Forman L, Kam I, Everson GT. Hepatitis C virus therapy, hepatocyte drug metabolism, and risk for acute cellular rejection. Liver Transpl. 2003;9:1159-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Berenguer M, Palau A, Fernandez A, Benlloch S, Aguilera V, Prieto M, Rayón JM, Berenguer J. Efficacy, predictors of response, and potential risks associated with antiviral therapy in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2006;12:1067-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Lange CM, Moradpour D, Doehring A, Lehr HA, Müllhaupt B, Bibert S, Bochud PY, Antonino AT, Pascual M, Farnik H. Impact of donor and recipient IL28B rs12979860 genotypes on hepatitis C virus liver graft reinfection. J Hepatol. 2011;55:322-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Allam SR, Krüger B, Mehrotra A, Schiano T, Schröppel B, Murphy B. The association of IL28B polymorphism and graft survival in patients with hepatitis C undergoing liver transplantation. PLoS One. 2013;8:e54854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Cisneros E, Baños I, Citores MJ, Duca A, Salas C, Noblejas A, Cañizares M, Millán I, Cuervas-Mons V, Vilches C. Increased risk of severe hepatitis C virus recurrence after liver transplantation in patients with a T allele of IL28B rs12979860. Transplantation. 2012;94:275-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, Uchiyama H, Soejima Y, Shirabe K, Matsuura Y. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139:1577-185, 1577-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, Ryland KL, Yataco ML, Satyanarayana R, Rosser BG, Vargas HE. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19:690-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, Radenne S, Pageaux GP, Si-Ahmed SN, Guillaud O, Antonini TM. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014;60:78-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 9. | Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 456] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 10. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 889] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 11. | Petta S, Cabibbo G, Enea M, Macaluso FS, Plaia A, Bruno R, Gasbarrini A, Craxì A, Cammà C. Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2014;59:1692-1705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Wei L, Lok AS. Impact of new hepatitis C treatments in different regions of the world. Gastroenterology. 2014;146:1145-50.e1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Bzowej N, Nelson DR, Terrault NA, Everson GT, Teng LL, Prabhakar A, Charlton MR. PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. Liver Transpl. 2011;17:528-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, Bosch J, Forns X. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, Riely C, Martin P, Teperman L, Jiao J. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005;41:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Dumortier J, Scoazec JY, Chevallier P, Boillot O. Treatment of recurrent hepatitis C after liver transplantation: a pilot study of peginterferon alfa-2b and ribavirin combination. J Hepatol. 2004;40:669-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Massoumi H, Elsiesy H, Khaitova V, Peterson B, Norkus E, Grewal P, Liu L, Chang C, Bach N, Schiano TD. An escalating dose regimen of pegylated interferon and ribavirin in HCV cirrhotic patients referred for liver transplant. Transplantation. 2009;88:729-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Dickson RC, Caldwell SH, Ishitani MB, Lau JY, Driscoll CJ, Stevenson WC, McCullough CS, Pruett TL. Clinical and histologic patterns of early graft failure due to recurrnet hepatitis C in four patients after liver transplantation. Transplantation. 1996;61:701-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Scheuer PJ, Standish RA, Dhillon AP. Scoring of chronic hepatitis. Clin Liver Dis. 2002;6:335-47, v-vi. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Ormonde DG, de Boer WB, Kierath A, Bell R, Shilkin KB, House AK, Jeffrey GP, Reed WD. Banff schema for grading liver allograft rejection: utility in clinical practice. Liver Transpl Surg. 1999;5:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Adeyi O, Fischer SE, Guindi M. Liver allograft pathology: approach to interpretation of needle biopsies with clinicopathological correlation. J Clin Pathol. 2010;63:47-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 23. | Fiel MI, Agarwal K, Stanca C, Elhajj N, Kontorinis N, Thung SN, Schiano TD. Posttransplant plasma cell hepatitis (de novo autoimmune hepatitis) is a variant of rejection and may lead to a negative outcome in patients with hepatitis C virus. Liver Transpl. 2008;14:861-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, Fung J, Gouw A, Gustafsson B, Haga H. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31:792-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 351] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 25. | Galmozzi E, Del Menico B, Rametta R, Dongiovanni P, Fracanzani AL, Benedan L, Borroni V, Maggioni P, Fargion S, Valenti L. A tetra-primer amplification refractory mutation system polymerase chain reaction for the evaluation of rs12979860 IL28B genotype. J Viral Hepat. 2011;18:628-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Hashemi M, Moazeni-Roodi A, Bahari A, Taheri M. A tetra-primer amplification refractory mutation system-polymerase chain reaction for the detection of rs8099917 IL28B genotype. Nucleosides Nucleotides Nucleic Acids. 2012;31:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Nakamoto S, Kanda T, Imazeki F, Wu S, Arai M, Fujiwara K, Yokosuka O. Simple assay based on restriction fragment length polymorphism associated with IL28B in chronic hepatitis C patients. Scand J Gastroenterol. 2011;46:955-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Berenguer M, Aguilera V, Rubín A, Ortíz C, Jimenez M, Prieto M. Comparison of two non-contemporaneous HCV-liver transplant cohorts: strategies to improve the efficacy of antiviral therapy. J Hepatol. 2012;56:1310-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Burton JR, O’Leary JG, Verna EC, Saxena V, Dodge JL, Stravitz RT, Levitsky J, Trotter JF, Everson GT, Brown RS. A US multicenter study of hepatitis C treatment of liver transplant recipients with protease-inhibitor triple therapy. J Hepatol. 2014;61:508-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Werner CR, Egetemeyr DP, Nadalin S, Königsrainer A, Malek NP, Lauer UM, Berg CP. Treatment of recurrent genotype 1 hepatitis C post-liver transplantation: single center experience with telaprevir-based triple therapy. Z Gastroenterol. 2014;52:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011;54:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 32. | Hulskotte E, Gupta S, Xuan F, van Zutven M, O’Mara E, Feng HP, Wagner J, Butterton J. Pharmacokinetic interaction between the hepatitis C virus protease inhibitor boceprevir and cyclosporine and tacrolimus in healthy volunteers. Hepatology. 2012;56:1622-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Tischer S, Fontana RJ. Drug-drug interactions with oral anti-HCV agents and idiosyncratic hepatotoxicity in the liver transplant setting. J Hepatol. 2014;60:872-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Coto-Llerena M, Pérez-Del-Pulgar S, Crespo G, Carrión JA, Martínez SM, Sánchez-Tapias JM, Martorell J, Navasa M, Forns X. Donor and recipient IL28B polymorphisms in HCV-infected patients undergoing antiviral therapy before and after liver transplantation. Am J Transplant. 2011;11:1051-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Duarte-Rojo A, Veldt BJ, Goldstein DD, Tillman HL, Watt KD, Heimbach JK, McHutchison JG, Poterucha JJ, Vargas-Vorackova F, Charlton MR. The course of posttransplant hepatitis C infection: comparative impact of donor and recipient source of the favorable IL28B genotype and other variables. Transplantation. 2012;94:197-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Charlton MR, Thompson A, Veldt BJ, Watt K, Tillmann H, Poterucha JJ, Heimbach JK, Goldstein D, McHutchison J. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology. 2011;53:317-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 37. | Firpi RJ, Dong H, Clark VC, Soldevila-Pico C, Morelli G, Cabrera R, Norkina O, Shuster JJ, Nelson DR, Liu C. CC genotype donors for the interleukin-28B single nucleotide polymorphism are associated with better outcomes in hepatitis C after liver transplant. Liver Int. 2013;33:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Ward SC, Schiano TD, Thung SN, Fiel MI. Plasma cell hepatitis in hepatitis C virus patients post-liver transplantation: case-control study showing poor outcome and predictive features in the liver explant. Liver Transpl. 2009;15:1826-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |