Published online Mar 14, 2015. doi: 10.3748/wjg.v21.i10.3132
Peer-review started: July 24, 2014
First decision: September 15, 2014
Revised: October 26, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: March 14, 2015
We herein present a case involving a 41-year-old woman in whom ultrasound examination revealed multiple liver hemangiomas more than 3 years ago. Follow-up ultrasound examination revealed that the masses had significantly increased; the largest was located in the right lobe (about 8.2 cm × 7.4 cm × 6.0 cm). Abdominal multidetector computed tomography revealed multiple well-circumscribed, heterogeneous, hypodense masses (largest, 6.4 cm × 6.3 cm × 5.0 cm) with significant contrast enhancement during the arterial and portal phases and with contrast wash-out and peripheral enhancement during the delayed phases. Magnetic resonance images demonstrated multiple well-circumscribed, heterogeneous, hypointense hepatic masses with significant contrast enhancement (largest, 6.4 cm × 6.5 cm × 5.1 cm); multiple enlarged porta lymph nodes; and multiple slightly enlarged retroperitoneal lymph nodes. Histological and immunohistochemical examination of the right mass biopsy specimen suggested a malignant neoplasm that had originated from a neuroendocrine cell type (grade 2 well-differentiated neuroendocrine carcinoma). After performing a systemic examination to exclude metastasis from an extrahepatic primary site, we considered that the masses had arisen from a primary hepatic neuroendocrine tumor with multiple liver metastases. The patient underwent transcatheter arterial chemoembolization using a combination of oxaliplatin (150 mg) mixed with one bottle of gelatin sponge particles (560-710 μm) and lipiodol (6 mL). Primary neuroendocrine tumors of the liver are extremely rare. This case is interesting because of the rarity of this neoplasm and previous misdiagnosis as multiple liver hemangiomas. Previously reported cases in the literature are also reviewed.
Core tip: Whereas more than 80% of the neuroendocrine tumors (NETs) found in the liver are metastatic, primary hepatic neuroendocrine tumors (PHNETs) are very rare, when a NET is found in the liver, it must be treated with great care to exclude metastasis from extrahepatic primary site, as that is a much more common occurrence. Only fewer than 100 cases of PHNETs have been reported in the English literatures and most was a case report. we report a case of PHNET with multiple liver metastases have focused on multiple imaging modalities, including computed tomography, magnetic resonance imaging, and digital subtraction angiography.
- Citation: Yang K, Cheng YS, Yang JJ, Jiang X, Guo JX. Primary hepatic neuroendocrine tumor with multiple liver metastases: A case report with review of the literature. World J Gastroenterol 2015; 21(10): 3132-3138
- URL: https://www.wjgnet.com/1007-9327/full/v21/i10/3132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i10.3132
Neuroendocrine tumors (NETs), not only behave like benign tumors and also they exhibit characteristics of carcinomas. Historically NETs known as carcinoid tumors or carcinoma-like tumors. These tumors develop secondary to neoplastic proliferation of neuroendocrine cells. Although the frequency of NETs has increased in recent years, they see only 2 of 100000 people on average. These tumors mainly arise in organs of the bronchopulmonary or gastrointestinal tract such as the pancreas, ileum, or appendix, but can occur in almost any organ including the bladder, prostate, rectum, stomach, bronchi, and biliary tree. NETs exhibit a varied malignant potential depending on their site of origin[1]. While more than 80% of the NETs found in the liver are metastatic, primary hepatic NETs (PHNETs) are very rare. When an NET is found in the liver, extrahepatic metastatic tumors must first be determined[2]. In 1958 Edmondson first reported a case of PHNET[3]. Since then, fewer than 60 cases of PHNET have been reported in the English-language literature[4], and these reports have mainly focused on computed tomography (CT) findings. While most are generally asymptomatic, the most frequent symptoms of PHNET are pain and a palpable mass. To the best of our knowledge, no reports of PHNET with multiple liver metastases have focused on magnetic resonance (MR) imaging using hepatobiliary-specific contrast agent or have described a misdiagnosis of multiple liver hemangiomas.
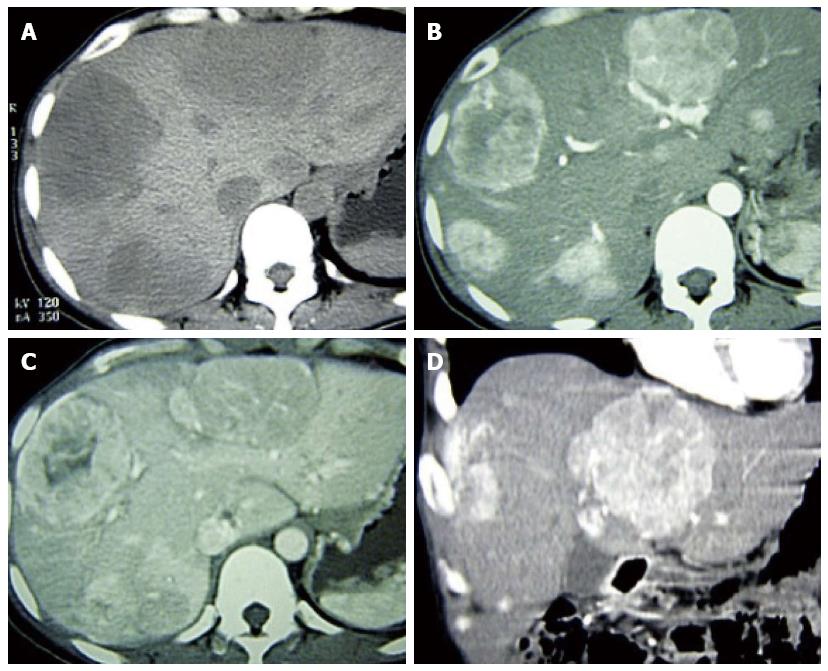
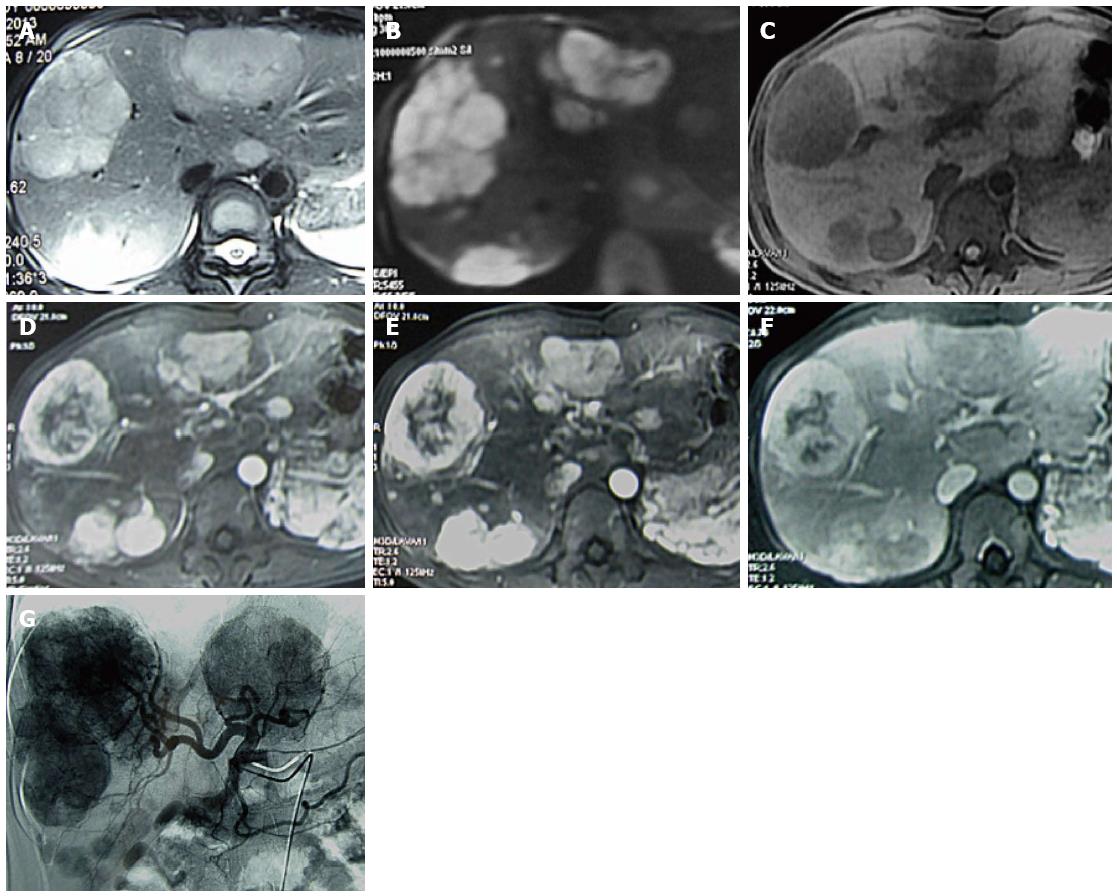
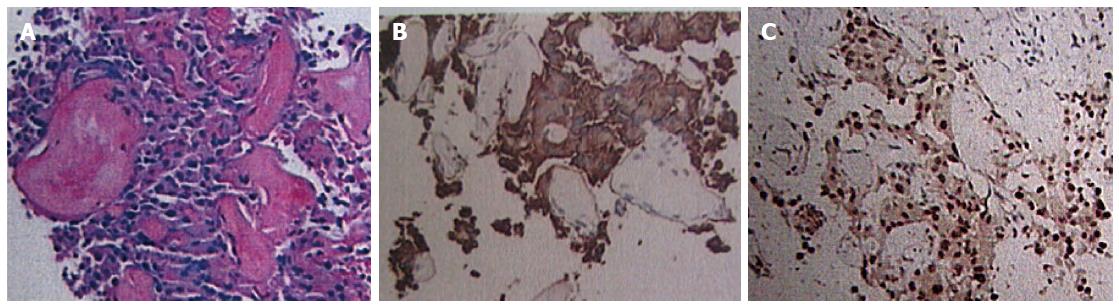
A 41-year-old woman underwent ultrasound examination more than 3 years ago. At that time, multiple solid masses were found in the right lobe; the largest measured about 2.2 cm × 2.0 cm × 1.7 cm. The masses were considered to be multiple hepatic hemangiomas. Two years later (1 year prior to the present report), follow-up ultrasound examination showed results similar to the first ultrasound; the largest mass measured about 2.8 cm × 2.5 cm × 2.1 cm. In April 2013, the patient reported right hypochondrial pain secondary to “stomach treatment”; this pain lessened slightly thereafter. In August 2013, another ultrasound examination revealed multiple solid liver masses in the right and left lobes (the largest was present in the right lobe and measured 8.2 cm × 7.4 cm × 6.0 cm). The intrahepatic lesions had significantly increased in size and were considered to be vascular tumors. Abdominal multidetector CT revealed multiple well-circumscribed, heterogeneous, and hypodense masses; the largest was present in the right lobe and measured 6.4 cm × 6.3 cm × 5.0 cm. The masses exhibited significant contrast enhancement during the arterial and portal phases and exhibited contrast washout and peripheral enhancement during the delayed phases (Figure 1). The liver background was not cirrhotic. MR images (Figure 2A-F) were obtained with a 3.0-T unit using a liver-specific contrast agent. On T2-weighted fast spin-echo MR images (TR/TE, 3400/100) and diffusion images (b = 800), the masses mainly showed high signal intensity that correlated with the normal liver parenchyma with hyperintense foci. On T1-weighted gradient echo images (TR/TE, 3.6/1.4), the masses were well-circumscribed, heterogeneous, and hypointense. The largest was present in the right lobe and measured 6.4 cm × 6.5 cm × 5.1 cm. Gadoxetic acid-enhanced T1-weighted MR images demonstrated significant enhancement of the solid tumor portion in the early arterial phase, continued enhancement in the portal venous phase, and a definite defect in the 20-min delayed hepatobiliary phase; multiple enlarged porta lymph nodes and multiple slightly enlarged retroperitoneal lymph nodes were also detected. The patient showed no clinical symptoms, such as nausea, vomiting, flushing, fever, diarrhea, constipation, or abdominal pain. No abnormal findings within physical examination. The laboratory evaluations, including liver tests and blood levels of tumor markers such as alpha-fetoprotein (AFP) (11.04 ng/mL; normal, < 7 ng/mL), carbohydrate antigen 19-9 (CA 19-9) (42.5 U/mL; normal, < 34 U/mL), neuron-specific enolase (NSE) (19.25 μg/L; < 17 μg/L), and others [i.e., CA-724 and carcinoembryonic antigen (CEA)] were negative. Hepatitis indices were negative, and liver and renal functions were normal. The patient underwent CT-guided biopsy of liver masses, and histological and immunohistochemical examinations suggested vasogenic tumors. The definitive diagnosis was multiple cavernous hemangiomas. She underwent transcatheter arterial chemoembolization (TACE) with a combination of bleomycin (16 mL) mixed with lipiodol (6 mL). For the treatment again, six weeks later, the patient was rehospitalized with no clinical symptoms and no abnormal findings. The levels of tumor markers including AFP (9.53 ng/mL), CA-19-9 (63.97 U/mL), NSE (21.55 μg/L), and others (i.e., CA-724 and CEA) were negative. Liver and renal functions were normal. MR images showed that the size of the largest mass was similar to that shown by the previous MR examination and that the intrahepatic lesions were slightly larger. We suspected the results of pathological and immunohistochemical examinations and thus decided to perform another ultrasound-guided biopsy of the hepatic masses. Histological and immunohistochemical examinations (Figure 3) demonstrated positive staining for the following markers within tumor cells: cell adhesion molecule, synaptophysin (Syn), pancreatic and duodenal homeobox 1 (PDX1), cytokeratin 8, cluster of differentiation 56, and beta-tubulin. The Ki-67 index was 5%. These findings suggested a malignant neoplasm that originated from a neuroendocrine cell type (grade 2 well-differentiated neuroendocrine carcinoma). After performing a systemic examination and follow-up of 14 mo to exclude metastasis from an extrahepatic primary site, we diagnosed a PHNET with multiple liver metastases. The patient underwent TACE (Figure 2G) with a combination of oxaliplatin (150 mg) mixed with one bottle of gelatin sponge particles (560-710 μm) and lipiodol (6 mL).
Neuroendocrine tumors mainly arise in organs of the bronchopulmonary or gastrointestinal systems. NETs are rarely seen and have an incidence of only 1% to 2% among all gastrointestinal tumors[4]. The most common occurrence site of NETs is small intestine (45%); NETs are seen less often in the rectum (20%), appendix (17%), colon (11%), and stomach (7%)[4]. Those tumors are classified according to their embryological origin and morphological pattern. The WHO found that it is more suitable to use the term NET instead of carcinoid tumor. Thus, the ongoing confusion has been partially reduced; however, neuroendocrine tumor and carcinoid tumor still appear to be used in the literature[5]. Our group is more appropriate to use the term NET for these tumor. The WHO updated the classification system in 2010, differentiating between the terms NET and neuroendocrine carcinoma. Proliferation indices (Ki-67 and MIB-1), angioinvasion, and mitoses are important factors. NETs are divided into three main categories based on the malignant potential of the tumor[6]: well-differentiated endocrine tumor (< 2 cm in size and Ki-67 index of < 2%, well-differentiated endocrine carcinoma (> 2 cm in size, Ki-67 index of > 2%, or presence of angioinvasion), and poorly differentiated endocrine carcinoma (Ki-67 index of > 20%).
NETs accounted for 1%-2% of all gastrointestinal tumors, and liver is the main metastasis organ. Nevertheless, PHNETs are much more rarely seen than other NETs. A diagnosis of PHNET must be excluded from extrahepatic metastasis. Currently distinguish between primary and metastatic NETs is still a thorny problem. Occurrence age of PHNET mainly in 40-50 years, although it may occur at any time. Although it does not show sex preference, but females more often develop the disease. The tumors are usually located in right liver lobe. Clinically asymptomatic patients with PHNET, the tumor may be found incidentally during the abdominal imaging. For few patients, right upper quadrant palpable mass and abdominal pain are the most common symptoms, may be associated with carcinoid syndrome symptoms[7]. This syndrome occurs in less than 10% of patients with gastrointestinal NETs; When this syndrome is found, it is always associated with hepatic metastasis, although the incidence of this syndrome in the gastrointestinal tract in less than 10%. But interestingly, in patients with PHNETs it is quite rarely seen[8].
The origin of PHNETs remains unclear. Currently there are three hypotheses been suggested. Firstly, the neuroendocrine cells scattered among the intrahepatic biliary tract and the epithelium manifest malignant transformation. Secondly, these tumors originate from adrenal tissue settling in the liver or heterotrophic pancreas tissue. Thirdly, malignant stem cells underwent neuroendocrine differentiation[9]. PHNETs are rarely seen and slow-growing tumors. If they has not yet resulted carcinoid syndrome, early diagnosis is difficult. When diagnosis they may be very large, because they progress asymptomatically. Nonetheless, several techniques are available to facilitate the diagnosis despite the fact that the carcinoid symptom is nonspecific in the early period.
Traditionally, diagnosis of NETs is based on the measurement of 5-hydroxyindoleacetic acid (5-HIAA), which is the inactive metabolite of serotonin in 24-h urine specimens. 5-HIAA, require the tumor secretes serotonin, cannot be measured in tumors when they do not show endocrine function, lowering the test sensitivity. 5-HIAA in 24-h urine specimens may be performed with high specificity (90%) and low sensitivity (73%), so it is still used[10]. Measurement of this metabolite in 24-h urine specimens is important because of the fluctuations in serotonin during 24-h.
Serum analysis of chromogranin A (CgA) is the most specific marker for NETs because of it secreted by neuroendocrine cells. Whereas the specificity of the serum CgA level ranges from 84% to 95%, the sensitivity ranges from 87% to 100%[11]. Moreover, unlike the measurement of 5-HIAA, the serum CgA level can be used to diagnose both tumors secreting serotonin and atypical or nonsecreting tumors. However, CgA measurement may obtain false-positive results in patients with hepatic and renal failure, atrophic gastritis, or chronic proton pump inhibitor use[12]. CgA also can be used for monitoring tumor recurrence. At present, the tumor markers CEA, CA 19-9, and AFP are nonspecific in PHNETs. Because a diagnosis of NET was not initially considered in the case, the urine 5-HIAA level and serum CgA level were not measurement in the preoperative. Tumor markers were negative in this case. The AFP, CA 19-9, and NSE levels were 9.53 ng/mL, 63.97 U/mL, and 21.55 μg/L, respectively.
Imaging findings of PHNETs often confused with other liver tumors. Ultrasound, multidetector CT (MDCT), and MR imaging on PHNETs often had low sensitivity[13]. Even so, MDCT is the most frequent use of radiological technique to show the localization and the prevalence of the disease[14]. Abdominal ultrasound, abdominopelvic MDCT and MRI examinations were carried out in the case. On the basis of those imagings, the lesions were misdiagnosed as liver hemangioma.
Although the role of positron emission tomography CT in the diagnosis of NETs is not clear, but the practice found that PHNETs usually reflect high update of 18F-fluorodeoxyglucose. Furthermore, the sensitivity and specificity of positron emission tomography CT may be increased in using this technology[15]. Scintigraphy is an imaging technique that provides both diagnostic and therapeutic information in patients with NETs. Octreotide, a somatostatin receptor analogue, was used in somatostatin receptor scintigraphy (OctreoScan) for this purpose and was more valuable than other techniques in detected the tumor. It had a sensitivity ranging from 85% to 90%[16]. In addition to identifying the locate of primary or recurrent, another benefit of the OctreoScan is the ability to predict the tumor’s response to the octreotide analogue therapy[17].
Histopathological examination is the most accurate diagnosis method. Used in routine pathological examination of hematoxylin-eosin staining method is not specific for NETs but only beneficial to tumor classification. NSE, CgA, and Syn are highly sensitive immunohistochemical markers used in the diagnosis of PHNETs[18]. In the case, Syn and PDX1 were the main markers in immunohistochemical examination.
PHNETs are rare and the best treatment is unclear. However, a multidisciplinary collaborative treatment is essential in the management. At present, surgery is the only approach that can provide a complete cure[2]. In one study, the 5-year recurrence and survival rates after complete resection were reportedly 18% and 74% to 78%, respectively[19]. Recent studies have shown that although debulking and removal of the tumor and its metastases is not completely curing unresectable tumors or metastatic disease, relative to the palliative effect, to extend the survival period was clinically meaningful[20].
The effect of chemotherapy for PHNETs is still unknown. Cytotoxic drugs can be used to treat tumors with a high proliferation index. However, cytotoxic drugs valid data is limited. Therefore, administration of a combination of agents such as 5-fluorouracil, doxorubicin, and streptozocin is the preferred treatment method[21,22]. Transcatheter arterial chemoembolization (TACE) is one of the most commonly used method in the management of patients with extrahepatic NET intrahepatic metastasis. TACE treatment of patients with PHNET has only been described in few case reports. In one study, TACE was performed to treat of 20 patients with hepatic metastases, and the radiological response and symptom improvement rates were 90%[23]. In the case, TACE was performed with a combination of oxaliplatin mixed with one bottle of gelatin sponge particles and lipiodol to reduce tumor bleeding and prolong survival.
Another technique used for the diagnosis and treatment of NETs is the administration of somatostatin analogues. Somatostatin regulates intracellular functions and shows its effect by connecting suddenly to somatostatin receptors 1 to 5. The results show that the effect on treatment was only through connection with somatostatin receptor 2. Two somatostatin analogues (octreotide, which has short-term effects, and lanreotide, which has long-term effects) are currently used for this purpose. Although this treatment effectively controls the symptoms of carcinoid syndrome, it is largely ineffective for tumor recession as shown radiologically[23]. Its therapeutic effect of PHNET remains unclear because of the lack of available data.
The role of liver transplantation for the treatment of PHNET is controversial. Some researchers have shown that the efficacy and survival rate of patients, with multiple liver tumors and poor liver function, who performed liver transplantation is higher than those who undergoing liver resection.
Radiofrequency ablation (RFA) is another treatment method for PHNETs[24]. The introduction of RFA has allowed physicians to surgically address a larger population of patients with curative intent. RFA may be performed alone or in combination with resection. To date, most reports on RFA management are single-institution retrospective series. Indications for RFA are the presence of three or fewer tumors and a tumor diameter of ≤ 5 cm. Tumors located near the major branches of the portal and hepatic veins have a higher potential for incomplete ablation[25]. These factors have limited the clinical application of RFA.
In conclusion, PHNETs are very rare and asymptomatic tumors. Distinguishing it from other liver tumors is quite difficult in medical imaging. Thus, highly sensitive laboratory and integrated imaging are required. However, a PHNETs should always be considered is detected in the liver when it is a solitary hypervascular tumor. Pathological and immunohistochemical are the most important method for clinical diagnosis. The main treatment methods that can be attempted for PHNETs with multiple metastases are hepatectomy, chemotherapy, TACE, somatostatin analogue administration, RFA, and liver transplantation. The optimal treatment method should be based on a comprehensive assessment of the patient’s condition.
The female patient presented with asymptomatic tumors, found multiple hypervascular tumors in live and alpha-fetoprotein/neuron-specific enolase [AFP (-)/NSE (+)], enhanced mode of computed tomography (CT) and magnetic resonance imaging (MRI) is “fast and slow out” and multi-nodular lesions on MRI.
The female patient had no obvious clinical symptoms and only right hypochondrial pain.
Benign neoplasms (Hepatic hemangioma), Malignant tumors (Hepatocellular carcinoma)
Serum analysis of AFP (11.04 ng/mL; normal, < 7 ng/mL), carbohydrate antigen 19-9 (42.5 U/mL; normal, < 34 U/mL), NSE (19.25 μg/L; < 17 µg/L), and others (i.e., CA-724 and carcinoembryonic antigen) were negative.
The enhanced mode of CT and MRI is “fast and slow out” and multi-nodular lesions on MRI.
Histological and immunohistochemical examinations demonstrated positive staining for the following markers within tumor cells: cell adhesion molecule, synaptophysin, pancreatic and duodenal homeobox 1 (PDX1), cytokeratin 8, cluster of differentiation 56, and beta-tubulin. The Ki-67 index was 5%.
The patient underwent transcatheter arterial chemoembolization (TACE) with a combination of oxaliplatin (150 mg) mixed with one bottle of gelatin sponge particles (560-710 μm) and lipiodol (6 mL).
Primary hepatic NETs (PHNETs) are very rare and asymptomatic tumors. Distinguishing these tumors radiologically from other liver tumors is quite difficult. PHNET should always be considered when a solitary hypervascular tumor is detected in the liver. Certain diagnoses can still be confirmed by pathological and immunohistochemical investigations. The best treatment method is unclear.
Neuroendocrine tumors (NETs), also known as carcinoid tumors, behave like benign tumors. NETs constitute 1%-2% of all gastrointestinal tumors, and they frequently metastasize to the liver. Whereas more than 80% of the NETs found in the liver are metastatic, primary hepatic NETs (PHNETs) are very rare.
This case report presents the clinical characteristics of PHNET and also discusses the treatment of PHNET. PHNET are very rare and asymptomatic tumors and it is quite difficult to distinguish these tumors radiologically from should be kept in mind; certain diagnosis can still be confirmed by pathological and immunohistochemical investigations when found hypervascular tumor of live and AFP (-)/NSE (+).
The authors have described a case of PHNET included imaging diagnosis, pathology diagnostic, immunohistochemical results and therapeutic method. The article highlights the imaging and immunohistochemical characteristics of this tumor and provides new therapeutic method.
P- Reviewer: Koutsampasopoulos K S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Scarsbrook AF, Ganeshan A, Statham J, Thakker RV, Weaver A, Talbot D, Boardman P, Bradley KM, Gleeson FV, Phillips RR. Anatomic and functional imaging of metastatic carcinoid tumors. Radiographics. 2007;27:455-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Iwao M, Nakamuta M, Enjoji M, Kubo H, Fukutomi T, Tanabe Y, Nishi H, Taguchi KI, Kotoh K, Nawata H. Primary hepatic carcinoid tumor: case report and review of 53 cases. Med Sci Monit. 2001;7:746-750. [PubMed] [Cited in This Article: ] |
| 3. | Kehagias D, Moulopoulos L, Smirniotis V, Pafiti A, Ispanopoulos S, Vlahos L. Imaging findings in primary carcinoid tumour of the liver with gastrin production. Br J Radiol. 1999;72:207-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Touloumis Z, Delis SG, Triantopoulou C, Giannakou N, Avgerinos C, Dervenis C. Primary hepatic carcinoid; a diagnostic dilemma: a case report. Cases J. 2008;1:314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Oberg K, Astrup L, Eriksson B, Falkmer SE, Falkmer UG, Gustafsen J, Haglund C, Knigge U, Vatn MH, Välimäki M. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part I-general overview. Acta Oncol. 2004;43:617-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Lüttges J. [What’s new? The 2010 WHO classification for tumours of the pancreas]. Pathologe. 2011;32 Suppl 2:332-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Donadon M, Torzilli G, Palmisano A, Del Fabbro D, Panizzo V, Maggioni M, Santambrogio R, Montorsi M. Liver resection for primary hepatic neuroendocrine tumours: report of three cases and review of the literature. Eur J Surg Oncol. 2006;32:325-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Mehta DC, Warner RR, Parnes I, Weiss M. An 18-year follow-up of primary hepatic carcinoid with carcinoid syndrome. J Clin Gastroenterol. 1996;23:60-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 9. | Kaya G, Pasche C, Osterheld MC, Chaubert P, Fontolliet C. Primary neuroendocrine carcinoma of the liver: an autopsy case. Pathol Int. 2001;51:874-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Lamberts SW, Hofland LJ, Nobels FR. Neuroendocrine tumor markers. Front Neuroendocrinol. 2001;22:309-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Campana D, Nori F, Piscitelli L, Morselli-Labate AM, Pezzilli R, Corinaldesi R, Tomassetti P. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007;25:1967-1973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62 Suppl 1:33-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | van der Hoef M, Crook DW, Marincek B, Weishaupt D. Primary neuroendocrine tumors of the liver: MRI features in two cases. Abdom Imaging. 2004;29:77-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Sippel RS, Chen H. Carcinoid tumors. Surg Oncol Clin N Am. 2006;15:463-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Orlefors H, Sundin A, Garske U, Juhlin C, Oberg K, Skogseid B, Langstrom B, Bergstrom M, Eriksson B. Whole-body (11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J Clin Endocrinol Metab. 2005;90:3392-3400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Kwekkeboom DJ, Krenning EP. Somatostatin receptor imaging. Semin Nucl Med. 2002;32:84-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Tamm EP, Kim EE, Ng CS. Imaging of neuroendocrine tumors. Hematol Oncol Clin North Am. 2007;21:409-432; vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Zhang LH, Chu Q, Huang ZY. Diagnostic effect of NSE, CgA, SYP in neuroendocrine carcinoma. Shanghai Med J. 1997;20:660-661. [Cited in This Article: ] |
| 19. | Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Woodside KJ, Townsend CM, Mark Evers B. Current management of gastrointestinal carcinoid tumors. J Gastrointest Surg. 2004;8:742-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Maroun J, Kocha W, Kvols L, Bjarnason G, Chen E, Germond C, Hanna S, Poitras P, Rayson D, Reid R. Guidelines for the diagnosis and management of carcinoid tumours. Part 1: the gastrointestinal tract. A statement from a Canadian National Carcinoid Expert Group. Curr Oncol. 2006;13:67-76. [PubMed] [Cited in This Article: ] |
| 22. | Kvols LK, Moertel CG, O’Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 548] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Yao KA, Talamonti MS, Nemcek A, Angelos P, Chrisman H, Skarda J, Benson AB, Rao S, Joehl RJ. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery. 2001;130:677-682; discussion 682-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 146] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Gamblin TC, Christians K, Pappas SG. Radiofrequency ablation of neuroendocrine hepatic metastasis. Surg Oncol Clin N Am. 2011;20:273-279, vii-viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Boonsirikamchai P, Loyer EM, Choi H, Charnsangavej C. Planning and follow-up after ablation of hepatic tumors: imaging evaluation. Surg Oncol Clin N Am. 2011;20:301-15, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |