Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.369
Peer-review started: April 25, 2014
First decision: May 29, 2014
Revised: June 19, 2014
Accepted: July 15, 2014
Article in press: July 16, 2014
Published online: January 7, 2015
A 79-year-old male was admitted to our hospital for the treatment of cancer of the gastric tube. Gastrointestinal examination revealed a T1b Union for International Cancer Control (UICC) tumor at the pyloric region of the gastric tube. Laparotomy did not reveal infiltration into the serosa, peritoneal dissemination, regional lymph node swelling, or distant metastasis. We performed a distal gastrectomy preserving the right gastroepiploic artery by referencing the preoperative three-dimensional computed tomoangiography. We also evaluated the blood flow of the right gastroepiploic artery and in the proximal gastric tube by using indocyanine green fluorescence imaging intra-operatively and then followed with a gastrojejunal anastomosis with Roux-en-Y reconstruction. The definitive diagnosis was moderately differentiated adenocarcinoma of the gastric tube, pT1bN0M0, pStage IA (UICC). His postoperative course was uneventful. Three-dimensional computed tomographic imaging is effective for assessing the course of blood vessels and the relationship with the surrounding structures. Intraoperative evaluation of blood flow of the right gastroepiploic artery and of the gastric tube in the anastomotic portion is very valuable information and could contribute to a safe gastrointestinal reconstruction.
Core tip: A report of a 79-year-old male who presented with a metachronous gastric tube cancer following an esophageal squamous cell carcinoma. It is necessary for less invasive surgery as the resection of the distal part of the tube to prevent right gastroepiploic artery. Curative resection of the distal part of the gastric tube was performed while safely preserving the right gastroepiploic artery by referencing the preoperative three-dimensional computed tomoangiography. The intraoperative evaluation of blood flow by using indocyanine green fluorescence imaging contributed critical information about the blood flow of right gastroepiploic artery and gastric tube; we then followed with the Roux-en-Y reconstruction.
- Citation: Nakano T, Sakurai T, Maruyama S, Ozawa Y, Kamei T, Miyata G, Ohuchi N. Indocyanine green fluorescence and three-dimensional imaging of right gastroepiploic artery in gastric tube cancer. World J Gastroenterol 2015; 21(1): 369-372
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/369.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.369
Long-term survival after esophagectomy has potentially increased the likelihood of metachronous malignancies. Secondary cancer in the reconstructed gastric tube after esophagectomy also should be considered and annual follow-up endoscopy can be useful in the detection of curable cancers[1]. It is important that surgical strategies are decided according to both physical and cancerous conditions[2]. Because the surgical invasion of a total gastric tube resection procedure for gastric tube cancer may be too intensive, minimally invasive therapy such as partial resection of the gastric tube may be desirable for the cancer that has not advanced. The reconstructed gastric tube is dominantly fed by the right gastroepiploic artery. It is necessary to preserve this artery to avoid ischemia of the gastric tube. There are few reports mention about the three dimensional computed tomoangiography and indocyanine green fluorescence for surgical navigation during the treatment for the gastric tube cancer. Here we report a case of distal gastrectomy with the safe preservation of the right gastroepiploic artery after using the preoperative three-dimensional CT for the confirmation of blood flow of the vessel and in the proximal gastric tube.
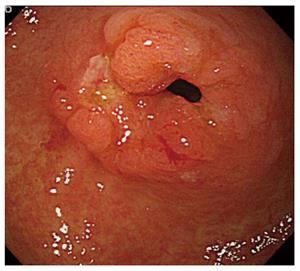
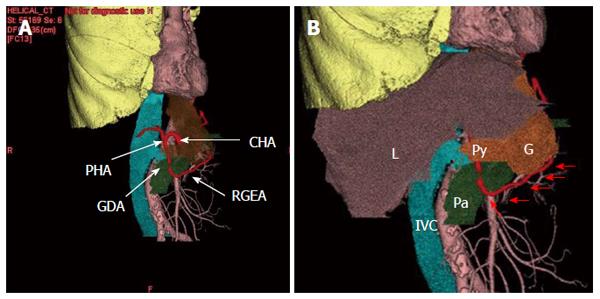
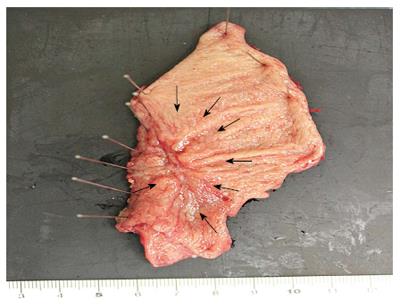
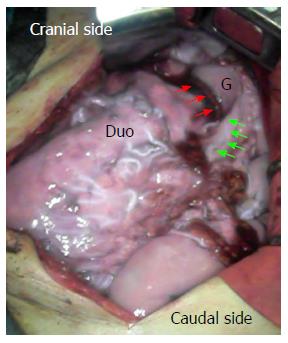
A 79-year-old male was admitted to our hospital for the treatment of cancer of the gastric tube after radical surgery for esophageal cancer. Five years previously, he had undergone subtotal esophagectomy for the treatment of esophageal squamous cell carcinoma followed by lifting the gastric tube through the posterior mediastinal route. The esophageal tumor stage was pT1pN1cM0 [Union for International Cancer Control (UICC)] and there was no adjuvant therapy. At a routine annual endoscopy, a superficial slightly depressed type tumor was found in a prepyloric lesion of the gastric tube. On admission, gastrointestinal examination revealed a cT1b tumor (UICC) at the pyloric region of the gastric tube that was very close to the pylorus ring; this was diagnosed as adenocarcinoma by endoscopic biopsy (Figure 1). Contrast-enhanced computed tomography of the abdomen and chest revealed no regional or distant signs of tumor spread, and enlarged regional lymph nodes; furthermore, there was no evidence of recurrence of esophageal cancer. Three-dimensional computed tomography (3D-CT) visualized the location of the course of the right gastroepiploic artery (Figure 2A) and relationship with pyloric region of gastric tube and neighboring organs (Figure 2B). The patient underwent transabdominal surgery. Laparotomy did not reveal infiltration into the serosa, peritoneal dissemination, lymph node swelling, or distant metastasis. We performed a distal gastrectomy (resection of the distal part of the gastric tube) safely preserving the right gastroepiploic artery with reference to the preoperative 3D-CT. The resected specimen showed a slightly depressed-type tumor, measuring 20 mm × 15 mm in size (Figure 3). Rapid histological diagnosis during surgery showed free-form cancer invasion of the proximal and distal surgical margins. We intraoperatively evaluated the blood flow along the gastric tube by using indocyanine green (ICG) fluorescence imaging (Figure 4). Intravenous ICG injection revealed good blood flow at the surgical margins of the proximal gastric tube and of the right gastroepiploic artery. We performed a gastrojejunal anastomosis with Roux-en-Y reconstruction. Histological examination showed modelately differentiated tubular adenocarcinoma. The resected gastric tube included small amount of adipose tissue containing no lymphoid tissue histologically. The definitive diagnosis was moderately differentiated adenocarcinoma of the gastric tube, pT1bN0M0, pStage IA (UICC). His postoperative course was uneventful. Postoperative upper gastrointestinal investigation did not reveal stenosis or leakage. The patient started a diet on post-operative day 3 and left the hospital on post-operative day 13.
In recent years, there has been an improved prognosis for esophageal cancer surgery patients[3]; therefore, there is a need to be aware of risk of metachronous cancers. Motoyama et al[1] has reported that 3.5% of the patients treated for esophageal cancer and reconstructed using a gastric tube were found to have carcinoma of the gastric tube.
Although we evaluated the invasion depth by upper gastrointestinal endoscopy, it was reported that endoscopic ultrasonography was useful in diagnosis of invasion depth and lymph node metastasis of esophageal cancer or stomach cancer to determine the clinical stage[4]. Early stage gastric tube cancer can be managed with endoscopic treatment. Endoscopic submucosal dissection for gastric tube cancer was feasible and effective for curative patients[5]. These patients who do not become the adaptation of ESD could be candidates for resection of the gastric tube. Total or partial gastrectomy for gastric tube cancer is a complex procedure and carries a high morbidity and mortality rate; it should still be considered as the treatment of choice in multifocal or locally advanced cases[6]. Although resection of the gastric tube with lymphadenectomy is standard and a reliable treatment for gastric tube cancer, resection of the tube through the mediastinal route exposes the patient to a great deal of surgical stress. The difficulty of the operative procedure differs according to the route of the reconstruction[7]. There have been reports about the significant surgical risks in total gastric tube resections because of the difficulty of the adhesiolysis necessary to reach the mediastinum[1,8,9]. Therapeutic strategies for gastric tube cancer should be determined depending on the curative effect and surgical stress. Reduction surgery such as partial resection of gastric tube or the omission of lymph node dissection should also be taken into account according to the patient status[10].
As a less invasive procedure, distal gastrectomy could be appropriate in some cases, in which the right gastroepiploic artery was preserved to maintain the blood supply for the proximal gastric tube[2,11]. The reconstructed gastric tube is dominantly fed by the right gastroepiploic artery. Knowledge of the vascular anatomy before surgery is of great importance in patients with previous surgery[12]. The 3D-computed tomographic image is very effective for assessing the blood vessel course and relationship with the surrounding structure while obtaining surgical navigation[13-15]. It has been reported that the ICG fluorescence method was useful in evaluating the blood flow of reconstructed gastric tube intraoperatively followed by esophageal resection[16]. Intravenous ICG injection for fluorescence imaging is also useful to verify the blood flow of the proximal gastric tube[10]. Intraoperative evaluation of the blood flow of the right gastroepiploic artery and of the gastric tube in the anastomotic portion is very valuable information and could contribute to a safe gastrointestinal reconstruction.
A 79-year-old male who presented with a metachronous gastric tube cancer, following an esophageal carcinoma after radical esophagectomy followed with reconstruction through mediastinal route.
Gastrointestinal examination revealed a superficial tumor at the pyloric region of the gastric tube that was very close to the pylorus ring.
Gastric tube ulcer, recurrence tumor derived from resected esophageal cancer.
WBC 3.7 k/μL; HGB 13.6 gm/dL; CEA 1.5 ng/mL; SCC 0.9 ng/mL; metabolic panel and liver function test were within normal limits.
Computed tomography scan of the abdomen and chest revealed no regional or distant signs of tumor spread, and enlarged regional lymph nodes.
The endoscopic biopsy specimen showed an adenocarcinoma of the gastric tube.
Distal gastrectomy preserving the right gastroepiploic artery was performed, and then followed with a gastrojejunal anastomosis with Roux-en-Y reconstruction.
A less invasive surgery such as the resection of the distal part of the gastric tube is essential to prevent right gastroepiploic artery.
Indocyanine green (ICG) bound to plasma proteins is visualized as a fluorescent image excited by near-infrared light.
The three-dimensional computed tomography is very effective for assessing the blood vessel course and intraoperative blood vessel detection and blood flow evaluation based on ICG would allow minimizing the risk of vascular damage, especially about the non-anatomical.
A distal gastrectomy was performed for the treatment of the gastric tube cancer, preserving the right gastroepiploic artery by referencing the preoperative three-dimensional computed tomoangiography. The blood flow of the right gastroepiploic artery was also evaluated by using indocyanine green fluorescence imaging intra-operatively.
P- Reviewer: Marrelli D S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Motoyama S, Saito R, Kitamura M, Suzuki H, Nakamura M, Okuyama M, Imano H, Inoue Y, Ogawa J. Prospective endoscopic follow-up results of reconstructed gastric tube. Hepatogastroenterology. 2003;50:666-669. [PubMed] [Cited in This Article: ] |
| 2. | Yoshida T, Nagahama T, Maruyama M, Ebuchi M. Endoscopic comparison of two cases: distal resection of reconstructed gastric tube. Hepatogastroenterology. 2002;49:371-374. [PubMed] [Cited in This Article: ] |
| 3. | Bamba T, Kosugi S, Takeuchi M, Kobayashi M, Kanda T, Matsuki A, Hatakeyama K. Surveillance and treatment for second primary cancer in the gastric tube after radical esophagectomy. Surg Endosc. 2010;24:1310-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Nonaka S, Oda I, Sato C, Abe S, Suzuki H, Yoshinaga S, Hokamura N, Igaki H, Tachimori Y, Taniguchi H. Endoscopic submucosal dissection for gastric tube cancer after esophagectomy. Gastrointest Endosc. 2014;79:260-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Ben-nun A, Soudack M, Best LA. Gastric tube gastrectomy. Dis Esophagus. 2000;13:243-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Koyanagi K, Ozawa S, Ando N, Shih CH, Nakamura E, Takeuchi H, Hayashi K, Kitajima M. Case report: Metachronous early gastric carcinoma in a reconstructed gastric tube after radical operation for oesophageal carcinoma. J Gastroenterol Hepatol. 1998;13:311-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Akita H, Doki Y, Ishikawa O, Takachi K, Miyashiro I, Sasaki Y, Ohigashi H, Murata K, Noura S, Yamada T. Total removal of the posterior mediastinal gastric conduit due to gastric cancer after esophagectomy. J Surg Oncol. 2004;85:204-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Okamoto N, Ozawa S, Kitagawa Y, Shimizu Y, Kitajima M. Metachronous gastric carcinoma from a gastric tube after radical surgery for esophageal carcinoma. Ann Thorac Surg. 2004;77:1189-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Ariyoshi Y, Fujiwara H, Shiozaki A, Konishi H, Komatsu S, Kubota T, Ichikawa D, Okamoto K, Morimura R, Murayama Y. [Minimally invasive surgery for cancer arising in a reconstructed gastric tube after esophagectomy based on evaluation of blood and lymphatic flow by indocyanine green fluorescence imaging]. Gan To Kagaku Ryoho. 2013;40:2170-2172. [PubMed] [Cited in This Article: ] |
| 10. | Motoyama S, Saito R, Okuyama M, Maruyama K, Ogawa J. Treating gastric tube cancer with distal gastrectomy preserving the gastroepiploic artery. Ann Thorac Surg. 2006;81:751-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Gockel I, Lang H, Mildenberger P. Necessity of preoperative imaging of the gastro-epiploic arcade prior to esophageal resection. Hepatogastroenterology. 2009;56:711-713. [PubMed] [Cited in This Article: ] |
| 12. | Kato T, Takase K, Ichikawa H, Satomi S, Takahashi S. Thoracic duct visualization: combined use of multidetector-row computed tomography and magnetic resonance imaging. J Comput Assist Tomogr. 2011;35:260-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Nakano T, Okamoto H, Maruyama S, Ohuchi N. Three-dimensional imaging of a thoracic duct cyst before thoracoscopic surgery. Eur J Cardiothorac Surg. 2014;45:585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Otani Y, Furukawa T, Suganuma K, Yoshida M, Saikawa Y, Kubota T, Kumai K, Mukai M, Kameyama K, Takami H. Minimally invasive surgery for gastric carcinoid tumor. Biomed Pharmacother. 2002;56 Suppl 1:217s-221s. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Murawa D, Hünerbein M, Spychała A, Nowaczyk P, Połom K, Murawa P. Indocyanine green angiography for evaluation of gastric conduit perfusion during esophagectomy--first experience. Acta Chir Belg. 2012;112:275-280. [PubMed] [Cited in This Article: ] |
| 16. | Shimada Y, Okumura T, Nagata T, Sawada S, Matsui K, Hori R, Yoshioka I, Yoshida T, Osada R, Tsukada K. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus. 2011;8:259-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |