Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3509
Peer-review started: October 6, 2014
First decision: October 29, 2014
Revised: November 6, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: March 28, 2015
AIM: To study the effects of entacapone, a catechol-O-methyltransferase inhibitor, on colon motility and electrolyte transport in Parkinson’s disease (PD) rats.
METHODS: Distribution and expression of catechol-O-methyltransferase (COMT) were measured by immunohistochemistry and Western blotting methods. The colonic smooth muscle motility was examined in vitro by means of a muscle motility recording device. The mucosal electrolyte transport of PD rats was examined by using a short-circuit current (ISC) technique and scanning ion-selective electrode technique (SIET). Intracellular detection of cAMP and cGMP was accomplished by radioimmunoassay testing.
RESULTS: COMT was expressed in the colons of both normal and PD rats, mainly on the apical membranes of villi and crypts in the colon. Compared to normal controls, PD rats expressed less COMT. The COMT inhibitor entacapone inhibited contraction of the PD rat longitudinal muscle in a dose-dependent manner. The β2 adrenoceptor antagonist ICI-118,551 blocked this inhibitory effect by approximately 67% (P < 0.01). Entacapone increased mucosal ISC in the colon of rats with PD. This induction was significantly inhibited by apical application of Cl- channel blocker diphenylamine-2, 2’-dicarboxylic acid, basolateral application of Na+-K+-2Cl-co-transporter antagonist bumetanide, elimination of Cl- from the extracellular fluid, as well as pretreatment using adenylate cyclase inhibitor MDL12330A. As an inhibitor of prostaglandin synthetase, indomethacin can inhibit entacapone-induced ISC by 45% (P < 0.01). When SIET was applied to measure Cl- flux changes, this provided similar results. Entacapone significantly increased intracellular cAMP content in the colonic mucosa, which was greatly inhibited by indomethacin.
CONCLUSION: COMT expression exists in rat colons. The β2 adrenoceptor is involved in the entacapone-induced inhibition of colon motility. Entacapone induces cAMP-dependent Cl- secretion in the PD rat.
Core tip: Entacapone, a catechol-O-methyltransferase (COMT) inhibitor, is an emerging drug for Parkinson’s disease (PD) patients. However, patients experience gastro-intestinal side effects with entacapone treatment and the reason for this is unknown. This study for the first time proved that COMT is expressed in normal and PD rat colons and that entacapone can inhibit PD rat muscle contraction through the β2 adrenoceptor. It was also discovered that entacapone can induce cAMP-dependent Cl- secretion in PD rats and that endogenous prostaglandin is involved in this process. These findings provided histological evidence of COMT in the colon, establishing an experimental basis for the mechanism of entacapone-induced PD gastro-intestinal side effects.
- Citation: Li LS, Liu CZ, Xu JD, Zheng LF, Feng XY, Zhang Y, Zhu JX. Effect of entacapone on colon motility and ion transport in a rat model of Parkinson’s disease. World J Gastroenterol 2015; 21(12): 3509-3518
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3509
Parkinson’s disease (PD) is one of the most common neurologic disorders, affecting approximately 1% of individuals older than 60 years[1]. L-DOPA is currently the most effective treatment for PD[2,3]. However, most patients with late-stage disease, or those who have been treated with L-DOPA for over 3 to 5 years, will develop “chronic syndromes”, such as desensitization to treatment or dyskinesia[4-6]. Entacapone, a catechol-O-methyltransferase (COMT) inhibitor, is an emerging drug that overcomes these problems[7-9]. However, patients experience gastro-intestinal side effects with entacapone treatment, especially diarrhea, with some patients displaying constipation and abdominal pain. Approximately 1% of patients discontinue entacapone treatment due to abdominal pain. Discontinuing use of entacapone for several days alleviates the adverse gastro-intestinal effects, although the mechanism for this observation is not clear. Abdominal pain can reach a moderate level, and taking large doses (> 1600 mg/d) of entacapone increases the occurrence and severity of the abdominal pain; the reason for this phenomenon is also unknown[10-12].
We hypothesized that the adverse gastro-intestinal effects of entacapone on PD patients was due to changes in intestinal smooth muscle motility, or to effects on ion transport in the intestinal epithelium. COMT is expressed in several tissues in humans and rodents. However, the precise localization of COMT in normal and PD rat intestine is not clear; there are also no studies reporting how entacapone affects ion transport in the intestines of PD patients or whether it affects smooth muscle motility[13-16]. Due to the above factors, this study utilized in vitro rat colon smooth muscle and mucosal samples to examine the precise localization of COMT in the rat colon. The effect of entacapone on colon smooth muscle and epithelial ion transport in PD rats was investigated. In addition, the main reasons responsible for entacapone-induced adverse intestinal effects were explored. This study provides experimental evidence for the prevention and treatment of these side effects.
The animals were purchased from the Department of Animal Science of Capital Medical University. Specific-pathogen-free (SPF) male SD rats with body weights of 200-300 g were randomly grouped. The animals were kept at room temperature, with normal light/dark cycle exposure and 24-h water and food access until the day of the experiment. The experiment was approved by the Laboratory Animal Welfare Committee.
The main reagent, entacapone, is a product of the Orion Corporation. Indomethacin, TTX, bumetanide, and DPC were purchased from Sigma (St. Louis, MO). All chemical reagents were dissolved in dimethyl sulfoxide (DMSO), and the DMSO volume fraction did not exceed 0.1%. Preliminary experiments showed that the solvent did not alter basic electrophysiologic parameters. In addition, NaCl, KCl, MgSO4·7H2O, KH2PO4, NaHCO3, CaCl2·2H2O, and glucose were purchased from Sigma.
The Krebs-Henseleit solution (K-HS) was prepared as follows: sodium chloride 117 mmol/L, potassium chloride 4.7 mmol/L, calcium chloride 2.5mmol/L, magnesium chloride 1.2 mmol/L, sodium bicarbonate 24.8 mmol/L, monopotassium phosphate 1.2 mmol/L, and glucose 11.1 mmol/L. For Cl-free K-HS, sodium gluconate, potassium gluconate, calcium gluconate, and magnesium gluconate were used to replace the sodium chloride, potassium chloride, calcium chloride, and magnesium chloride, respectively.
Male SD rats weighing 210 to 240 g were selected. First, the weights were taken, and 0.4 mL of 10% chloral hydrate/100 g body weight was injected for anesthetization. The animals were placed on a Kopf stereotaxic apparatus. According to the coordinates, the positions posterior to the frontal suture 5.6 mm, shifted 2 mm laterally, or AP = -5.6 (posterior to the frontal suture is negative and anterior to the frontal suture is positive), ML = ± 2 mm (right is negative and left is positive) were located by adjusting the guide bars and were marked with a marker. Four microliters of 6-OHDA (2 μg/μL), a total of 8 μg of drug, was administered at an even speed of 1 μL/min. The needle was kept at the position for 2 min. The needle was then lifted slowly, and a small amount of saline was scattered to hydrate the incision. A dried saline saturated gelatin sponge was used to seal the incision. Penicillin powder was scattered before the skin was closed by suture. Then, an intraperitoneal injection of penicillin (0.5 mL/animal) was administered. The procedures of the normal control group were the same as those of the experimental model group, except that the normal control group was administered saline.
Western blot analysis and immunohistochemistry were performed as previously described[17,18]. Information on antibodies used in this study is summarized in Tables 1 and 2.
| Antigen | Immunizing antigen | Host species | Dilution | Source/Catalog No. | |
| IHC | WB | ||||
| COMT | Synthetic peptide corresponding to amino acid residues 60-76 of rat COMT conjugated to KLH | Rabbit | 1:200 | 1:1000 | Santa Cruz/sc-25844 |
| COMT | Synthesized peptide derived from human COMT | Rabbit | 1:400 | 1:1000 | Sigma/SAB4500401 |
| GAPDH | A synthetic peptide containing 314-333 amino acids of mouse GAPDH with a 407972 gene ID | Rabbit | - | 1:5000 | Sigma/G9545 |
| NF | Pellet of pig brain cold stable proteins after depolymerization of microtubules | Mouse | 1:400 | - | Abcam/ab7794 |
| NF | Recombinant C-terminal segment of rat NF-M | Rabbit | 1:400 | - | Novus/NB300-133 |
| Antibody | Conjugation | Dilution | Source/Catalog No. |
| Goat anti-rabbit IgG | Alexa Fluor 488 | 1:100 | Beyotime/A0423 |
| Goat anti-rabbit IgG | Alexa Fluor 488 | 1:100 | Beyotime/A0428 |
| Donkey anti-rabbit IgG | IRDyeTM 800 | 1:10000 | Rockland/16747 |
The rats were killed and then distal colon was quickly removed. The luminal contents of the colon were washed with Krebs solution. The 1st to 4th segments of the distal colon were collected. Each segment was cut open along the mesentery border and spread evenly over silica gel with the apical membrane facing down. The samples were surrounded by ice-cold Krebs solution to maintain tissue activity. The entire colon was cut into 2-mm-wide, 1-1.5-cm-long strips along the longitudinal or circular muscles. Surgical sutures were used to tie a piece of thread to each end of the muscle strip and connect one end to a specimen support rod and the other end to a pressure transducer. The muscle strips were incubated in 10 mL of Krebs solution at a constant temperature (37 ± 0.5 °C) under continuous gas ventilation (95% oxygen and 5% carbon dioxide). After applying 1g of resting muscle tension, the change in length was recorded by the pressure transducer. With 2000 mg of weight for calibration, intensity of muscle strip contraction was measured with mg as the basic unit. After the muscle strips were adapted in a water bath for 120 min, the autonomous contraction intensity was officially recorded.
For the preparation of the rat colon mucosa sample, roughly 7 cm of colon from above the rectal lymph node was obtained (this lymph node is often located roughly 3 cm up the rectum). Using fine tip forceps under a dissection microscope to carefully separate the submucosa, muscularis, and serosa from the mucosa with blunt dissection, a thin layer of tissue that included epithelia and some residual connective tissue was obtained. This tissue comprised the rat colon mucosa sample.
This experiment utilized a constant temperature perfusion system to record the ISC in vitro. The colon mucosa sample was placed in the Ussing chamber of the perfusion system. Both sides of the colon mucosa were injected with 5 mL of K-HS, and 95% oxygen and 5% carbon dioxide were administered simultaneously to maintain the solution pH at approximately 7.4. The samples were incubated in a 37 °C water bath for 30 min to allow the electrical parameters to stabilize. A voltage clamp was utilized to maintain the epithelial voltage at zero potential, short-circuiting the tissue, and the transepithelial current measured at this time was the ISC[19].
This experiment utilized SIET (BIO-001A, YoungerUSA Sci. & Tech. Corp., United States). The noninvasive microelectrode (model XY-H-01) used for measuring the Cl- concentration and flux was provided by Xuyue (Beijing) Sci. & Tech. Co., Ltd. The tip of the Cl- selective microelectrode was filled with a 15-25 μm chloride selective liquid ion exchanger column (LIX) and was subsequently filled with an approximately 10 mmol/L electrolyte solution column. The Cl- selective microelectrode must be calibrated before use; only Nernstian Slope > 56 mV/decade electrodes can be used, and the distance between the Cl- electrode and cells must be controlled to approximately 30 μm. The absolute concentration, flux direction, and flux rate difference before and after drug treatment were compared[20].
The rat colon mucosa samples were collected and placed in a 37 °C K-HS solution gassed with 5% CO2 and 95% O2 for incubation; each sample weighed approximately 150 mg. After incubation and stabilization, the tissue was treated with 0.9% NaCl and indomethacin (10 μmol/L) for 5 min. Next, 200 μmol/L entacapone was added to react for 15 min. To observe the effect of various drugs on the entacapone-induced intracellular cAMP/cGMP level changes, after drug pretreatment, the tissues were cut quickly, with excess water blotted on filter paper, and flash frozen in liquid nitrogen. The tissues were then homogenized on ice and centrifuged (10620 ×g, 5 min) for analysis. Intracellular cAMP/cGMP detection was accomplished using a commercial radioimmunoassay kit (RIA) (Beijing Huaying Biotechnology Co. Ltd., Beijing, China).
GraphPad Prism 5.0 software was used for the statistical analysis. Data are expressed as the mean ± SE. The means between two groups were analyzed by t-test. When the variance was unequal, the Wilcoxon rank-sum test was used for comparison of the means of two groups. The significance level was set at P = 0.05.
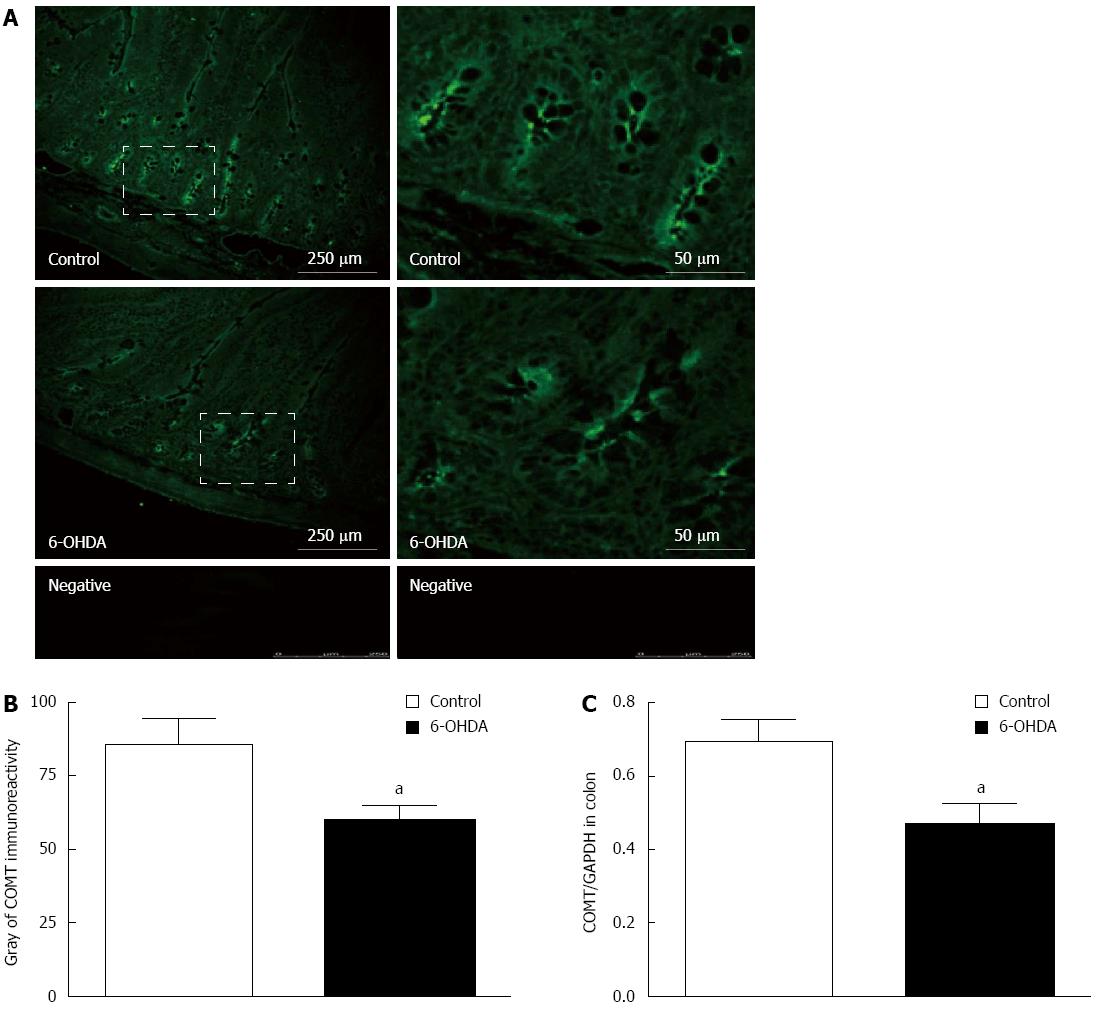
To provide morphological evidence, this study used immunofluorescence labeling and immunohistochemistry, utilizing rat colon cryosections to determine the localization of COMT. It was discovered that in both normal and 6-OHDA PD model rats, COMT was expressed more abundantly on the villi and crypts at the apical membrane (Figure 1A). The gray analysis of COMT immunoreactivity in normal rats (78.23 ± 4.63, 48 fields of vision COMT from 8 rats) was higher than that in the PD group (60.27 ± 3.96, 48 fields of vision COMT from 8 rats) (Figure 1B).
Immunoblotting similarly confirmed that COMT is localized in colon tissue. Compared to normal rats, 6-OHDA PD model rats expressed slightly less COMT. A quantitative comparison using an internal reference by GAPDH showed a 27% decrease in COMT expression, from 0.67 ± 0.04 to 0.49 ± 0.06 in PD model rats (Figure 1C) (n = 8, P < 0.05).
Entacapone concentrations of 2 μmol/L, 20 μmol/L, 100 μmol/L, 200 μmol/L, 400 μmol/L and 1000 μmol/L were used individually to observe their effects on intestine longitudinal muscle in PD model rats. It was observed that longitudinal muscle contraction was decreased from baseline (1233 ± 50.13 mg) to 1150 ± 64.96 mg, 859.9 ± 43.54 mg, 566.8 ± 92.6 mg, 450.6 ± 74.86 mg, 304.9 ± 53.7 mg and 307.98 ± 63.4 mg, respectively (n = 30, Figure 2A). This result indicates that entacapone has an inhibitory effect on colon longitudinal muscle contraction with a dose-dependent pattern, by EC50 of 200 μmol/L (Figure 2B). However, the different doses of entacapone did not significantly alter the PD rat colon circular muscle (data not shown).
To investigate the mechanism of entacapone-induced smooth muscle inhibition, the samples were pretreated with β2 adrenoceptor antagonist ICI-118,551 (10-5 mol/L), and it was found that entacapone-induced colon longitudinal muscle inhibition was decreased by 67%, from 782.4 ± 34.24 mg to 258.39 ± 39.47 mg (n = 9, Figure 2C and D). However, with pretreatment with α adrenoceptor antagonist phentolamine (10-5 mol/L), entacapone-induced smooth muscle inhibition was not affected (n = 9). We also found that using acetylcholine (10-6 mol/L) for pretreatment or administering entacapone before adding acetylcholine did not significantly affect entacapone-induced smooth muscle inhibition (data not shown). The results show that entacapone might exert its inhibitory effects on PD rat colon smooth muscle through the β2 adrenoceptor.
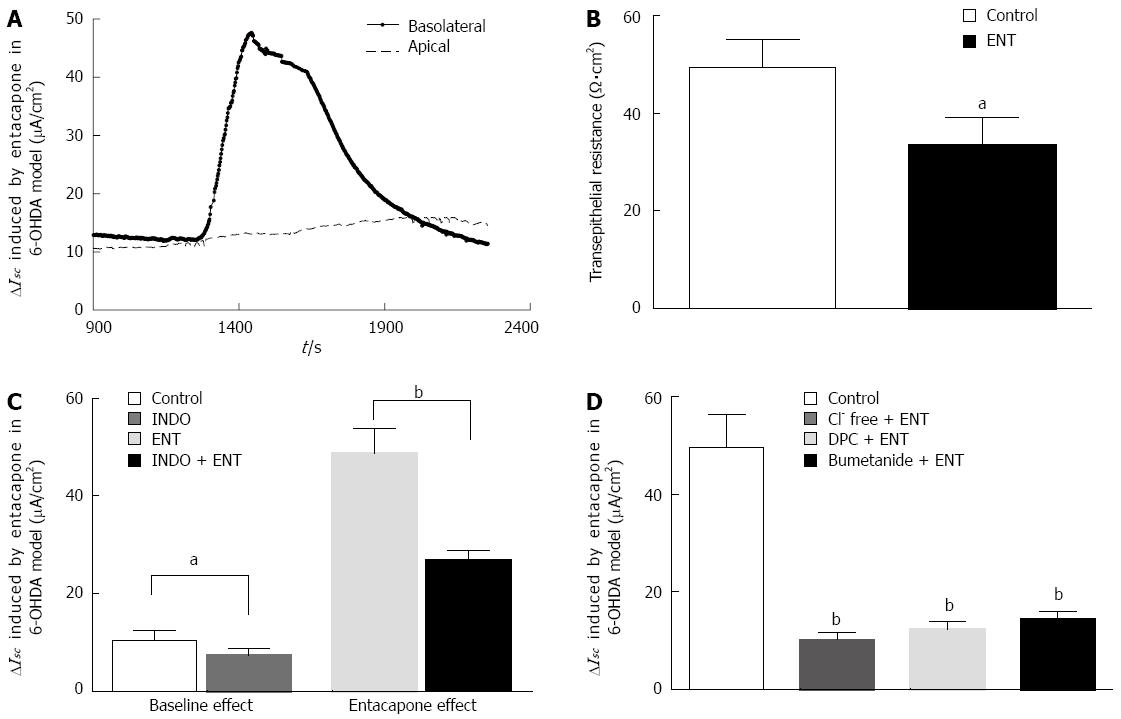
PD rat colon mucosa samples were placed in the ISC apparatus. After approximately 30 min, the basal electrical activity of the samples reached stability. In this experiment, all mucosa samples were pretreated with TTX to eliminate the effects from residual neural activity. The basal potential difference, ISC, and transepithelial resistance (Rt) were -0.8 ± 0.3 mV, 10.1 ± 3.2 μA/cm2, and 49.6 ± 5.4 Ω·cm2, respectively. Adding 200 μmol/L entacapone to the basolateral (serosal) side can induce a 47.69 ± 8.91 μA/cm2 increase in ISC (Figure 3A). Simultaneously, a significant decrease in the Rt from 49.6± 5.4 Ω·cm2 to 36.84 ± 3.94 Ω·cm2 (n = 13, P < 0.05) was observed (Figure 3B). When entacapone was added to the apical (mucosal) side, there was no significant change in the ISC (Figure 3A).
To investigate the effect of endogenous prostaglandin on entacapone-induced ISC changes in PD rat colons, the cyclooxygenase (COX) inhibitor indomethacin (10 μmol/L) was added to the basolateral side of the mucosa for observation. As shown in Figure 3C, after pretreatment with indomethacin, the mucosal basal ISC decreased from 10.1 ± 2.14 μA/cm2 to 7.28 ± 2.06 μA/cm2 (n = 9, P < 0.05), reaching a 28% reduction; the pretreatment was able to decrease the entacapone-induced ISC from 47.69 ± 8.91 μA/cm2 to 26.14 ± 2.67 μA/cm2 (n = 9, P < 0.01), reaching a 45% reduction. These results show that endogenous prostaglandin was involved in PD rat colon basal ISC formation and entacapone-induced ISC change.
Generally speaking, an upward ISC curve reflects the occurrence of cation absorption or anion secretion with electrogenic features. In order to study the properties of the electric activities and involvement in ion channels or transporters, the following experiment was performed (Figure 3D). Epithelial Na+ channel inhibitor amiloride (10 μmol/L) or DIDS (Ca2+-dependent Cl-channel inhibitor, 200 μmol/L) was applied to the apical side of mucosa, and it was observed that they did not reduce the entacapone-induced increase of the ISC (n = 9, data not shown). However, removal of Cl- from the mucosa sample bath buffer could reduce entacapone-induced ISC by 79%, from 47.69 ± 8.91 μA/cm2 to 10.01 ± 2.53 μA/cm2 (n = 9, P < 0.01). When DPC (1 mmol/L) (a nonselective Cl- channel blocker) was added to the apical side or when bumetanide (100 μmol/L) (a Na+-K+-2Cl- cotransporter inhibitor) was added to the basolateral side, this also significantly reduced the entacapone-induced ISC by 74% and 68% to 12.4 ± 3.78 (n = 9, P < 0.01) and 15.26 ± 3.52 μA/cm2μA/cm2 (n = 9, P < 0.01), respectively. These results show that entacapone-induced ISC changes in PD rat colons are mainly mediated by Cl- secretion.
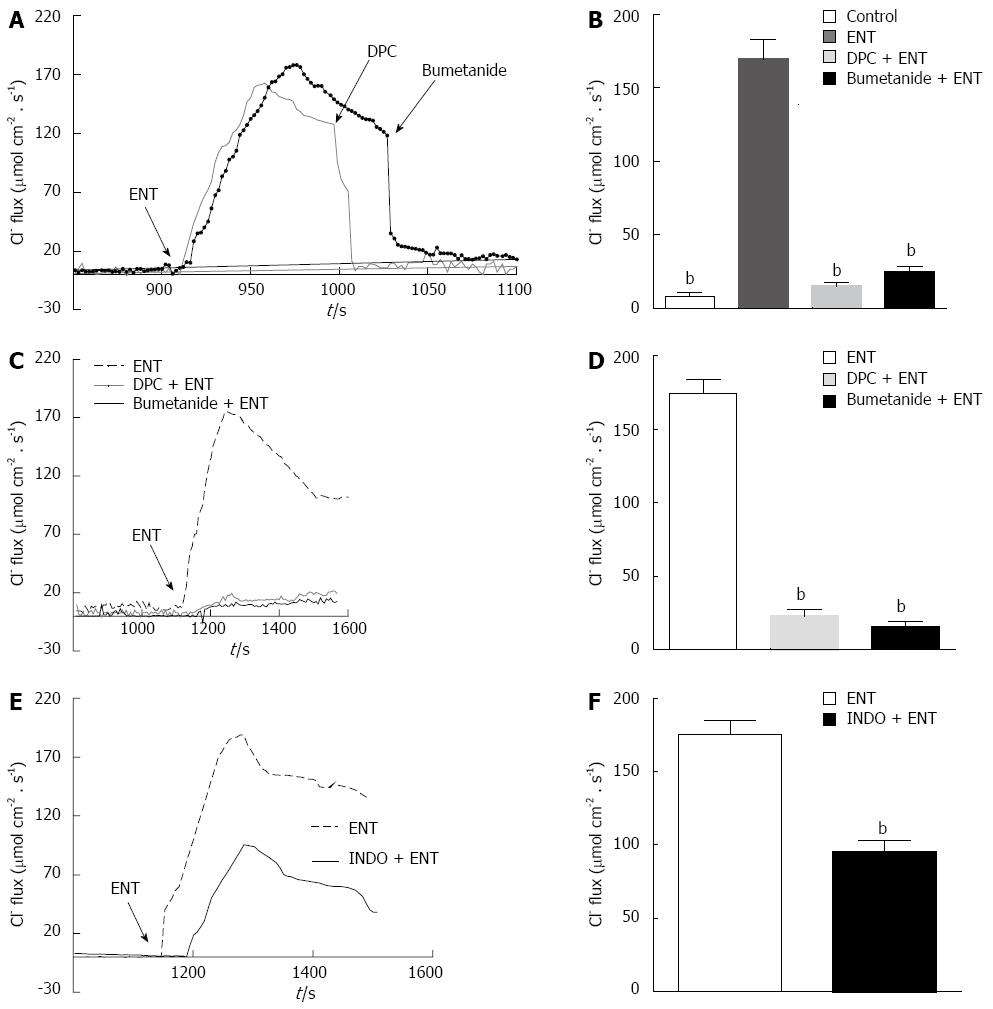
SIET is a new research method. Using specialized ion-selective electrodes, it is possible to obtain information regarding ion movements. Thus, we used this method to directly measure entacapone-induced PD rat colon Cl- transport.
As Figure 4 shows, using Cl- sensitive electrodes at the basolateral side of PD rat colon mucosa, a small, stable Cl--flux can be recorded. The addition of entacapone (200 μmol/L) could induce a significant Cl--flux from the basolateral side of the colon mucosa into the cells, showing a significant increase from 6.37 ± 3.01 μmol·cm-2·s-1 to 172.3 ± 14.86 μmol·cm-2·s-1. This Cl--flux could be inhibited by DPC (1 mmol/L) and bumetanide (100 μmol/L), reducing the concentrations to 13.69 ± 3.12 μmol·cm-2·s-1 and 22.3 ± 3.72 μmol·cm-2·s-1, respectively (Figure 4A and B). If pretreated with DPC and bumetanide, similar results were observed; the entacapone-induced flux was reduced to 22.07 ± 3.59 μmol·cm-2·s-1 and 18.35 ± 3.1 μmol·cm-2·s-1 (n = 10, P < 0.001), separately (Figure 4C and D). The pretreatment by indomethacin (10 μmol/L) was able to decrease significantly the entacapone-induced ISC (n = 9, P < 0.01) (Figure 4E and F). Apical application of Ca2+-dependent Cl-channel blocker DIDS (200 μmol/L) did not affect entacapone-caused Cl--flux (data not shown).
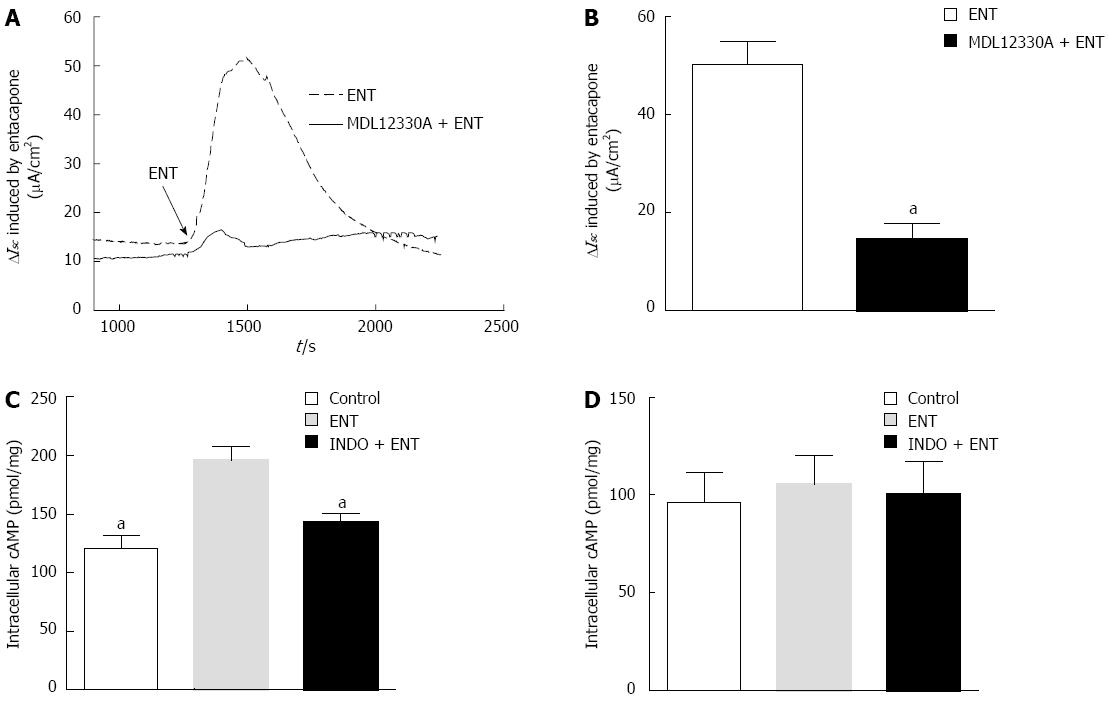
We know that colon mucosa has mainly Ca2+-dependent or cAMP-dependent Cl-channels. cAMP-dependent Cl- channels play an important role in the regulation of mammalian Cl- transport. After the application of 20 μmol/L MMDL-12330A (an adenylate cyclase inhibitor), entacapone-induced Cl- flux was inhibited by 70.69%, from 47.69 ± 8.91 μmol·cm-2·s-1 to 13.98 ± 3.42 μmol·cm-2·s-1 (Figure 5A and B, n = 6, P < 0.01).
Furthermore, a radioimmunoassay was used to detect the amount of cAMP in PD rat colon mucosa (Figure 5C); the basal expression was 132.1 pmol·mg-1 (n = 6). After treatment with 200 μmol/L entacapone, the expression was increased to 181.7 pmol·mg-1, and indomethacin pretreatment significantly reduced the entacapone-induced cAMP increase. However, with entacapone or indomethacin pre-treatment, the content of CGMP did not obviously change (Figure 5D).
As an important metabolic enzyme for neurotransmitters such as dopamine, COMT is not only expressed abundantly in the central nervous system but is also widely expressed in peripheral tissues, such as liver, kidney, stomach, and duodenum[21]. This study is the first to describe COMT expression in normal and PD rat colon and the characteristics of its localization. This provides histological evidence for further investigation of COMT-related functions.
Currently, clinical studies and application have proven that the COMT inhibitor entacapone has outstanding clinical relevance for the treatment of PD. When entacapone is used in conjunction with L-DOPA, significant improvement is noted in the clinical symptoms[22,23]. However, gastro-intestinal side effects are also common[24]. This manuscript utilized in vitro colon tissue and investigated the mechanism for entacapone-induced side effects in PD model rats.
The experimental results from this study show that entacapone inhibited colon longitudinal muscle contraction in a dose-dependent manner. This inhibitory effect may be achieved through the β2 adrenoceptor, subsequently causing the gastro-intestinal smooth muscle of PD patients to contract and relax irregularly, displaying abnormal motility and obstructing luminal content transport, with a proportion of patients displaying constipation.
ISC recording techniques have been widely utilized to measure epithelial cell electrolyte transport. However, this type of technique lacks chemical selectivity and can only be used to detect electrogenic ion transport[25,26]. In contrast, SIET is a novel recording method that can overcome the shortcomings of ISC recording techniques using specialized ion-sensitive electrodes, such as Cl--sensitive electrodes, to achieve ion flux detection. In this study, in order to make the results more comprehensive, we combined these two methods for determination of ion transport.
In fresh in vitro PD rat colon tissue, it was proven that the COMT inhibitor induced colon Cl- secretion. This type of Cl- secretion is mainly electrogenic ion transport mediated by basolateral NKCC and apical membrane Cl- channels, since DPC and bumetanide can block approximately 70% of entacapone-induced Cl- secretion.
The colon mucosa can induce intracellular cAMP expression by exogenous stimulation or endogenous prostaglandin, therefore promoting Cl- secretion[27,28]. The cyclooxygenase inhibitor indomethacin can block Cl- secretion by 45%, indicating that prostaglandin is involved in PD rat colon mucosa Cl- secretion. In addition, we found that entacapone-induced Cl- secretion in PD rat colons was mainly transduced through the secondary messenger cAMP, because pretreatment with the AC inhibitor MDL-12330A significantly reduced this Cl- secretion and entacapone can induce colon epithelial cell cAMP expression. In the regulation of intestinal water-electrolyte metabolism, the Cl- channel plays an important role. Water also spreads to the enteric cavity due to the osmotic gradient of Cl-, causing secretory diarrhea[29,30]. This may be one of the mechanisms by which entacapone induces diarrhea in PD patients.
In conclusion, this study is the first to prove that COMT is expressed in normal and PD rat colons and that entacapone can inhibit PD rat longitudinal muscle contraction through the β2 adrenoceptor. It was also discovered that entacapone can induce cAMP-dependent Cl- secretion in PD rats and that endogenous prostaglandin is involved in this process. This study has provided histological evidence for functional studies of COMT in the colon, establishing an experimental basis for the mechanism of entacapone-induced PD gastro-intestinal side effects.
Catechol-O-methyltransferase (COMT) is expressed in several tissues in humans and rodents. However, the precise localization of COMT in normal and Parkinson’s disease (PD) rat intestine is not clear; there are also no studies reporting how entacapone affects ion transport in the intestines of PD patients or whether it affects smooth muscle motility. The effect of entacapone on colon smooth muscle and epithelial ion transport in PD rats was investigated in this study. In addition, the main reasons responsible for entacapone-induced adverse intestinal effects were explored.
The authors combined ISC recording technique with a novel scanning ion-selective electrode technique method to determine colonic ion transport in PD rats and establish a mechanism of entacapone-induced PD gastro-intestinal side effects.
COMT plays a crucial role in the regulation of dopaminergic systems by catalyzing the inactivation of catecholamines. Entacapone, as a COMT inhibitor, is an emerging drug for PD patients. However, patients experience gastro-intestinal side effects with entacapone treatment; the reasons are unknown. This study was the first to prove that COMT is expressed in normal and PD rat colons and that entacapone can inhibit PD rat longitudinal muscle contraction through the β2 adrenoceptor. It was also discovered that entacapone can induce cAMP-dependent Cl- secretion in PD rats and that endogenous prostaglandin is involved in this process.
The study established an experimental basis for the mechanism of entacapone-induced gastro-intestinal side effects with Parkinson’s disease.
COMT inhibitor refers to a drug that can inhibit catecholamine degradation. Tolcapone and entacapone can be regarded as representative of a new generation of COMT inhibitor. Using a combination of these two drugs with levodopa, the clinical symptoms of patients with Parkinson’s disease can be improved remarkably, significantly improving life quality of the patients. However, tolcapone may cause liver damage in individual patients, and entacapone can induce gastro-intestinal side effects in some patients. The further study of related mechanisms is needed to overcome these issues.
The authors have exerted a substantial effort to analyze the effects of entacapone on colon motility and ion transport in Parkinson’s disease rats and introduced their findings. This manuscript has a good novel idea. The paper is well organized and written methodology is systematized.
P- Reviewer: Sgourakis G, Shehata MMM S- Editor: Ma YJ L- Editor: Logan S E- Editor: Zhang DN
| 1. | Rajput AH, Uitti RJ, Rajput A, Offord KP. Mortality in Parkinson’s disease. Mov Disord. 2010;25:507-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Carecchio M, Collini A, Comi C, Cantello R, Bhatia KP, Monaco F. Levodopa-induced belly dancer’s dyskinesias in Parkinson’s disease: report of one case. Mov Disord. 2010;25:1760-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Othman AA, Dutta S. Population pharmacokinetics of levodopa in subjects with advanced Parkinson’s disease: levodopa-carbidopa intestinal gel infusion vs. oral tablets. Br J Clin Pharmacol. 2014;78:94-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Tambasco N, Belcastro V, Gallina A, Castrioto A, Calabresi P, Rossi A. Levodopa-induced breathing, cognitive and behavioral changes in Parkinson’s disease. J Neurol. 2011;258:2296-2299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Kurtis MM, Franch O. Tardive syndrome and Parkinson’s disease responsive to concomitant tetrabenazine and levodopa therapy. Parkinsonism Relat Disord. 2011;17:774-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Lim SY, Tan ZK, Ngam PI, Lor TL, Mohamed H, Schee JP, Tan AK, Goh JY, Ooi E, Soh PC. Impulsive-compulsive behaviors are common in Asian Parkinson’s disease patients: assessment using the QUIP. Parkinsonism Relat Disord. 2011;17:761-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Tambasco N, Muti M, Chiarini P, Tarducci R, Caproni S, Castrioto A, Nigro P, Parnetti L, Floridi P, Rossi A. Entacapone reduces cortical activation in Parkinson’s disease with wearing-off: a f-MRI study. PLoS One. 2014;9:e96806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Salat D, Tolosa E. Levodopa in the treatment of Parkinson’s disease: current status and new developments. J Parkinsons Dis. 2013;3:255-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Reichmann H, Emre M. Optimizing levodopa therapy to treat wearing-off symptoms in Parkinson’s disease: focus on levodopa/carbidopa/entacapone. Expert Rev Neurother. 2012;12:119-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Marsala SZ, Gioulis M, Ceravolo R, Tinazzi M. A systematic review of catechol-0-methyltransferase inhibitors: efficacy and safety in clinical practice. Clin Neuropharmacol. 2012;35:185-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Ingman K, Naukkarinen T, Vahteristo M, Korpela I, Kuoppamäki M, Ellmén J. The effect of different dosing regimens of levodopa/carbidopa/entacapone on plasma levodopa concentrations. Eur J Clin Pharmacol. 2012;68:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Müller T. Entacapone. Expert Opin Drug Metab Toxicol. 2010;6:983-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Baeken C, Marinazzo D, Claes S, Wu GR, Van Schuerbeek P, De Mey J, Luypaert R, De Raedt R. COMT Val(158)Met genotypes differentially influence subgenual cingulate functional connectivity in healthy females. Front Hum Neurosci. 2014;8:481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kelm MK, Boettiger CA. Effects of acute dopamine precusor depletion on immediate reward selection bias and working memory depend on catechol-O-methyltransferase genotype. J Cogn Neurosci. 2013;25:2061-2071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 167] [Reference Citation Analysis (0)] |
| 15. | Baumann C, Klauke B, Weber H, Domschke K, Zwanzger P, Pauli P, Deckert J, Reif A. The interaction of early life experiences with COMT val158met affects anxiety sensitivity. Genes Brain Behav. 2013;12:821-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Müller T, van Laar T, Cornblath DR, Odin P, Klostermann F, Grandas FJ, Ebersbach G, Urban PP, Valldeoriola F, Antonini A. Peripheral neuropathy in Parkinson’s disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord. 2013;19:501-507; discussion 501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Zheng LF, Wang ZY, Li XF, Song J, Hong F, Lian H, Wang Q, Feng XY, Tang YY, Zhang Y. Reduced expression of choline acetyltransferase in vagal motoneurons and gastric motor dysfunction in a 6-OHDA rat model of Parkinson’s disease. Brain Res. 2011;1420:59-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Zheng LF, Song J, Fan RF, Chen CL, Ren QZ, Zhang XL, Feng XY, Zhang Y, Li LS, Zhu JX. The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiol (Oxf). 2014;211:434-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Yang N, Liu SM, Zheng LF, Ji T, Li Y, Mi XL, Xue H, Ren W, Xu JD, Zhang XH. Activation of submucosal 5-HT(3) receptors elicits a somatostatin-dependent inhibition of ion secretion in rat colon. Br J Pharmacol. 2010;159:1623-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Sun J, Chen S, Dai S, Wang R, Li N, Shen X, Zhou X, Lu C, Zheng X, Hu Z. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 2009;149:1141-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Mueller EM, Burgdorf C, Chavanon ML, Schweiger D, Hennig J, Wacker J, Stemmler G. The COMT Val158Met polymorphism regulates the effect of a dopamine antagonist on the feedback-related negativity. Psychophysiology. 2014;51:805-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Maltête D, Cottard AM, Mihout B, Costentin J. Erythrocytes catechol-o-methyl transferase activity is up-regulated after a 3-month treatment by entacapone in parkinsonian patients. Clin Neuropharmacol. 2011;34:21-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lew MF, Somogyi M, McCague K, Welsh M. Immediate versus delayed switch from levodopa/carbidopa to levodopa/carbidopa/entacapone: effects on motor function and quality of life in patients with Parkinson’s disease with end-of-dose wearing off. Int J Neurosci. 2011;121:605-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Tarrants ML, Denarié MF, Castelli-Haley J, Millard J, Zhang D. Drug therapies for Parkinson’s disease: A database analysis of patient compliance and persistence. Am J Geriatr Pharmacother. 2010;8:374-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Yang N, Xue H, Guo H, Chen X, Zhu JX. Segmental heterogeneity of epithelial ion transport induced by stimulants in rat distal colon. Biol Pharm Bull. 2006;29:1825-1829. [PubMed] [Cited in This Article: ] |
| 26. | Feng XY, Li Y, Li LS, Li XF, Zheng LF, Zhang XL, Fan RF, Song J, Hong F, Zhang Y. Dopamine D1 receptors mediate dopamine-induced duodenal epithelial ion transport in rats. Transl Res. 2013;161:486-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Thibodeau PH, Butterworth MB. Proteases, cystic fibrosis and the epithelial sodium channel (ENaC). Cell Tissue Res. 2013;351:309-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | O’Hara JR, Ho W, Linden DR, Mawe GM, Sharkey KA. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G998-1007. [PubMed] [Cited in This Article: ] |
| 29. | Moskwa A, Boznańska P. [Role of serotonin in the pathophysiology of the irritable bowel syndrome]. Wiad Lek. 2007;60:371-376. [PubMed] [Cited in This Article: ] |
| 30. | Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245-289. [PubMed] [Cited in This Article: ] |