Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.6065
Peer-review started: October 28, 2014
First decision: November 14, 2014
Revised: December 3, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: May 21, 2015
In this case report, we examined the levels of cytokines expressed before and during fecal stream diversion and after intestinal continuity was restored in a patient with collagenous colitis. We report the case of a 46-year-old woman with chronic, active collagenous colitis who either failed to achieve clinical remission or experienced adverse effects with the following drugs: loperamide, cholestyramine, budesonide, methotrexate and adalimumab. Due to the intractable nature of the disease and because the patient was having up to 15 watery bowel movements per day, she underwent a temporary ileostomy. Colonic biopsies were analyzed for mucosal cytokine protein levels before and during fecal stream diversion and after intestinal continuity was restored. Mucosal protein levels of interleukin (IL)-1β, IL-2, IL-6, IL-12, IL-17 A, IL-23, TNF, IFN-γ, IL-4, IL-5, IL-10 and IL-13 were all higher during active disease and decreased to non-detectable or considerably lower levels during fecal stream diversion. One month after the restoration of bowel continuity, when the patient experienced a relapse of symptoms, IL-2, IL-23 and IL-21 levels were again increased. Our results indicate that fecal stream diversion in this patient suppressed the levels of all cytokines analyzed in colonic biopsies. With the recurrence of clinical symptoms and histological changes after bowel reconstruction, the levels of primarily proinflammatory cytokines increased. Our findings support the hypothesis that a luminal factor triggers the inflammation observed in collagenous colitis.
Core tip: The pathophysiology of collagenous colitis remains poorly understood. We describe a patient with chronic, active collagenous colitis who either failed to achieve clinical remission or experienced adverse effects with all medications given; therefore the patient was treated with a temporary loop ileostomy. We analyzed cytokine protein production with Luminex assays in colonic biopsy tissues obtained before and during fecal stream diversion (FSD) and after intestinal continuity was restored. Because FSD leads to clinical and histological remission, this study protocol provided a unique opportunity to study cytokine dynamics during different stages of disease. We were thus able to demonstrate that FSD was followed by a decrease in the levels of nearly all cytokines and that the restoration of bowel continuity increased the levels of the proinflammatory cytokines interleukin IL-2, IL-21 and IL-23.
- Citation: Daferera N, Kumawat AK, Hultgren-Hörnquist E, Ignatova S, Ström M, Münch A. Fecal stream diversion and mucosal cytokine levels in collagenous colitis: A case report. World J Gastroenterol 2015; 21(19): 6065-6071
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/6065.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.6065
Collagenous colitis (CC) was first described in 1976 by Lindström et al[1] in rectal biopsies of a woman with chronic diarrhoea. In recent years, the incidence of CC has increased, and it is currently approximately 6 per 100000 inhabitants in Sweden[2]. The pathogenesis of the disease remains unknown, but it is considered to be multifactorial and to involve luminal agents that trigger a mucosal inflammatory reaction[3]. Furthermore, a mucosal barrier defect appears to be present in CC, resulting in an increased uptake of antigens and bacteria[4]. Little is known about the immunological mechanisms that drive the inflammation observed in CC, but recent studies have reported that a Th1 immunological reaction[5] or a mixed Th1/Th17 and Tc1/Tc17 reaction is present in microscopic colitis (MC)[6]. Medical treatment of CC is effective in the majority of patients, but in rare cases, if treatment fails, a temporary or permanent ileostomy (fecal stream diversion) may be necessary. Fecal stream diversion has been shown to lead to clinical and histological remission in patients with CC[7]; this phenomenon was first observed in patients with Crohn’s disease[8]. It was also reported that mucosal barrier dysfunction and altered mucosal permeability normalized during fecal stream diversion in one patient with CC but reappeared with bowel continuity[9]. Thus, fecal stream diversion offers an opportunity to study the immunological processes in inflammatory bowel diseases such as CC. In this case report, we describe the dynamics of cytokine production before and during fecal stream diversion and after bowel reconstruction in a patient with CC.
Our case report involves a 46-year-old woman with a history of diarrhoea that began in 2006 after one week of antibiotic treatment with amoxicillin. Stool cultures were negative, and the patient received the diagnosis irritable bowel syndrome (IBS) without endoscopic evaluation. Her previous medical history included asthma and Raynaud’s syndrome, for which she had been treated with verapamil on a continuous basis. In 2010, she was admitted to the gastroenterology clinic at the Linköping University Hospital because she was passing 10 watery stools per day and was consequently experiencing social disability as well. A colonoscopy was performed, and a diagnosis of CC was confirmed histologically.
She had already been given loperamide and cholestyramine for her IBS, with no symptomatic improvement. An induction treatment with 9 mg/d budesonide was initiated, which initially resulted in clinical improvement, but the patient’s condition stopped responding to treatment after one month. Repeated stool samples ruled out infectious causes, a SeHCAT test ruled out bile salt malabsorption, and a gastroscopy with duodenal biopsies revealed no sign of celiac disease. Human immunodeficiency virus with secondary chronic diarrhoea and hyperthyroidism were also ruled out.
A new colonoscopy was performed, and biopsy tissue was collected; subsequent analysis of the biopsy tissue revealed no sign of lymphoma, although the tissue was positive for Congo red staining, confirming the presence of amyloidosis. Primary amyloidosis was ruled out, and the increased amyloid deposition observed in the colonic biopsy tissue was considered to be secondary to prolonged mucosal inflammation.
Therapy with subcutaneous injections of methotrexate was initiated while the patient was still receiving 6 mg of budesonide daily as maintenance therapy. After 6 wk on 15 mg/wk methotrexate and a further 6 wk on 25 mg/wk methotrexate, no symptomatic improvement was observed, and all medication was discontinued. As third-line therapy, adalimumab was administered at an initial dose of 160 mg/wk, followed by 80 mg/wk after 2 wk and finally 40 mg/wk after 2 more weeks. After induction therapy with adalimumab, the patient’s symptoms improved significantly; the patient’s mean stool frequency fell from an initial > 10 watery stools per day to 2.8 soft stools per day. Clinical remission was maintained for 2 mo after discontinuation of adalimumab before the patient relapsed again. During relapse, she experienced fever, muscle and joint pain, and vomiting. Extensive blood analysis revealed elevated transaminase levels. Blood samples were tested for evidence of infection with CMV, EBV, HSV 1, HSV 2, rotavirus and parasites; the patient was positive for CMV-specific serum IgM, but CMV DNA was not detected in blood. An additional colonoscopy was performed, and biopsies were analyzed for the presence of CMV; all analyses were normal. At the same time, a consultation with a rheumatologist ruled out serum sickness and other potential underlying causes of her symptoms. No explanation of her symptoms was found, but it seemed unlikely that adalimumab had a causative role. Nevertheless, the decision was made not to continue with biological treatment. Because the patient continued to suffer from persisting, excessive joint pain, 30 mg of prednisolone daily was prescribed; her pain symptoms improved gradually, but her diarrhoea remained unchanged. Because all treatment regimens so far had failed and because the patient had developed severe anal skin ulcerations, a loop ileostomy was performed. Prednisolone treatment was tapered and stopped one month after surgery. Initially, there was a high flow of watery stool in the ileostomy, but normal ileac mucosa was found in biopsy tissue collected during an ileoscopy. After treatment with loperamide and a proton pump inhibitor, a normal flow was achieved. One year after the ileostomy, the patient experienced a herniation of the stoma, which caused stool leakage and consequent social and hygiene problems; thus, she requested bowel reconstruction. After bowel continuity was restored, her diarrhea reappeared immediately.
The patient gave written consent, and the regional ethical committee of Linkoping, Sweden consented to our obtaining extra mucosal biopsies for research purposes (Dnr 2012/216-31). A sigmoidoscopy with biopsies of the sigmoid colon was performed a week before and 4 mo after the ileostomy operation and 1 mo after bowel continuity was restored. The week before the operation, the patient was on 20 mg prednisolone per day (she had initially been receiving 30 mg prednisone, which was tapered down over a period of 2 mo before the first sigmoidoscopy); 4 mo after ileostomy and one month after bowel restoration, she was receiving no corticosteroid or other immunomodulating drug.
Biopsy tissues were immediately placed in Allprotect and stored at -80 °C until use. The biopsy tissues were homogenized using a TissueLyser II (Qiagen, Hilden, Germany) at 25 Hz for 5 × 1 min in radioimmunoprecipitation (RIPA) buffer containing 1 mmol/L Mini Protease Inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany). The homogenization mixture was centrifuged for 5 min at 10000 rpm, and the supernatant was divided into aliquots and stored at -80 °C until further analysis.
Tissue levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17A, IL-21, IL-23, IFN-γ and TNF proteins were analyzed using the xMAP technology developed by Luminex® (Austin, TX, United States). The concentrations were determined using the Milliplex® Map Kit (cat. no- #HCYTOMAG-60K and #HCYP2MAG-62K, Millipore, MA, United States) according to the manufacturer’s instructions.
The assays were performed in duplicates. The levels of cytokines in samples were expressed as pg/mg tissue, based on a standard curve constructed using known amounts of each analyte (Millipore).
All biopsies were analyzed by one pathologist with experience with microscopic colitis.
As shown in the Table 1 above, the first histological analysis performed before the ileostomy showed a picture of CC, but the classical signs of CC were not very apparent, most likely because the patient was taking 20 mg of prednisolone daily. The collagen layer thickness normalized during fecal diversion but increased to a clearly pathological level after intestinal reconstruction. The numbers of intraepithelial lymphocytes followed a similar pattern, with a considerable increase in their numbers observed after intestinal continuity was restored. However, the degree of lamina propria infiltration and epithelial degeneration remained unchanged throughout the study.
| Ileostomy | IEL | Collagen layer thickness | Lamina propria infiltration | Epithelial degeneration |
| Before | 58/100 enterocytes | 10 μm | 1 | 2 |
| During | 48/100 enterocytes | 3 μm | 1 | 2 |
| After | 108/100 enterocytes | 25 μm | 1 | 2 |
The mean ± SE weight of biopsy specimens used for cytokine quantification was 10 ± 0.98 mg. The levels of different cytokines in samples were expressed as pg/mg tissue.
Most tissue cytokine levels decreased substantially after fecal stream diversion (Figure 1). These decreases resulted in non-detectable concentrations for some cytokines, including IL-4, IL-10, and IL-1β. The levels of other cytokines, including IL-13, 1L-17 and TNF, decreased by 22%-40%, while the levels of IL-6, IL-12p70 and IFN-γ decreased by 75%-80%. IL-5 protein was not detectable at any time.
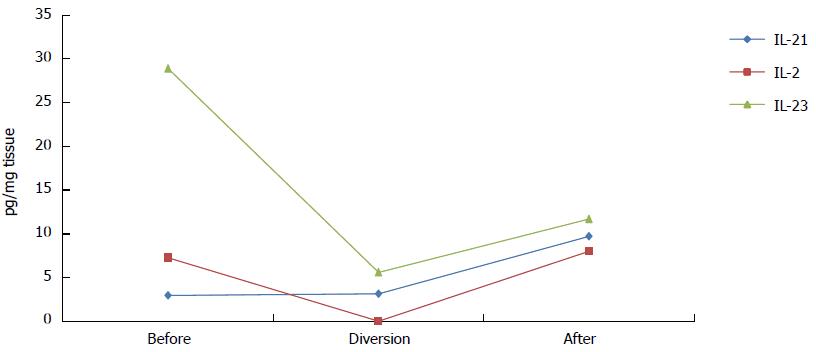
In comparison with all other measured cytokines (Figure 1), IL-21 was the only cytokine whose levels were unchanged during diversion (Figure 2). One month after intestinal continuity was restored; most cytokines remained at approximately the same low levels that were measured during fecal stream diversion (data not shown). However, IL-2, IL-21 and IL-23 exhibited a different trend; the levels of these cytokines increased after the fecal stream was re-established, as demonstrated in Figure 2.
Fecal stream diversion is known to cause clinical and histological remission in patients with CC[10]. Moreover, fecal stream diversion also appears to normalize mucosal barrier dysfunction in CC[4], but active disease unfortunately reoccurs after intestinal continuity is restored.
To our knowledge, this is the first case report to examine the dynamics of mucosal cytokine levels in a patient with collagenous colitis before and during fecal stream diversion and after bowel continuity was re-established. Fecal stream diversion in this patient offered an opportunity to investigate the levels of cytokines involved in immunological processes in the disease and the molecular basis of the effects of fecal stream diversion. We showed that diversion of the fecal stream led to a clear reduction in the protein levels of almost all analyzed cytokines and that the reoccurrence of symptoms and classical histological findings following the restoration of bowel continuity is accompanied by increases in mucosal IL-2, IL-21 and IL-23 levels. These results further demonstrate the importance of luminal agents as triggers of intestinal inflammation.
The role of fecal material in patients with Crohn’s disease has been studied by Rutgeerts et al[8]. These investigators reported that inflammation in the neoterminal ileum in patients with Crohn’s disease who underwent ileocecal resection was dependent on the fecal stream because inflammation did not appear in patients who were treated with a temporary proximal ileostomy. Harper et al[11] went a step further by either introducing either intestinal effluent or a sterile ultrafiltrate into the defunctioned colon of Crohn’s disease patients who had undergone a split ileostomy. These investigators concluded that particles greater than 0.22 μm were the luminal triggers responsible for disease because the effluent caused a clinical response as well as changes in laboratory values in comparison with the ultrafiltrate.
Few studies have analyzed cytokine profiles in MC, and those that do exist have focused primarily on mRNA and not protein levels. In the one study that analyzed both, Kumawat et al[6] found a Th1/Tc1 and Th17/Tc17 mRNA mucosal cytokine profile in both CC and LC, although the protein concentrations of these cytokines were not always increased[6]. Protein levels depend not only on mRNA levels but also on regulatory processes after mRNA is produced, e.g., the protein degradation rate; these differences can explain the discrepancies found in vivo[12]. The relationship between mRNA and protein levels is complex, but protein levels may reflect biological activity and the cytokine milieu more accurately than mRNA levels. Tagkalidis et al[5] also described a Th1 reaction based on mucosal mRNA cytokine values; Th2 cytokines were difficult to detect, with the exception of IL-10, whose levels tended to be increased in MC patients compared with controls. Dey et al[13] described increased expression of TNF, IFN-γ and IL-8 mRNA in patients with lymphocytic colitis (LC), while IL-1β, IL-4, IL-10, IL-12 and IL-23 mRNA levels were not found to be significantly different in patients with LC compared with controls. These studies focused on patients with active MC and not on patients in remission. Only Tagkalidis et al[5] followed 6 of 18 patients and noted a decrease in IFN-γ mRNA levels over time; however, the dynamics of IL-10, TNF and IL-15 mRNA levels were not reported.
Although the patient in this study received 20 mg of prednisolone initially, all protein levels of cytokines were elevated during active disease compared to the period of fecal stream diversion. Surprisingly, little is known about the effect of prednisolone on cytokine levels in the intestinal mucosa in vivo, but in the bronchoalveolar lavage fluid of allergic patients, Liu et al[14] showed that treatment with prednisolone reduced mRNA and protein levels of IL-4, IL-5 and IL-2. Because we analyzed protein levels of cytokines, it is hard to compare our results with those of other studies; also, because we analyzed only one patient, our primary focus was to describe the dynamics of cytokine levels at different stages of treatment and disease. Most striking were the obvious increases in mucosal IL-2, IL-21 and IL-23 levels after intestinal continuity was restored, which paralleled a prompt relapse of symptoms and the reestablishment of the classical histological signs of CC.
The most pronounced changes were observed in the levels of IL-23 during the course of the study; IL-23 levels exhibited the largest increase among all cytokine levels one month after intestinal restoration. Studies in murine models have shown the importance of IL-23 in inducing and enhancing chronic intestinal inflammatory disease[15]. Furthermore, inhibition of the IL-23 p19 subunit has been shown to be effective both in preventing and treating active colitis in murine models[16]. This cytokine is thought to mediate communication between the innate and adaptive immune systems and is produced by activated dendritic cells and macrophages within hours after encountering a pathogen[17]. IL-23 also plays a role by inducing CD4+ T cells to produce IL-17, and IL-17 levels are increased in another inflammatory bowel disease, Crohn’s disease[18]. Kumawat et al[6] previously found colonic levels of both IL-17 and IL-23 mRNA to be increased in both CC and LC, while protein levels were not found to be markedly different from those in healthy controls[6]. These findings, together with our present results, indicate that IL-23 is involved in the inflammatory process.
The dynamics of IL-2 levels after bowel reconstruction resembled those of IL-23 but not to the same extent. IL-2 is a cytokine that has been studied extensively in the past 25 years. IL-2 levels have been found to be altered in autoimmune diseases; positive correlations between serum levels of IL-2 and both skin progression in scleroderma[19] and joint destruction in rheumatoid disease have been observed[20]. In gastrointestinal disorders, enhanced serum IL-2 is associated with advanced gastric cancer[21]. Using IL-2R-/- mice, Malek et al[22] showed that IL-2 and/or IL-2R act via stimulating the production of Tregs.
Finally, IL-21 is a cytokine known to be involved in a variety of tissues in many inflammatory diseases[23,24]. Although IL-21 is found in mucosal biopsies from healthy individuals, the expression of this cytokine is enhanced in the colonic mucosa of Crohn’s patients[25]. It is thought to be produced by activated Th17 cells[26] and activated NKT cells[27]. In a murine experimental model of colitis, wild-type mice develop colitis when treated with dextran sulphate sodium and trinitrobenzene sulfonic acid, whereas IL-21-deficient mice do not[28]. A proposed mechanism of action of IL-21 in IBD is that colonic fibroblasts, when stimulated with IL-21, produce matrix metalloproteinases[29], which are involved in the epithelial damage that causes IBD[30].
In conclusion, our observations demonstrate that fecal stream diversion affects inflammation both histologically and on a molecular level by reducing the levels of cytokines involved in the inflammatory process in CC. Our results show that the levels of all cytokines, except IL-21, decreased during diversion and remained at lower levels after bowel reconstruction, with the exception of IL-2, IL-21 and IL-23, whose levels increased. These three cytokines are all known to be proinflammatory. Fecal stream diversion and bowel reconstruction provides a unique opportunity to investigate immunological processes involved in inflammatory bowel disorders.
The patient experienced diarrhea with 10 watery stools per day, which eventually caused anal skin ulceration and a decreased quality of life.
Collagenous colitis, a subtype of microscopic colitis.
Repeated stool samples ruled out infectious causes. A SeHCAT investigation ruled out bile salt malabsorption, and gastroscopy with duodenal biopsies revealed no sign of celiac disease. Human immunodeficiency virus with secondary chronic diarrhea and hyperthyroidism were also ruled out by blood analyses. Finally, primary amyloidosis was ruled out with a biopsy of adipose tissue.
Luminex assays were used to analyze cytokine levels, and while almost all cytokines levels decreased during ileostomy; only the levels of interleukin (IL)-21, IL-23 and IL-2 increased after intestinal continuity was restored.
Histological examination revealed classical findings of collagenous colitis.
The patient was initially treated with budesonide, but the patient’s symptoms stopped responding to treatment. Methotrexate had no effect on symptoms, and adalimumab did not have a long-lasting effect. Finally, a loop ileostomy was performed.
Fecal stream diversion is known to induce remission in patients with collagenous colitis, but in this study, cytokine levels were measured throughout this process for the first time.
Collagenous colitis is an inflammatory intestinal disease that presents with non-bloody, watery diarrhea. Fecal stream diversion refers to the creation of a terminal ileostomy that excludes the colon from intestinal transit.
Collagenous colitis can be refractory to treatment, leading to severely impaired quality of life. IL-2, IL-21 and IL-23 appear to be involved in disease pathogenesis, and disease processes should be analyzed further in patients with this disease.
It is well written, comprehensively reviewed and potentially helpful in the understanding of basic mechanisms in inflammation of the bowel.
P- Reviewer: Ahluwalia NK S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Lindström CG. ‘Collagenous colitis’ with watery diarrhoea--a new entity? Pathol Eur. 1976;11:87-89. [PubMed] [Cited in This Article: ] |
| 2. | Wickbom A, Bohr J, Eriksson S, Udumyan R, Nyhlin N, Tysk C. Stable incidence of collagenous colitis and lymphocytic colitis in Örebro, Sweden, 1999-2008: a continuous epidemiologic study. Inflamm Bowel Dis. 2013;19:2387-2393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Bohr J. A review of collagenous colitis. Scand J Gastroenterol. 1998;33:2-9. [PubMed] [Cited in This Article: ] |
| 4. | Münch A, Söderholm JD, Ost A, Ström M. Increased transmucosal uptake of E. coli K12 in collagenous colitis persists after budesonide treatment. Am J Gastroenterol. 2009;104:679-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Tagkalidis PP, Gibson PR, Bhathal PS. Microscopic colitis demonstrates a T helper cell type 1 mucosal cytokine profile. J Clin Pathol. 2007;60:382-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Kumawat AK, Strid H, Tysk C, Bohr J, Hörnquist EH. Microscopic colitis patients demonstrate a mixed Th17/Tc17 and Th1/Tc1 mucosal cytokine profile. Mol Immunol. 2013;55:355-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Järnerot G, Bohr J, Tysk C, Eriksson S. Faecal stream diversion in patients with collagenous colitis. Gut. 1996;38:154-155. [PubMed] [Cited in This Article: ] |
| 8. | Rutgeerts P, Goboes K, Peeters M, Hiele M, Penninckx F, Aerts R, Kerremans R, Vantrappen G. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet. 1991;338:771-774. [PubMed] [Cited in This Article: ] |
| 9. | Münch A, Söderholm JD, Wallon C, Ost A, Olaison G, Ström M. Dynamics of mucosal permeability and inflammation in collagenous colitis before, during, and after loop ileostomy. Gut. 2005;54:1126-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Järnerot G, Tysk C, Bohr J, Eriksson S. Collagenous colitis and fecal stream diversion. Gastroenterology. 1995;109:449-455. [PubMed] [Cited in This Article: ] |
| 11. | Harper PH, Lee EC, Kettlewell MG, Bennett MK, Jewell DP. Role of the faecal stream in the maintenance of Crohn’s colitis. Gut. 1985;26:279-284. [PubMed] [Cited in This Article: ] |
| 12. | Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2493] [Cited by in F6Publishing: 2623] [Article Influence: 218.6] [Reference Citation Analysis (0)] |
| 13. | Dey I, Beck PL, Chadee K. Lymphocytic colitis is associated with increased pro-inflammatory cytokine profile and up regulation of prostaglandin receptor EP4. PLoS One. 2013;8:e61891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Liu MC, Proud D, Lichtenstein LM, Hubbard WC, Bochner BS, Stealey BA, Breslin L, Xiao H, Freidhoff LR, Schroeder JT. Effects of prednisone on the cellular responses and release of cytokines and mediators after segmental allergen challenge of asthmatic subjects. J Allergy Clin Immunol. 2001;108:29-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1153] [Cited by in F6Publishing: 1179] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 16. | Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 362] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 17. | Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 559] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 18. | Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jürgens M, Schmechel S, Konrad A. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14:437-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Kahaleh MB, LeRoy EC. Interleukin-2 in scleroderma: correlation of serum level with extent of skin involvement and disease duration. Ann Intern Med. 1989;110:446-450. [PubMed] [Cited in This Article: ] |
| 20. | Tebib JG, Boughaba H, Letroublon MC, Bienvenu J, Noel E, Armanet P, Colson F, Roullet A, Bouvier M. Serum IL-2 level in rheumatoid arthritis: correlation with joint destruction and disease progression. Eur Cytokine Netw. 1991;2:239-243. [PubMed] [Cited in This Article: ] |
| 21. | Forones NM, Mandowsky SV, Lourenço LG. Serum levels of interleukin-2 and tumor necrosis factor-alpha correlate to tumor progression in gastric cancer. Hepatogastroenterology. 2001;48:1199-1201. [PubMed] [Cited in This Article: ] |
| 22. | Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167-178. [PubMed] [Cited in This Article: ] |
| 23. | Diaz-Gallo LM, Simeon CP, Broen JC, Ortego-Centeno N, Beretta L, Vonk MC, Carreira PE, Vargas S, Román-Ivorra JA, González-Gay MA. Implication of IL-2/IL-21 region in systemic sclerosis genetic susceptibility. Ann Rheum Dis. 2013;72:1233-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Sglunda O, Mann HF, Hulejová H, Pecha O, Pleštilová L, RůŽičková O, Fojtíková M, Sléglová O, Forejtová S, Pavelka K. Decrease in serum interleukin-21 levels is associated with disease activity improvement in patients with recent-onset rheumatoid arthritis. Physiol Res. 2014;63:475-481. [PubMed] [Cited in This Article: ] |
| 25. | Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, Caruso R, Tersigni R, Alessandroni L, Biancone L, Naccari GC. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology. 2005;128:687-694. [PubMed] [Cited in This Article: ] |
| 26. | Spolski R, Leonard WJ. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20:295-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827-2834. [PubMed] [Cited in This Article: ] |
| 28. | Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Monteleone G, Caruso R, Fina D, Peluso I, Gioia V, Stolfi C, Fantini MC, Caprioli F, Tersigni R, Alessandroni L. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut. 2006;55:1774-1780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Pender SL, MacDonald TT. Matrix metalloproteinases and the gut - new roles for old enzymes. Curr Opin Pharmacol. 2004;4:546-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |