Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.653
Peer-review started: May 22, 2014
First decision: June 18, 2014
Revised: July 3, 2014
Accepted: July 25, 2014
Article in press: July 25, 2014
Published online: January 14, 2015
AIM: To observe the effect of response-guided add-on therapy with adefovir (ADV) and lamivudine (LAM) in cirrhotic hepatitis B (CHB) patients.
METHODS: A total of 100 patients with CHB and cirrhosis were divided into three arms according to hepatitis B virus (HBV) DNA level after 24 wk LAM monotherapy: Arm A (complete response, HBV DNA ≤ 60 IU/mL, n = 49), Arm B (partial response, HBV DNA: 60-2000 IU/mL, n = 31) and Arm C (inadequate response, HBV DNA > 2000 IU/mL, n = 20). ADV was added to LAM at week 48 in Arms A and B, but at week 24 in Arm C. Virological response, YMDD mutations, biochemical response, and liver function were evaluated.
RESULTS: Comparison of the three arms demonstrated that early complete virologic response at week 24 was associated with maintained viral suppression (undetectable rate of HBV DNA at week 144 was 95.96%, 66.67% and 35.29%, respectively, P = 0.000) and reduced YMDD mutations (mutation rate at week 144 was 0%, 3.23% and 15%, respectively, P = 0.015) after 144 wk treatment. For patients who failed to achieve complete virological response at week 24, switching to combination therapy further decreased HBV DNA level by 1 log10 IU/mL. All three arms obtained biochemical benefits including decline of alanine aminotransferase and elevation of albumin. In patients who developed HBV DNA breakthrough for YMDD mutations, ADV add-on therapy did not induce further multiple drug resistance to LAM or ADV.
CONCLUSION: Optimized response-guided add-on therapy of ADV and LAM maintains long-term suppression of HBV DNA and improves liver function in CHB patients with compensated liver cirrhosis.
Core tip: We conducted this prospective cohort study to explore an optimized strategy of adding adefovir (ADV) and lamivudine (LAM) at different time points according to the early virological response, and to observe its association with long-term treatment outcomes in cirrhotic hepatitis B (CHB) patients with compensated cirrhosis. We found that optimized response-guided add-on therapy of ADV and LAM maintains long-term suppression of hepatitis B virus DNA and improves liver function in CHB patients with compensated liver cirrhosis.
- Citation: Gu EL, Yu YQ, Wang JL, Ji YY, Ma XY, Xie Q, Pan HY, Wu SM, Li J, Chen CW, Xu XW, Wang YE, Yao GB, Wang H, Zhang WH. Response-guided treatment of cirrhotic chronic hepatitis B patients: Multicenter prospective study. World J Gastroenterol 2015; 21(2): 653-660
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/653.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.653
Hepatitis B virus (HBV) remains a major public health problem, with 350-400 million people infected chronically worldwide[1]. In China, most HBV infection occurs perinatally or in early childhood, usually with a long period of immune tolerance before immune clearance[2]. Patients with CHB are at an increased risk of major complications as disease duration extends, including hepatic cirrhosis, liver failure, and hepatocellular carcinoma (HCC). The REVEAL study in Taiwan demonstrated that an elevated level of serum HBV DNA was associated with disease progression in patients with cirrhotic hepatitis B, and antiviral therapy prevented progression of liver disease[3,4]. Currently, there are several choices of nucleos(t)ide analogs (NAs) in antiviral treatment of CHB, including lamivudine (LAM), adefovir dipivoxil (ADV), entecavir (ETV), telbivudine and tenofovir disoproxil fumarate[5]. LAM was the first NA introduced into clinical use and is still a common choice for CHB patients in China as well as the Asia-Pacific region. However, administration of LAM is limited by its high rate of drug resistance, with a 3-year resistance rate of approximately 50%[6,7]. For patients with CHB and cirrhosis, the emergence of drug resistance and resumption of viral replication might lead to hepatic flares, exacerbation of liver function, or even death in some liver failure cases. Add-on ADV can effectively suppress viral replication and reduce the risk of drug resistance to LAM[8,9].
The primary aim of antiviral therapy is to suppress HBV replication and prevent disease progression. Many studies have shown that the undetectable rate of HBV DNA at week 24 was associated with reduced drug-resistance-associated mutations[10], but its predictive value for the treatment outcomes after long-term add-on ADV-LAM combination therapy has not been clarified, especially for the subgroup of CHB patients with liver cirrhosis. We conducted this prospective cohort study to explore an optimized strategy of adding ADV to LAM at different time points according to the early virological response, and to observe its association with long-term treatment outcomes in CHB patients with compensated cirrhosis.
This prospective, multicenter cohort study was conducted at eight medical centers in China. Hepatitis B e antigen (HBeAg) positive or negative CHB patients with compensated cirrhosis were enrolled from June 2007 to Febrary 2009. All patients were given LAM 100 mg/d (Heptodin; GlaxoSmithKline China Investment Co. Ltd., Beijng, China). Patients were assigned into three arms according to serum HBV DNA levels at week 24, with Arm A ≤ 60 IU/mL (complete virological response), Arm B 60-2000 IU/mL (partial virological response), and Arm C > 2000 IU/mL (inadequate virological response). Patients in Arm C were treated with ADV 10 mg/d (Hepsera; GlaxoSmithKline China Investment Co. Ltd.) in addition to on-going LAM at week 24, while patients in Arms A and B continued LAM monotherapy until week 48, and at the end of 48 wk LAM monotherapy, ADV was added in these two arms. All the patients were monitored until week 144 (Figure 1). The study was approved by the Ethics Review Committee of Jing’an Central Hospital (Certification No. Ethic-07-05) and all the patients gave their written informed consent before enrolment in the study. The procedures were in accordance with the Helsinki Declaration of 1975.
Patients eligible for the study were diagnosed with compensated liver cirrhosis by clinical evidence, which was defined as platelet count < 100000/L with ultrasonographical findings suggestive of cirrhosis, including a blunted, nodular liver edge accompanied by splenomegaly (> 12 cm), or by liver biopsy showing an Ishak fibrosis score > 4[11]. Patients included in this study were Child-Pugh class A. The inclusion criteria included hepatitis B surface antigen (HBsAg) positivity for at least 6 mo; baseline HBV DNA levels > 2000 IU/mL; compensated liver cirrhosis (indicated by routine laboratory tests together with ultrasound or computed tomography results) with Child-Pugh class A; absence of co-infection with hepatitis C virus, hepatitis D virus or HIV; no previous NA treatment. Major exclusion criteria included evidence of HCC; alanine aminotransferase (ALT) > 10 times upper limit of normal; decompensated liver cirrhosis; comorbidity with other liver diseases, severe physical or mental disorders; or pregnancy.
Serum HBV DNA levels (Cobas Taqman; Roche Diagnostics Shanghai Co. Ltd., China) with the lower limit of detection (LLOD) of 12 IU/mL, liver functions [including platelet count (PLT), prothrombin time (PT), albumin, total bilirubin, ALT, aspartate aminotransferase, alkaline phosphatase and γ-glutamyl transpeptidase], and HBV serological markers (HBsAg, anti-HBs, HBeAg, anti-HBe; Abbott Architect System; Abbott China Co. Ltd., Shanghai, China) testing as well as ultrasound examination were performed every 12 wk within the first 48 wk and every 24 wk thereafter.
Complete virological response or undetectable HBV DNA was defined as serum HBV DNA levels no more than 60 IU/mL. Virological breakthrough was defined as any increase in serum HBV DNA by > 1 log10 IU/mL from nadir, or redetection of serum HBV DNA at levels 10 times the LLOD after having an undetectable result[12].
Statistical analyses were performed by Stata version 11. Continuous variables were expressed as median (25-75 percentile). Categorical variables were summarized as counts and percentages. Continuous variables were compared using two-tailed Student’s t test, analysis of variance, Mann-Whitney test, or Kruskal-Wallis test, depending on their distribution, while categorical variables were compared by χ2 test or Fisher’s exact test. Serum HBV DNA level was expressed as log10 IU/mL, and we regarded an HBV DNA level as 10 IU/mL when it was below LLOD for the sake of description and statistical analysis.
A cohort of 113 CHB patients with compensated cirrhosis were enrolled at baseline, and 100 underwent allocation according to serum HBV DNA levels at week 24 (Figure 1). Age, sex ratio, and liver function results of the three arms were comparable at baseline. The proportion of HBeAg-positive CHB in Arms B and C was significantly higher than that in Arm A. Baseline HBV DNA level in Arm C was significantly higher than that in Arms A and B. The demographic characteristics of the three arms, as well as their laboratory results at baseline, are demonstrated in Table 1.
| Arm A (n = 49) | Arm B (n = 31) | Arm C (n = 20) | Statistics | P value | |
| Median age (yr) | 45 (38-52) | 40 (36-50) | 43.5 (40.5-55) | F = 0.8400 | 0.4345 |
| Male:Female | 41:8 | 25:6 | 15:5 | χ2 = 0.6979 | 0.7050 |
| HBeAg-positive, n (%) | 13 (26.53) | 21 (67.74)a | 14 (70)a | χ2 = 17.7676 | 0.0000 |
| Median PLT (× 109/L) | 109 (73-150) | 109 (89-144) | 98.25 (76.5-122) | χ2 = 2.4880 | 0.2882 |
| Median PT (s) | 13.40 (12.6-14.3) | 12.75 (12-13.7)c | 14.30 (13-15.5) | χ2 = 10.1270 | 0.0063 |
| Median albumin (g/L) | 44 (39-46.8) | 43 (40-47.6) | 43.5 (40.9-47.3) | F = 0.0400 | 0.9600 |
| Median TB (μmol/L) | 17.35 (14.5-23.35) | 15.8 (13.1-20.1) | 18.35 (15.9-23.6) | χ2 = 2.1780 | 0.3366 |
| Median ALT (U/L) | 55 (43.2-87.5) | 51 (34.5-82) | 64 (48.5-91.6) | F = 0.400 | 0.6730 |
| Median AST (U/L) | 55 (41.05-68.5) | 49 (30-57) | 54.15 (45.5-66.5) | F = 1.5100 | 0.2251 |
| Median HBV DNA (log10 IU/mL) | 5.86 (5.16, 6.58)c | 6.06 (5.77, 6.39)c | 6.7 (6.29, 7.17) | F = 5.0300 | 0.0084 |
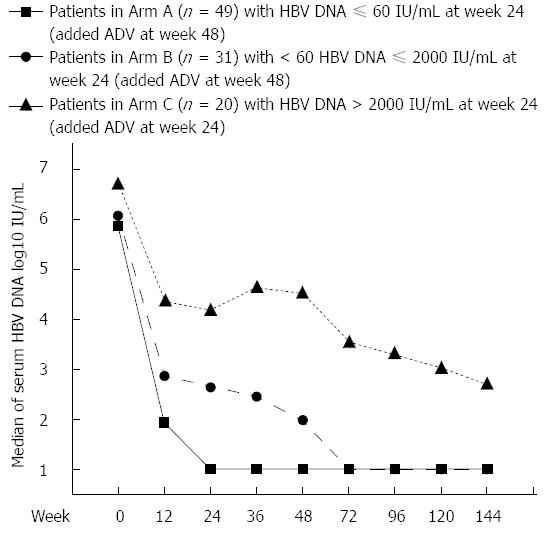
Baseline HBV DNA levels of Arms A and B were comparable, while those in of Arm C were significantly higher at baseline as well as each time point during treatment (P < 0.05). Viral load of Arms A and B both decreased sharply after initiation of LAM monotherapy. For Arm A, serum HBV DNA levels progressively decreased until median HBV DNA level was below LLOD at week 24, and the suppression of viral replication was maintained thereafter. However, in Arm B, further reduction of HBV DNA was minimally observed after week 24 (week 24 vs week 48, P = 0.2059) with LAM monotherapy. After adding ADV to LAM at week 48, a reduction of approximately 1 log10 IU/mL of serum HBV DNA levels was resumed (week 48 vs week 72, P = 0.0001), and median HBV DNA level below LLOD was achieved at week 72. Serum HBV DNA level in Arm A was significantly lower than that in Arm B at each time point from weeks 12 to 144 (P < 0.05 at each time point). In Arm C, serum HBV DNA levels decreased with slow and fluctuating kinetics. After ADV was added to LAM at week 24, further reduction of approximately 1 log10 IU/mL serum HBV DNA levels was observed, but the median HBV DNA level of Arm C did not reach LLOD throughout treatment. Serum HBV DNA levels at each time point are depicted in Figure 2.
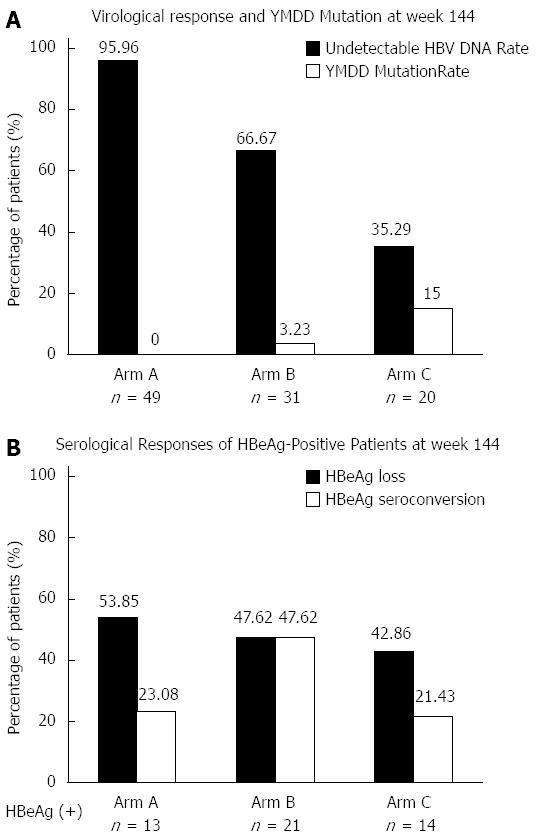
The undetectable rate of HBV DNA (≤ 60 IU/mL) at week 144 was 95.56%, 66.67% and 35.29% for Arm A, B and C, respectively (P = 0.000), as shown in Figure 3. At each time point during treatment, the differences in undetectable HBV DNA rates among the three arms were statistically significant (Figure 3). The YMDD mutation rate at week 144 was 0%, 3.23% and 15% for Arm A, B and C, respectively (P = 0.015), which is shown in Figure 4. For patients with virological breakthrough due to YMDD mutations, ADV add-on therapy did not further induce multiple drug resistance to both LAM and ADV. Early complete virological response at week 24 seemed to be associated with maintained viral suppression and reduced YMDD mutations at week 144. None of the patients who achieved complete virological response at week 24 (Arm A) developed YMDD mutation at week 144. For patients who failed to achieve complete virological response at week 24, undetectable rate of HBV DNA was increased after switching to LAM and ADV combination therapy, but still far from satisfactory when compared with Arm A.
The number of patients with HBeAg-positive CHB at baseline was 13, 21 and 14 for Arm A, B and C, respectively. At week 144, HBeAg loss rate was 53.85% (7/13), 47.62% (10/21) and 42.86 (6/14) (P = 0.993), and HBeAg seroconversion rate was 23.08% (3/13), 47.62% (10/21) and 21.43% (3/14) (P = 0.245) in the three arms, respectively (Figure 4).
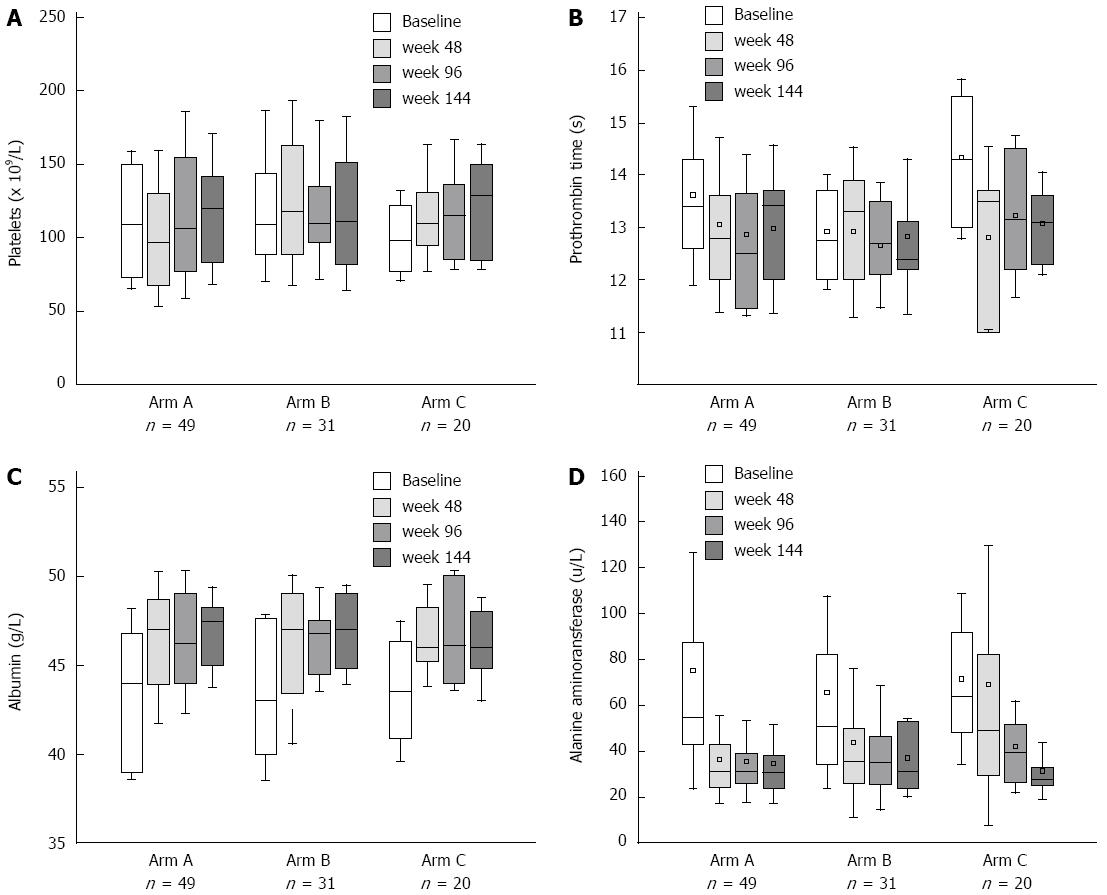
Biochemical response and improvement of liver function were achieved after NA treatment, and no biochemical breakthrough was observed during 144-wk follow-up. There was no significant difference in PLT and PT at week 48 compared to baseline in each arm (Figure 5A and B). Serum ALT levels decreased significantly at week 48, which reduced from 55 (43.2-87.5) U/L to 31.75 (24.5-43.1) U/L (P = 0.0000) and from 51 (34.5-82) U/L to 35.8 (26-50) U/L (P = 0.0092) in Arm A and B, respectively. For Arm C, reduction of serum ALT levels occurred after LAM and ADV combination therapy [week 0 vs 48: 64 (48.5-91.6) U/L vs 49.3 (29.5-82) U/L, P = 0.2471; week 0 vs 96: 64 (48.5-91.6) U/L vs 39.55 (26.9-52) U/L, P = 0.0130] (Figure 5D). For the parameters of liver function, the increase in serum levels of albumin was the most remarkable. At week 48, serum levels of albumin rose from 44 (39-46.75) g/L to 47 (43.9-48.65) g/L in Arm A (P = 0.0006), 43 (40-47.6) g/L to 47 (43.4-49) g/L in Arm B (P = 0.0029), and 43.5 (40.9-47.3) g/L to 46 (45.2-48.25) g/L in Arm C (P = 0.0045) (Figure 5C). Biochemical results of the three arms were comparable at each time point.
Several clinical studies have suggested that ADV add-on therapy is associated with a higher rate of virological response and reduced antiviral resistance, compared with sequential montherapy with LAM and ADV[8,9,13,14] in patients with CHB. According to the roadmap concept proposed by Keeffe et al[15], assessment of virological response at week 24, which is predictive of long-term treatment outcomes, is of significant importance for further treatment decisions. However, no prospective study has been reported to evaluate the response-guided strategy of ADV add-on therapy to LAM-based treatment in CHB patients with compensated cirrhosis. We conducted this multicenter, prospective cohort study to explore the optimal strategy of ADV add-on LAM combination therapy in the specified subgroup of CHB patients with cirrhosis, using the roadmap concept.
All the patients started with LAM monotherapy and had ADV added at different times, according to the virological response at week 24. The total treatment duration of the study was 144 wk. Our findings indicated that long-term antiviral therapy was effective in suppressing HBV replication, achieving serological and biochemical responses, and improving liver functions in CHB patients with compensated cirrhosis. Early virological response was associated with sustained viral suppression and a lower rate of drug resistance during long-term treatment[16,17].
Early ADV add-on can prevent the emergence of resistance to LAM[18]. In this study, add-on therapy of ADV to LAM was conducted even in patients with complete response after week 48. In the Asia-Pacific region, especially in economically undeveloped areas, LAM is still the first option for CHB and HBV-related cirrhosis. The resistance rate was 24% after 1 year LAM treatment and rose to 70% after 5 years treatment[6,19]. Emergence of resistance may exacerbate disease, reduce the benefit of antiviral therapy, and even threaten the life of patients with liver cirrhosis. Therefore, add-on therapy of ADV to LAM should be conducted even in patients with a complete response. For patients with compensated cirrhosis who achieved a complete virological response at week 24, the risk of drug resistance was reduced significantly by prolonged treatment. ADV add-on LAM combination therapy could maintain virological response and prevent the emergence of drug resistance, with an undetectable rate of HBV DNA of 95.96% and none of the patients developed YMDD mutations at week 144.
The ADV add-on strategy was also considered effective for patients with partial virological response at week 24. Further reduction of HBV DNA levels was observed and most of the patients with compensated cirrhosis achieved sustained undetectable HBV DNA after week 72. In addition, the YMDD mutation rate at week 144 was only 3.23%. However, serum HBV DNA levels did not further decrease with the continuation of LAM monotherapy from weeks 24 to 48, which suggests that immediate add-on of ADV at week 24 might be beneficial for patients with partial response.
After long-term therapy, patients in Arm C with inadequate virological response at week 24 achieved significantly lower rates of undetectable HBV DNA at week 144 than did patients in Arms A and B, even though combination therapy was initiated at an earlier stage. Despite a reduction of approximately 1 log10 IU/mL of serum HBV DNA after combination therapy, the median HBV DNA level did not reach LLOD throughout treatment. Moreover, the YMDD mutation rate at week 144 was 15%, which was significantly higher than that of Arms A and B. Thus, for patients with inadequate virological response at week 24, switching to more potent antiviral agents with a high genetic barrier and without cross-resistance to LAM would be a better choice[20].
In conclusion, most CHB patients with compensated liver cirrhosis benefited from long-term antiviral therapy. Significant improvement of liver function was observed in all patients, regardless of the degree of HBV DNA reduction. Early virological response at week 24 was associated with satisfactory long-term treatment outcomes and was valuable for individualized treatment decisions. To avoid HBV DNA breakthrough, add-on therapy of ADV to LAM was effective in maintaining viral suppression and reducing YMDD mutations in patients with complete or partial virological response. Meanwhile, switching to more potent antiviral monotherapy or combination therapy was suggested for patients with inadequate response at week 24.
We thank Professor Guang-Bi Yao who designed and initiated this prospective cohort study.
Many studies have shown that the undetectable rate of hepatitis B virus (HBV) DNA at week 24 was associated with reduced drug-resistance-associated mutations. According to the roadmap concept, assessment of virological response at week 24, which was predictive of long-term treatment outcomes, was of significant importance for further treatment decisions. However, no prospective study has been reported to evaluate the response-guided strategy of adefovir (ADV) add-on therapy to lamivudine (LAM)-based treatment in chronic hepatitis B (CHB) patients with compensated cirrhosis.
LAM can achieve sustained viral suppression, slow down progression of liver disease, and prevent development of major complications or hepatocellular carcinoma. However, long-term LAM may induce selective drug-resistant mutations, reduce the clinical benefit, and lead to exacerbation of liver functions, or even death. The incidence of viral breakthrough was 5.1%-36.0% in LAM-resistant CHB patients treated with entecavir (ETV), compared to 0%-3% in those receiving LAM plus ADV combination therapy. Therefore, ADV add-on therapy seemed to achieve more sustained suppression of viral replication than sequential ETV monotherapy in LAM-resistant patients. Considering the fact that tenofovir disoproxil fumarate has not come to market and the reality of the economic situation in China, ADV add-on therapy is still likely to be the optimal choice for LAM-resistant patients for the forseeable future.
Several clinical studies have suggested that ADV add-on strategy is associated with a higher rate of virological response and reduced antiviral resistance, compared with sequential monotherapy of LAM and ADV in patients with CHB. However, few prospective studies have been reported to evaluate the response-guided strategy of ADV add-on therapy to LAM-based treatment in CHB patients with compensated cirrhosis. This was a multicenter, prospective cohort study to explore the optimal strategy of ADV add-on LAM combination therapy in the specified subgroup of cirrhotic CHB patients, using the roadmap concept.
In the Asia-Pacific region, especially in the economically undeveloped areas, LAM is still the first option for CHB and HBV-related cirrhosis. Nucleot(s)ide analogs with a low-resistance barrier represent the first-line therapy for 65% of patients in China. The study was of importance for choosing the optimal strategy for response-guided treatment to avoid drug resistance in CHB patients with compensated cirrhosis.
Complete virological response or undetectable HBV DNA was defined as serum HBV DNA levels no more than 60 IU/mL. Virological breakthrough was defined as any increase in serum HBV DNA by > 1 log10 IU/mL from nadir or redetection of serum HBV DNA at levels 10 times the lower limit of detection after having an undetectable result.
This type of study may provide important information or guidelines to treat CHB patients with compensated liver cirrhosis.
P- Reviewer: Decena Sollano JD, Kamal SA, Kim K S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Ma S
| 1. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] [Cited in This Article: ] |
| 2. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 528] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 3. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [PubMed] [Cited in This Article: ] |
| 4. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [PubMed] [Cited in This Article: ] |
| 5. | Ahn SH, Chan HL, Chen PJ, Cheng J, Goenka MK, Hou J, Lim SG, Omata M, Piratvisuth T, Xie Q. Chronic hepatitis B: whom to treat and for how long? Propositions, challenges, and future directions. Hepatol Int. 2010;4:386-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687-696. [PubMed] [Cited in This Article: ] |
| 7. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 929] [Cited by in F6Publishing: 936] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 8. | Yatsuji H, Suzuki F, Sezaki H, Akuta N, Suzuki Y, Kawamura Y, Hosaka T, Kobayashi M, Saitoh S, Arase Y. Low risk of adefovir resistance in lamivudine-resistant chronic hepatitis B patients treated with adefovir plus lamivudine combination therapy: two-year follow-up. J Hepatol. 2008;48:923-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81-90. [PubMed] [Cited in This Article: ] |
| 10. | Keeffe EB, Zeuzem S, Koff RS, Dieterich DT, Esteban-Mur R, Gane EJ, Jacobson IM, Lim SG, Naoumov N, Marcellin P. Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5:890-897. [PubMed] [Cited in This Article: ] |
| 11. | Kim MN, Lee CK, Ahn SH, Lee S, Kim SU, Kim do Y, Kim HS, Han KH, Chon CY, Park JY. Maintaining remission in lamivudine-resistant patients with a virological response to adefovir add-on lamivudine after stopping lamivudine therapy. Liver Int. 2014;34:1543-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Ridruejo E, Adrover R, Silva MO. Virological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practice. Hepatology. 2011;54:1104-115; author reply 1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385-1391. [PubMed] [Cited in This Article: ] |
| 14. | Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307-313. [PubMed] [Cited in This Article: ] |
| 15. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315-141; quiz 1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 362] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 16. | Shin JW, Jung SW, Park BR, Kim CJ, Eum JB, Kim BG, Du Jeong I, Bang SJ, Park NH. HBV DNA level at 24 weeks is the best predictor of virological response to adefovir add-on therapy in patients with lamivudine resistance. Antivir Ther. 2012;17:387-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Yuen MF, Fong DY, Wong DK, Yuen JC, Fung J, Lai CL. Hepatitis B virus DNA levels at week 4 of lamivudine treatment predict the 5-year ideal response. Hepatology. 2007;46:1695-1703. [PubMed] [Cited in This Article: ] |
| 18. | Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34:785-791. [PubMed] [Cited in This Article: ] |
| 19. | Yao GB, Zhu M, Cui ZY, Wang BE, Yao JL, Zeng MD. A 7-year study of lamivudine therapy for hepatitis B virus e antigen-positive chronic hepatitis B patients in China. J Dig Dis. 2009;10:131-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Liaw YF. On-treatment outcome prediction and adjustment during chronic hepatitis B therapy: now and future. Antivir Ther. 2009;14:13-22. [PubMed] [Cited in This Article: ] |