Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4696
Peer-review started: August 31, 2014
First decision: September 27, 2014
Revised: December 9, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: April 21, 2015
AIM: To investigate the impact of telaprevir-based triple therapy on the serum alpha-fetoprotein (AFP) level of chronic hepatitis C patients.
METHODS: A total of 210 patients with chronic hepatitis C genotype 1 of high viral load (baseline serum hepatitis C virus RNA > 5.0 log10 IU/mL) were divided into two groups by type of treatment: triple therapy with telaprevir, pegylated-interferon-α (PEG-IFNα), and ribavirin (RBV) for 24 wk (n = 88), or dual therapy with PEG-IFNα and RBV for 48 wk (n = 122). The relationship between virological response and the change in the serum AFP level from baseline to 24 wk after the end of treatment was examined.
RESULTS: No significant difference in mean baseline AFP level was found between the triple and dual therapy groups (8.8 ng/mL vs 7.8 ng/mL). Triple therapy produced significant declines in the AFP level in sustained virological response (SVR) and non-SVR patients (7.8 ng/mL at baseline to 3.5 ng/mL at 24 wk after the end of treatment, P < 0.001 and 14.3 ng/mL to 9.5 ng/mL, P = 0.004, respectively). In contrast, dual therapy resulted in a significant decline in AFP level only in SVR patients (4.7 ng/mL to 2.8 ng/mL, P < 0.001), but not in non-SVR patients (10.2 ng/mL to 10.1 ng/mL). Among patients with a high-baseline AFP level (≥ 10 ng/mL), the decline in the AFP level was significantly higher in the triple therapy than in the dual therapy group (15.9 ng/mL vs 1.6 ng/mL, P = 0.037).
CONCLUSION: Regardless of virological response, telaprevir-based triple therapy reduced the serum AFP level.
Core tip: Patients with hepatocellular carcinoma often have elevated serum alpha-fetoprotein concentrations, for which a high level is a risk factor for developing hepatocellular carcinoma in chronic hepatitis C patients. A recently introduced direct-acting antiviral agent, telaprevir, has been included in triple therapy regimens using a protease inhibitor with conventional pegylated-interferon-α and ribavirin, and has significantly improved the sustained virological response rate, up to 80% for patients with hepatitis C virus genotype 1. This study shows that regardless of virological response, telaprevir-based triple therapy more effectively reduces the serum alpha-fetoprotein level than dual therapy with pegylated-interferon-α and ribavirin.
- Citation: Takayama K, Furusyo N, Ogawa E, Ikezaki H, Shimizu M, Murata M, Hayashi J. Direct-acting antiviral-based triple therapy on alpha-fetoprotein level in chronic hepatitis C patients. World J Gastroenterol 2015; 21(15): 4696-4706
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4696.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4696
Chronic hepatitis C virus (HCV) infection affects approximately 170 million people worldwide and often causes liver cirrhosis and hepatocellular carcinoma (HCC), a leading cause of cancer-related death[1,2]. One of the goals of chronic hepatitis C treatment is to achieve sustained viral response (SVR), which leads to the suppression of liver cirrhosis and HCC.
The combination therapy of pegylated-interferon-α (PEG-IFNα) and ribavirin (RBV) that has been used for the last decade has improved the SVR rate to about 50% for chronic hepatitis C patients infected with HCV genotype 1[3-5]. Interferon therapy is effective in reducing the development of HCC, not only for SVR patients but also for non-SVR patients[6]. Recently, direct-acting antiviral agents (DAAs), such as telaprevir (TVR) and boceprevir, which are HCV non-structural 3/4A (NS3/4A) protease inhibitors, have been included in triple-therapy regimens with conventional PEG-IFNα and RBV, thus significantly improving the SVR rate, up to 80%, for patients with HCV genotype 1[7-11].
We previously reported the results of a prospective, long-term follow-up study conducted to evaluate the effect of treatment outcome on the development of HCC in 1013 Japanese patients with chronic hepatitis C treated with PEG-IFNα2b and RBV[6]. Patients who achieved SVR or relapsed had a significantly reduced risk of HCC development within five years after the end of treatment when compared with patients without virological response, with or without cirrhosis. However, there is no data on the efficacy of NS3/4A protease inhibitors for reducing the number of patients who develop HCC.
Serum concentration of alpha-fetoprotein (AFP) is often elevated in patients with HCC[12], and a high AFP level has been identified as a risk factor for the development of HCC in chronic hepatitis C patients[13]. In addition, accumulating evidence indicates that interferon treatment reduces serum AFP levels, and the post-interferon treatment AFP level is strongly associated with the development of HCC, regardless of whether or not SVR is achieved[14-17].
The aim of this prospective study was to investigate the impact of DAA-based triple therapy on serum AFP levels. We also compare the post-treatment serum AFP levels in patients receiving triple therapy with those receiving dual PEG-IFNα and RBV therapy.
Patients were enrolled at Kyushu University Hospital and four affiliated hospitals and one clinic in the northern Kyushu area of Japan (Mitsutake, Yokota, Kyushu Central, and Hara-Doi Hospitals, and the Kyushu General Internal Medicine Clinic). This multicenter cohort study consisted of 354 genotype 1 chronic hepatitis C patients, without human immunodeficiency virus or hepatitis B virus infection, who received TVR-based triple therapy or dual therapy with PEG-IFNα2b and RBV. Triple therapy was initiated between December 2011 and March 2013 and was completed by the end of September 2013. Dual therapy was initiated between June 2005 and August 2011 and was completed by the end of July 2012. In order to investigate the impact of treatment on the serum AFP level, measurements were taken at baseline, at the end of treatment (EOT), and at 24 wk after EOT. ∆AFP decline, defined as the AFP level at 24 wk after EOT subtracted from baseline, and %∆AFP decline, defined as the percentage of AFP at 24 wk after EOT subtracted from baseline, were also calculated. Similarly, the alanine aminotransferase (ALT) level was measured, and ∆ALT decline and %∆ALT decline, during the same period, were calculated. The study design was approved by the ethics committee of Kyushu University Hospital, and it was carried out in accordance with the 1975 Declaration of Helsinki, as updated in 2008. The study was registered as a clinical trial on the University Hospital Medical Information Network (ID 000013784).
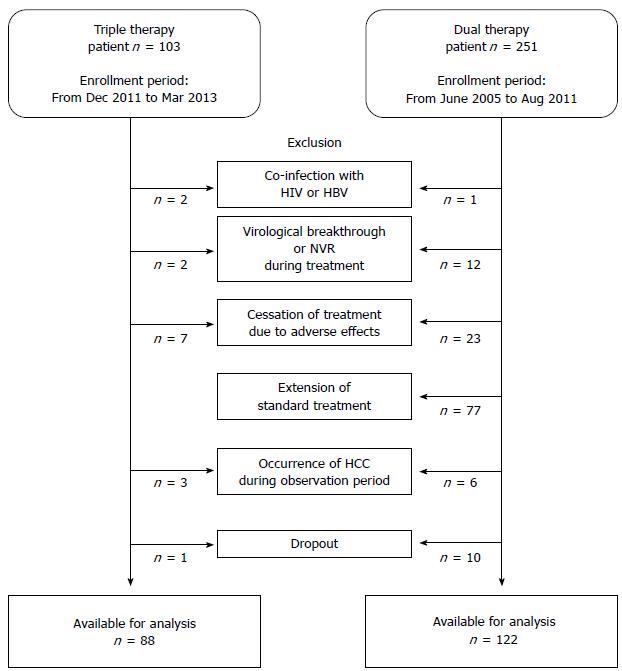
Patient inclusion criteria for the study required patients to be infected with HCV genotype 1 and to have baseline serum HCV RNA > 5.0 log10 IU/mL. The exclusion criteria were as follows: (1) presence of HCC at enrollment, or development of HCC within 24 wk after EOT; (2) shortening of treatment due to adverse effects, virological breakthrough, non-virological response (NVR), or dropout; (3) in the dual therapy group, extension of the standard 48-wk therapy; (4) inadequate treatment dosage of 80% or less of the assigned total cumulative dosage of each drug; (5) positive for antibody to human immunodeficiency virus or positive for hepatitis B surface antigen; (6) clinical or biochemical evidence of hepatic decompensation; (7) excessive active alcohol consumption (≥ 60 g alcohol/d) or drug abuse; (8) other forms of liver disease (e.g., autoimmune hepatitis, alcoholic liver disease, hemochromatosis); or (9) treatment with antiviral or immunosuppressive agents prior to enrollment. Of the 354 patients screened, 144 were excluded because they did not meet the above criteria, leaving the data of 210 available for analysis. Figure 1 shows a flowchart of the study design.
All patients in the triple therapy group received 12 wk of TVR (Telavic; Mitsubishi Tanabe Pharma, Osaka, Japan), PEG-IFNα2b (PEG-Intron; MSD, Tokyo, Japan), and RBV (Rebetol; MSD) followed by an additional 12 wk of PEG-IFNα2b and RBV alone. All patients in the dual therapy group received 48 wk of PEG-IFNα2b and RBV. TVR 750 mg was administered orally three times a day, after meals at 8-h intervals. If marked anorexia, an elevation of serum creatinine, or severe anemia developed, the TVR dose could be reduced to 1500 mg/d (750 mg at a 12-h interval after each meal). PEG-IFNα2b was injected subcutaneously once weekly at a dose of 1.5 μg/kg. RBV was given orally at a daily dose of 600-1000 mg based on body weight (600 mg for patients weighing < 60 kg, 800 mg for those weighing 60-80 kg, and 1000 mg for those weighing > 80 kg). The above durations and dosages are those approved by the Japanese Ministry of Health, Labor, and Welfare.
Successful treatment was SVR, defined as undetectable serum HCV RNA at 24 wk after EOT. Relapse was defined as undetectable HCV RNA at EOT, but detectable at some time within the 24 wk of follow-up. Non-virological response (NVR) was defined as a < 2 log10 IU/mL decrease in the HCV RNA level from baseline to week 12 of therapy (null response) or a > 2 log10 IU/mL decrease in the HCV RNA level from baseline to week 12 of therapy, but still detectable at weeks 12 and 24 (partial response). Virological breakthrough was defined as the reappearance of HCV RNA at any time during the course of treatment. EOT response is defined as undetectable HCV RNA at EOT. We excluded 14 patients with NVR or virological breakthrough who stopped treatment and met the exclusion criteria. The virological response rates were analyzed by per-protocol analysis.
Blood samples were taken at baseline, every week thereafter to week 24 for the triple therapy group and to week 48 for the dual therapy group, and at 24 wk after EOT. Laboratory parameters included serum albumin, creatinine, total bilirubin, ALT, aspartate aminotransferase, γ-glutamyl transpeptidase, estimated glomerular filtration rate, white blood cell count, hemoglobin, platelet count, and serum HCV markers.
HCV genotyping was performed by sequence determination in the 5’-nonstructural region of the HCV genome. The serum HCV RNA level of each patient was determined by COBAS TaqMan HCV Test (Roche Diagnostics, Basel, Switzerland). The test has lower and upper limits of quantitation of 15 IU/mL and 6.9 × 106 IU/mL, respectively, (1.2-7.8 log IU/mL referred to log10 IU/mL).
Human genomic DNA was extracted from peripheral blood. Genotyping by the single nucleotide polymorphism (SNP) of the interleukin 28B (IL28B) (rs8099917) gene was performed using the TaqMan Allelic Discrimination Demonstration Kit (7500 Real-Time PCR System; Applied Biosystems of Thermo Fisher Scientific, Waltham, MA, United States). Patients were genotyped as TT, TG, or GG at the polymorphic site. The IL28B SNPs data were available for 192/210 (91.4%) participants.
Liver biopsy was conducted at enrollment for 148/210 (70.5%) participants by two or more experienced hepatologists. The minimum length of the liver biopsy was 15 mm and at least ten complete portal tracts were necessary for inclusion. For each specimen, the stage of fibrosis (F0-4) and the grade of activity (A0-3) were established according to the METAVIR score[18].
All data were analyzed using JMP statistical software (SAS Institute, Cary, NC, United States). Continuous data are expressed as median (first-third quartiles) or mean ± SE, and compared using Student’s t tests or Mann-Whitney U tests. Categorical variables are reported as frequencies and percentages and compared using χ2 or Fisher’s exact tests. The serum AFP and ALT levels at each test point are expressed as mean ± SE, and comparison between these levels at baseline and at 24 wk after EOT was performed by paired t-test. ∆AFP decline, %∆AFP decline, ∆ALT decline, and %∆ALT decline are expressed using a mean value histogram and standard error bar, and comparison of each subgroup was performed using Student’s t tests or Mann-Whitney U tests. A P < 0.05 was regarded as statistically significant in all analyses.
The patient characteristics are summarized in Table 1 according to the treatment given. The 63 triple therapy patients with prior relapse, NVR, or unknown response had previously undergone a 48-wk course PEG-IFNα2b plus RBV combination treatment. The baseline HCV RNA level of the triple therapy group was significantly higher than that of the dual therapy group (P = 0.010). The baseline platelet count of the triple therapy group was significantly lower than that of the dual therapy group (P = 0.040). Thus, the triple therapy group had more “difficult-to-treat” patients than did the dual therapy group. There was no significant difference between the groups in the number of patients tested for liver histology, IL28B SNPs, or other laboratory data. No significant difference in the baseline AFP level was observed between the two groups, and the percentage of patients with AFP < 5 ng/mL, 5-9.9 ng/mL, or ≥ 10 ng/mL did not differ.
| Baseline characteristics | Triple therapy (n = 88) | Dual therapy (n = 122) | P value |
| Age, yr | 63 (53-68) | 60 (52-65) | 0.101 |
| Male | 37 (42.1) | 52 (42.6) | 0.933 |
| Body weight, kg | 56 (49-67) | 58 (50-66) | 0.782 |
| Body mass index, kg/m2 | 22.7 (20.7-25.1) | 22.8 (20.6-24.9) | 0.859 |
| Serum AFP level, ng/mL | 4.5 (2.8-9.0) | 4.0 (2.8-8.9) | 0.677 |
| Serum AFP level < 5 ng/mL | 50 (56.8) | 70 (57.4) | 0.967 |
| Serum AFP level 5-9.9 ng/mL | 18 (20.5) | 26 (21.3) | |
| Serum AFP level ≥ 10 ng/mL | 20 (20.7) | 26 (21.3) | |
| HCV RNA level, log10 IU/mL | 6.5 (6.1-6.9) | 6.3 (5.8-6.6) | 0.010 |
| HCV genotype 1a | 1 (1.1) | 3 (2.5) | 0.476 |
| HCV genotype 1b | 87 (98.9) | 119 (97.5) | |
| Serum albumin, g/L | 41 (38-43) | 41 (39-43) | 0.376 |
| Alanine aminotransferase, U/L | 46 (25-73) | 51 (33-81) | 0.176 |
| Alanine aminotransferase ≥ 40 IU/L | 49 (55.7) | 78 (63.9) | 0.228 |
| γ-glutamyl-transpeptidase, U/L | 39 (23-76) | 37 (21-63) | 0.199 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 80 (68-94) | 81 (71-94) | 0.464 |
| Total cholesterol, mg/dL | 175 (153-192) | 164 (146-192) | 0.216 |
| Triglycerides, mg/dL | 94 (68-124) | 79 (57-121) | 0.070 |
| Aspartate aminotransferase to platelet ratio index | 0.86 (0.51-1.85) | 0.76 (0.50-1.40) | 0.306 |
| White blood cell count, × 106/L | 4780 (4038-5890) | 4600 (3890-5645) | 0.447 |
| Hemoglobin level, g/L | 137 (129-144) | 135 (126-145) | 0.319 |
| Platelet count, × 109/L | 156 (122-195) | 178 (136-209) | 0.040 |
| Diabetes | 10 (11.3) | 7 (5.7) | 0.144 |
| Stage of fibrosis | |||
| F0-2 | 52 (81.2) | 76 (90.5) | 0.105 |
| F3-4 | 12 (18.8) | 8 (9.5) | |
| Not determined, n | 24 | 38 | |
| Previous treatment response | |||
| Naïve | 25 (28.4) | ||
| Prior relapse | 34 (38.6) | ||
| Prior NVR | 27 (30.7) | ||
| Prior unknown response | 2 (2.3) | ||
| IL28B SNP (rs8099917) | |||
| TT | 49 (55.7) | 73 (70.2) | 0.113 |
| TG/GG | 39 (44.3) | 31 (29.8) | |
| Not determined, n | 0 | 18 |
To investigate the impact of treatment on the serum AFP level, we excluded from the analysis patients with shortened or extended treatment or inadequate treatment dose (Figure 1). The SVR rate was significantly higher in the triple therapy group [85.2% (75/88) vs 42.6% (52/122), P < 0.001]. The relapse rate was lower in the triple therapy group [12.5% (11/88) vs 23.0% (28/122), P = 0.050]. Table 2 shows the virological response of each treatment group according to previous treatment response, IL28B SNPs, stage of fibrosis, baseline HCV RNA level, baseline platelet count, and baseline serum AFP level. Significant differences in the SVR rates were observed for all of the subgroups (all P < 0.05), except for advanced fibrosis (F3-4), probably due to the small number analyzed.
| Group | Triple therapy | Dual therapy | P value | ||
| n | SVR | n | SVR | ||
| All patients | 88 | 75 (85.2) | 122 | 52 (42.6) | < 0.001 |
| Previous treatment response | |||||
| Naïve/prior relapse | 59 | 56 (94.9) | |||
| Prior NVR | 27 | 17 (63.0) | |||
| Prior unknown response | 2 | 2 (100) | |||
| IL28B SNP (rs8099917) | |||||
| TT | 49 | 48 (98.0) | 73 | 45 (61.6) | < 0.001 |
| TG/GG | 39 | 27 (69.2) | 31 | 1 (3.2) | < 0.001 |
| Not determined, n | 0 | - | 18 | 6 (33.3) | - |
| Stage of fibrosis | |||||
| F0-2 | 52 | 48 (92.3) | 76 | 43 (56.6) | < 0.001 |
| F3-4 | 12 | 9 (75.0) | 8 | 4 (50.0) | 0.279 |
| Not determined, n | 24 | 18 (75.0) | 38 | 5 (13.2) | |
| Baseline HCV RNA level | |||||
| ≥ 6 log10 IU/mL | 69 | 58 (84.1) | 86 | 39 (45.3) | < 0.001 |
| < 6 log10 IU/mL | 19 | 17 (89.5) | 36 | 23 (63.8) | 0.032 |
| Baseline platelet count | |||||
| ≥ 100 × 109/L | 80 | 71 (88.8) | 117 | 62 (53.0) | < 0.001 |
| < 100 × 109/L | 8 | 4 (50.0) | 5 | 0 - | 0.043 |
| Baseline serum AFP level | |||||
| < 10 ng/mL | 68 | 63 (92.6) | 96 | 49 (51.0) | < 0.001 |
| ≥ 10 ng/mL | 20 | 12 (60.0) | 26 | 3 (11.5) | < 0.001 |
Table 3 shows the serum AFP levels at baseline, EOT, and 24 wk after EOT according to the treatment outcome, baseline AFP level, previous treatment response, IL28B SNPs, and stage of fibrosis. The AFP level of all subgroups within the triple therapy group decreased significantly from baseline to 24 wk after EOT (all P < 0.05). In the dual therapy group, a significant decline in the AFP level was only seen for patients with SVR, low baseline serum AFP (< 10 ng/mL), IL28B SNPs TT, and mild fibrosis (F0-2) (all P < 0.001).
| Group | Triple therapySerum AFP level (ng/mL) | Dual therapySerum AFP level (ng/mL) | ||||||||
| n | At baseline | At end of treatment | At 24 wk after the end of treatment | P value1 | n | At baseline | At end of treatment | At 24 wk after the end of treatment | P value1 | |
| All patients | 88 | 8.8 ± 1.4 | 5.1 ± 0.4 | 4.4 ± 0.4 | < 0.001 | 122 | 7.8 ± 0.9 | 6.2 ± 1.3 | 7.0 ± 1.5 | 0.316 |
| Treatment outcome | ||||||||||
| SVR | 75 | 7.8 ± 1.5 | 4.5 ± 0.4 | 3.5 ± 0.2 | 0.004 | 52 | 4.7 ± 0.6 | 3.1 ± 0.2 | 2.8 ± 0.2 | < 0.001 |
| Non-SVR | 13 | 14.3 ± 2.3 | 8.3 ± 1.1 | 9.5 ± 1.2 | 0.007 | 70 | 10.2 ± 1.5 | 8.7 ± 2.2 | 10.1 ± 2.5 | 0.953 |
| Baseline AFP level | ||||||||||
| ≥ 10 ng/mL | 20 | 24.8 ± 4.4 | 10.5 ± 1.0 | 8.9 ± 0.8 | 0.003 | 26 | 21.8 ± 3.0 | 17.1 ± 5.3 | 20.2 ± 6.4 | 0.701 |
| < 10 ng/mL | 68 | 4.1 ± 0.2 | 3.4 ± 0.2 | 3.1 ± 0.2 | < 0.001 | 96 | 4.1 ± 0.2 | 3.2 ± 0.2 | 3.4 ± 0.2 | < 0.001 |
| Previous treatment response | ||||||||||
| Naïve/prior relapse | 59 | 8.8 ± 1.9 | 4.8 ± 0.5 | 3.8 ± 0.3 | 0.007 | |||||
| Prior NVR | 27 | 9.1 ± 1.5 | 5.5 ± 0.8 | 5.8 ± 0.9 | < 0.001 | |||||
| IL28B SNP (rs8099917) | ||||||||||
| TT | 49 | 6.2 ± 1.0 | 3.9 ± 0.4 | 3.6 ± 0.3 | < 0.001 | 73 | 6.0 ± 0.7 | 3.7 ± 0.3 | 3.9 ± 0.3 | < 0.001 |
| TG/GG | 39 | 12.0 ± 2.8 | 6.5 ± 0.8 | 5.5 ± 0.6 | < 0.001 | 31 | 10.2 ± 1.6 | 8.8 ± 2.9 | 9.3 ± 2.2 | 0.520 |
| Stage of fibrosis | ||||||||||
| F0-2 | 52 | 6.5 ± 1.4 | 4.2 ± 0.5 | 3.3 ± 0.3 | 0.014 | 76 | 6.0 ± 0.7 | 3.7 ± 0.3 | 4.3 ± 0.5 | < 0.001 |
| F3-4 | 12 | 12.2 ± 3.0 | 6.5 ± 0.8 | 6.8 ± 1.1 | 0.023 | 8 | 13.6 ± 3.2 | 9.2 ± 3.2 | 9.9 ± 3.8 | 0.284 |
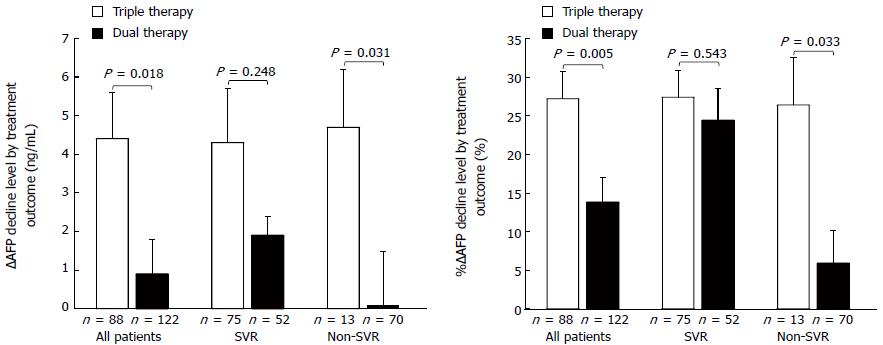
Figure 2 shows ∆AFP decline and %∆AFP decline by treatment and virological outcome. ∆AFP decline and %∆AFP decline did not significantly differ between the triple and dual therapy SVR patients (4.3 ± 1.4 ng/mL vs 1.9 ± 0.5 ng/mL, and 27.4% ± 3.5% vs 24.4% ± 4.1%, respectively). However, among the non-SVR patients, ∆AFP decline and %∆AFP decline were significantly higher in the triple than in the dual therapy group (4.7 ± 1.5 ng/mL vs 0.1 ± 1.4 ng/mL; P = 0.031, and 26.4% ± 6.1% vs 5.9% ± 4.3%; P = 0.033, respectively).
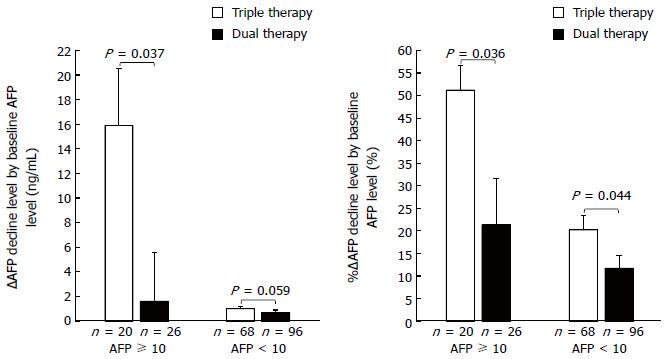
Figure 3 shows ∆AFP decline and %∆AFP decline by baseline AFP level (≥ 10 ng/mL and < 10 ng/mL). ∆AFP decline did not significantly differ between the two groups for patients with a low baseline AFP level (< 10 ng/mL) (1.0 ± 0.2 ng/mL vs 0.7 ± 0.2 ng/mL). However, among patients with a high baseline AFP level (≥ 10 ng/mL), ∆AFP decline was significantly higher in the triple than in the dual therapy group (15.9 ± 4.6 ng/mL vs 1.6 ± 4.0 ng/mL, P = 0.037). %∆AFP decline was significantly higher in the triple than in the dual therapy group (among patients with a low baseline AFP level, 20.2% ± 3.2% vs 11.7% ± 2.9%, P = 0.044, among patients with a high baseline AFP level, 51.2% ± 5.5% vs 20.2% ± 3.2%, P = 0.036).
Table 4 shows the ALT levels at baseline, EOT, and 24 wk after EOT according to treatment outcome, baseline ALT level, previous treatment response, IL28B SNPs, and stage of fibrosis. In the triple therapy group, all of the subgroups except non-SVR achieved a significant decline of the ALT level (all P < 0.01). However, in the dual therapy group, patients with SVR, high baseline ALT level (≥ 40 U/L), IL28B SNPs TT, and mild fibrosis (F0-2) achieved a significant decline of the ALT level (all P < 0.001).
| Group | Triple therapyALT level (U/L) | Dual therapyALT level (U/L) | ||||||||
| n | At baseline | At end of treatment | At 24 wk after the end of treatment | P value1 | n | At baseline | At end of treatment | At 24 wk after the end of treatment | P value1 | |
| All patients | 88 | 56.6 ± 4.1 | 25.9 ± 2.4 | 22.7 ± 1.8 | < 0.001 | 122 | 64.1 ± 4.7 | 29.4 ± 4.0 | 35.3 ± 3.0 | < 0.001 |
| Treatment outcome | ||||||||||
| SVR | 75 | 54.3 ± 4.3 | 25.4 ± 2.7 | 17.8 ± 1.1 | < 0.001 | 52 | 74.0 ± 7.3 | 18.8 ± 1.6 | 14.9 ± 0.8 | < 0.001 |
| Non-SVR | 13 | 69.8 ± 12.2 | 28.8 ± 3.8 | 50.6 ± 6.0 | 0.147 | 70 | 56.8 ± 4.9 | 37.4 ± 6.7 | 50.4 ± 4.3 | 0.169 |
| Baseline ALT level | ||||||||||
| ≥ 40 U/L | 49 | 81.4 ± 5.1 | 32.9 ± 3.5 | 28.5 ± 2.8 | < 0.001 | 78 | 84.3 ± 5.4 | 36.6 ± 5.9 | 40.0 ± 4.2 | < 0.001 |
| < 40 U/L | 39 | 25.4 ± 1.2 | 17.1 ± 2.6 | 15.4 ± 1.3 | < 0.001 | 44 | 28.3 ± 1.1 | 16.0 ± 1.7 | 26.8 ± 3.0 | 0.637 |
| Previous treatment response | ||||||||||
| Naïve/Prior relapse | 59 | 56.5 ± 5.2 | 25.5 ± 2.7 | 19.4 ± 1.5 | < 0.001 | |||||
| Prior NVR | 27 | 56.7 ± 7.5 | 21.0 ± 2.5 | 28.6 ± 4.5 | < 0.001 | |||||
| IL28B SNP (rs8099917) | ||||||||||
| TT | 49 | 56.9 ± 5.4 | 26.6 ± 3.8 | 17.4 ± 1.3 | < 0.001 | 73 | 63.6 ± 5.4 | 18.9 ± 1.4 | 25.9 ± 2.4 | < 0.001 |
| TG/GG | 39 | 56.2 ± 6.5 | 24.9 ± 2.6 | 29.2 ± 3.5 | < 0.001 | 31 | 65.9 ± 9.4 | 38.8 ± 6.2 | 50.9 ± 5.2 | 0.073 |
| Stage of fibrosis | ||||||||||
| F0-2 | 52 | 51.6 ± 5.2 | 23.1 ± 3.2 | 17.6 ± 1.5 | < 0.001 | 76 | 63.4 ± 5.4 | 21.1 ± 1.7 | 25.4 ± 2.4 | < 0.001 |
| F3-4 | 12 | 68.8 ± 11.6 | 32.3 ± 5.6 | 27.5 ± 5.4 | 0.008 | 8 | 93.0 ± 25.8 | 34.8 ± 7.6 | 46.5 ± 11.8 | 0.172 |
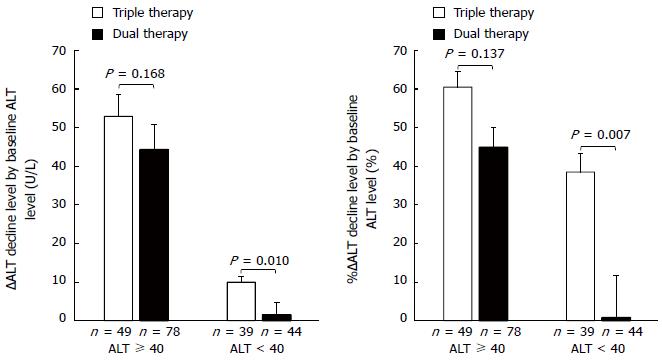
Figure 4 shows ∆ALT decline and %∆ALT decline by baseline ALT level (≥ 40 U/L and < 40 U/L). They did not significantly differ between the two groups for patients with a high baseline ALT level (≥ 40 U/L) (52.9 ± 5.7 U/L vs 44.3 ± 6.5 U/L, and 60.5% ± 4.1% vs 44.9 ± 5.2%, respectively). However, among patients with a low baseline ALT level (< 40 U/L), both were significantly greater in the triple than in the dual therapy group (10.0 ± 1.4 U/L vs 1.5 ± 3.1 U/L, P = 0.010, and 38.5% ± 4.7% vs 0.8% ± 10.9%, P = 0.007, respectively).
∆ALT decline and %∆ALT decline did not significantly differ between the non-SVR patients of the two groups (19.2 ± 12.4 U/L vs 6.5 ± 4.7 U/L, and 11.5% ± 11.2% vs -2.7% ± 7.3%, respectively). However, among the SVR patients, ∆ALT decline and %∆ALT decline were significantly higher in the dual than in the triple therapy group (59.0 ± 7.1 U/L vs 36.5 ± 4.1 U/L, P = 0.003, and 71.7% ± 2.6% vs 57.5% ± 2.7%, P < 0.001, respectively). This may be related to the significant difference in the baseline ALT level between the SVR patients (triple therapy group 54.3 ± 4.3 U/L and dual therapy group 74.0 ± 7.3 U/L; P = 0.018).
One of the most commonly used surveillance tests for HCC is measurement of serum AFP level. To our knowledge, this is the first study to compare the treatment effect of therapy for HCV that includes a DAA and conventional PEG-IFNα plus RBV dual therapy on serum AFP. We documented that, regardless of virological response and ALT change, the serum AFP level of all categories in the TVR-based triple therapy group decreased significantly from baseline to 24 wk after EOT, but no significant decline was found among non-SVR patients of the dual therapy group with a high baseline serum AFP (≥ 10 ng/mL), who were IL28B SNPs TG/GG (non-TT), or stage of fibrosis F3-4.
For chronic hepatitis C patients whose serum AFP level before interferon therapy is ≥ 10 ng/mL, a reduction of the average AFP integration value to < 10 ng/mL by treatment has been strongly associated with a reduced rate of the development of HCC[14]. Moreover, a significantly higher cumulative development of HCC was found for patients with an increased serum AFP level (≥ 10 ng/mL) at EOT, and virological response was not a significant predictive factor[17]. Our study shows a significantly higher decline of the serum AFP level in the TVR-based triple therapy than in the dual therapy group among non-SVR patients or those who have a higher baseline AFP level (≥ 10 ng/mL). This finding suggests the possibility that TVR-based triple therapy has a greater benefit than PEG-IFNα and RBV therapy for the suppression of HCC, via a greater reduction of the serum AFP level.
For chronic hepatitis C patients, the HCV-coding core protein is known to upregulate the transcription of several molecules that activate the cell cycle and induce proliferation in hepatocytes, and it may also upregulate AFP transcription[19]. Therefore, mild elevation of the serum AFP level is sometimes seen in patients with chronic active hepatitis C but without HCC[15,20]. The percentage of chronic hepatitis C patients with an elevated AFP level (≥ 10 ng/mL) ranges from 11.6% to 43%[20-22]. Interferon upregulate p53 gene transcription to halt the cell cycle, or to evoke an apoptotic response of cancerous cells[23,24], and these antitumor effects probably play an important role in reducing the AFP level after interferon therapy[19]. RBV enhances the curative effect of interferon in the treatment of chronic hepatitis C[25,26], however, there is no evidence to date for its efficacy in reducing serum AFP levels. RBV monotherapy has no effect on the serum HCV RVA level, even though a transient decline in the ALT level was observed during the treatment period[27,28]; thus, a reduction in the serum AFP level by RBV seems unlikely. In this study, TVR-based triple therapy resulted in a larger reduction in the serum AFP level than did PEG-IFNα plus RBV dual therapy, despite the shorter therapy duration. We presume the reason for this is that TVR works directly as an HCV NS3/4A protease inhibitor to inhibit replication of HCV. NS3/4A is thought to contribute to the development of HCV-associated promotion of hepatocyte growth, which may promote hepatocarcinogenesis, as do HCV core, NS5A, and NS5B proteins[29,30]. Therefore, other promising DAAs, such as HCV NS5A or NS5B inhibitors, as well as TVR, should have potentially similar effects that would result in a reduction of the serum AFP level, with the potential to reduce the risk of developing HCC.
The persistence of ALT elevation is a convenient marker for identifying an increased risk for HCC[31], as chronic inflammation in the liver is related to HCC development. In fact, the cumulative development of HCC was significantly higher for SVR patients whose post-interferon treatment AFP or ALT levels were high (≥ 10 ng/mL and ≥ 40 U/L, respectively), as well as for non-SVR patients (≥ 6.0 ng/mL and ≥ 40 U/L, respectively)[15]. Our study shows that TVR-based triple therapy does not significantly decrease the ALT level in non-SVR patients, and no significant difference in the decline of the ALT level after treatment is observed between patients in the triple or dual therapy groups with high baseline ALT (≥ 40 U/L). However, regardless of virological response and ALT decline, TVR-based triple therapy reduces the serum AFP level. These findings show that the significant reduction in the serum AFP level by DAA-based therapy for chronic hepatitis C patients may not be related to an anti-inflammatory effect.
The IL28B (rs8099917) non-TT genotype is related to reduced rates of successful treatment in both TVR-based triple therapy and dual therapy of PEG-IFNα and RBV[5,8,32]. Moreover, a high level of baseline AFP is a surrogate marker for predicting treatment failure in TVR-based triple therapy for patients with the IL28B non-TT genotype[33]. To apply this to our study, the SVR rate of IL28B non-TT genotype patients undergoing TVR-based triple therapy was significantly lower in the high baseline AFP level group (i.e., ≥ 10 ng/mL) than in the low baseline AFP level group (46.2% vs 80.8%, P = 0.027). These findings suggest that a high baseline AFP level may be a useful surrogate marker for predicting treatment failure in other DAA-based triple therapy regimens for patients with the IL28B non-TT genotype.
Recent developments in molecular biology have led to the identification of new tumor markers for HCC (heat shock protein 70, AFP-L3, fucosylated GP73, α-L-fucosidase, squamous cell carcinoma antigen, glypican-3, transforming growth factor-β1, and endothelial growth factor), including proteantigens, cytokines, enzymes, and isoenzymes, as well as related genes that can be used in the treatment and prognosis of liver cancer[34]. Additional studies are likely to show these novel markers are improved by antiviral treatment.
Limitations of this study are that it was not randomized and that our participants were all Japanese with HCV genotype 1. In addition, the age of our participants is relatively old [108/210 (51.4%) participants > 60 years of age], thus it will be necessary to determine if our results apply to younger patients. However, older aged chronic hepatitis C patients have a higher risk for the development of HCC[6,14,15]. We believe that our study provides valuable information on the efficacy of DAAs in reducing the serum AFP level.
In conclusion, TVR-based triple therapy more effectively reduces serum AFP levels, even in non-SVR patients, than does dual therapy with PEG-IFNα and RBV.
The authors thank Drs. Mosaburo Kainuma, Kazuhiro Toyoda, Haru Mukae, Takeshi Ihara, Takeo Hayashi, Yuji Harada, Sakiko Hayasaki, Norihito Satoh, Fujiko Mitsumoto, Satoshi Hiramine, Kazuya Ura, Yoshifumi Kato, and Masaru Yamasaki from our department for assistance with data collection. The authors also thank Mr. Yoshitaka Etoh for his excellent lab work on IL28B SNPs.
The serum concentration of alpha-fetoprotein (AFP) is often elevated in patients with hepatocellular carcinoma (HCC), and a high AFP level is a risk factor for the development of HCC in chronic hepatitis C patients. Accumulating evidence indicates that interferon treatment reduces serum AFP levels, and the post-interferon treatment AFP level is strongly associated with the suppression of HCC, regardless of whether or not sustained virological response (SVR) is achieved.
The combination of direct-acting antiviral agents (DAAs) with pegylated-interferon-α (PEG-IFNα) and ribavirin (RBV) has resulted in an improved SVR rate. However, there is no data on the efficacy of DAAs for reducing the development of HCC. This cohort study was conducted to compare serum AFP levels and SVR in patients treated with telaprevir-based triple therapy or conventional dual therapy with PEG-IFNα and RBV.
This is the first study to compare the treatment effect of therapy for hepatitis C virus infections on serum AFP that includes a DAA and conventional PEG-IFNα plus RBV dual therapy.
By understanding the efficacy of DAAs on reducing serum AFP levels, this study provides evidence that therapies that include a DAA have the potential to dramatically reduce the risk of developing HCC.
The combination therapy of PEG-IFNα and RBV that has been used for the last decade has improved the SVR rate to about 50% for chronic hepatitis C patients infected with HCV genotype 1. Interferon therapy is effective in reducing the number of patients who develop HCC, not only for SVR patients but also for non-SVR patients. DAAs, which are HCV non-structural 3/4A (NS3/4A) protease inhibitors, have recently been introduced, and triple therapy regimens that include a protease inhibitor with conventional PEG-IFNα and RBV have significantly improved the SVR rate, up to 80% for patients with HCV genotype 1.
This manuscript is an interesting study that aims to investigate the effect of TVR-based triple therapy on the serum AFP level.
P- Reviewer: Bolhassani A, Lopez-Rodriguez R, Tong HL S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 919] [Cited by in F6Publishing: 929] [Article Influence: 71.5] [Reference Citation Analysis (2)] |
| 2. | Thomas DL. Global control of hepatitis C: where challenge meets opportunity. Nat Med. 2013;19:850-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Furusyo N, Kajiwara E, Takahashi K, Nomura H, Tanabe Y, Masumoto A, Maruyama T, Nakamuta M, Enjoji M, Azuma K. Association between the treatment length and cumulative dose of pegylated interferon alpha-2b plus ribavirin and their effectiveness as a combination treatment for Japanese chronic hepatitis C patients: project of the Kyushu University Liver Disease Study Group. J Gastroenterol Hepatol. 2008;23:1094-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] [Cited in This Article: ] |
| 5. | Ogawa E, Furusyo N, Murata M, Ikezaki H, Ihara T, Hayashi T, Toyoda K, Taniai H, Okada K, Kainuma M. Insulin resistance undermines the advantages of IL28B polymorphism in the pegylated interferon alpha-2b and ribavirin treatment of chronic hepatitis C patients with genotype 1. J Hepatol. 2012;57:534-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Ogawa E, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Maruyama T, Tanabe Y, Satoh T, Nakamuta M, Kotoh K. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: a prospective, multicenter study. J Hepatol. 2013;58:495-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Manns MP, Gane E, Rodriguez-Torres M, Stoehr A, Yeh CT, Marcellin P, Wiedmann RT, Hwang PM, Caro L, Barnard RJ. Vaniprevir with pegylated interferon alpha-2a and ribavirin in treatment-naïve patients with chronic hepatitis C: a randomized phase II study. Hepatology. 2012;56:884-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Furusyo N, Ogawa E, Murata M, Toyoda K, Ohnishi H, Eiraku K, Shimizu M, Harada Y, Mitsumoto F, Takayama K. Therapeutic drug monitoring of telaprevir in chronic hepatitis C patients receiving telaprevir-based triple therapy is useful for predicting virological response. J Antimicrob Chemother. 2014;69:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Furusyo N, Ogawa E, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, Takahashi K, Satoh T, Azuma K, Kawano A. Telaprevir can be successfully and safely used to treat older patients with genotype 1b chronic hepatitis C. J Hepatol. 2013;59:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918-1929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1948] [Cited by in F6Publishing: 1949] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 12. | Peterson ML, Ma C, Spear BT. Zhx2 and Zbtb20: novel regulators of postnatal alpha-fetoprotein repression and their potential role in gene reactivation during liver cancer. Semin Cancer Biol. 2011;21:21-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Tateyama M, Yatsuhashi H, Taura N, Motoyoshi Y, Nagaoka S, Yanagi K, Abiru S, Yano K, Komori A, Migita K. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46:92-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Osaki Y, Ueda Y, Marusawa H, Nakajima J, Kimura T, Kita R, Nishikawa H, Saito S, Henmi S, Sakamoto A. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: a single center study. J Gastroenterol. 2012;47:444-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, Nakanishi H. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 16. | Oze T, Hiramatsu N, Yakushijin T, Miyazaki M, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H. Post-treatment levels of α-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12:1186-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Tamura Y, Yamagiwa S, Aoki Y, Kurita S, Suda T, Ohkoshi S, Nomoto M, Aoyagi Y. Serum alpha-fetoprotein levels during and after interferon therapy and the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig Dis Sci. 2009;54:2530-2537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] [Cited in This Article: ] |
| 19. | Murashima S, Tanaka M, Haramaki M, Yutani S, Nakashima Y, Harada K, Ide T, Kumashiro R, Sata M. A decrease in AFP level related to administration of interferon in patients with chronic hepatitis C and a high level of AFP. Dig Dis Sci. 2006;51:808-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Hu KQ, Kyulo NL, Lim N, Elhazin B, Hillebrand DJ, Bock T. Clinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol. 2004;99:860-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [PubMed] [Cited in This Article: ] |
| 22. | Kobeisy MA, Morsy KH, Galal M, Sayed SK, Ashmawy MM, Mohammad FM. Clinical significance of elevated alpha-foetoprotein (AFP) in patients with chronic hepatitis C without hepatocellular carcinoma in upper EGYPT. Arab J Gastroenterol. 2012;13:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 663] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 24. | Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2420] [Cited by in F6Publishing: 2380] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 25. | Zoulim F, Haem J, Ahmed SS, Chossegros P, Habersetzer F, Chevallier M, Bailly F, Trépo C. Ribavirin monotherapy in patients with chronic hepatitis C: a retrospective study of 95 patients. J Viral Hepat. 1998;5:193-198. [PubMed] [Cited in This Article: ] |
| 26. | Schalm SW, Hansen BE, Chemello L, Bellobuono A, Brouwer JT, Weiland O, Cavalletto L, Schvarcz R, Ideo G, Alberti A. Ribavirin enhances the efficacy but not the adverse effects of interferon in chronic hepatitis C. Meta-analysis of individual patient data from European centers. J Hepatol. 1997;26:961-966. [PubMed] [Cited in This Article: ] |
| 27. | Lee JH, von Wagner M, Roth WK, Teuber G, Sarrazin C, Zeuzem S. Effect of ribavirin on virus load and quasispecies distribution in patients infected with hepatitis C virus. J Hepatol. 1998;29:29-35. [PubMed] [Cited in This Article: ] |
| 28. | Bodenheimer HC, Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology. 1997;26:473-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 279] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Banerjee A, Ray RB, Ray R. Oncogenic potential of hepatitis C virus proteins. Viruses. 2010;2:2108-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | El-Shamy A, Shindo M, Shoji I, Deng L, Okuno T, Hotta H. Polymorphisms of the core, NS3, and NS5A proteins of hepatitis C virus genotype 1b associate with development of hepatocellular carcinoma. Hepatology. 2013;58:555-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Hayashi J, Furusyo N, Ariyama I, Sawayama Y, Etoh Y, Kashiwagi S. A relationship between the evolution of hepatitis C virus variants, liver damage, and hepatocellular carcinoma in patients with hepatitis C viremia. J Infect Dis. 2000;181:1523-1527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1747] [Cited by in F6Publishing: 1746] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 33. | Shimada N, Tsubota A, Atsukawa M, Abe H, Ika M, Kato K, Sato Y, Kondo C, Sakamoto C, Tanaka Y. α-Fetoprotein is a surrogate marker for predicting treatment failure in telaprevir-based triple combination therapy for genotype 1b chronic hepatitis C Japanese patients with the IL28B minor genotype. J Med Virol. 2014;86:461-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol. 2013;1:593-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |