Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.1923
Revised: December 6, 2013
Accepted: January 14, 2014
Published online: February 28, 2014
The evasion from controlled cell death induction has been considered as one of the hallmarks of cancer cells. Defects in cell death signaling are a fundamental phenomenon in colorectal cancer. Nearly any non-invasive cancer treatment finally aims to induce cell death. However, apoptosis resistance is the major cause for insufficient therapeutic success and disease relapse in gastrointestinal oncology. Various compounds have been developed and evaluated with the aim to meet with this obstacle by triggering cell death in cancer cells. The aim of this review is to illustrate current approaches and future directions in targeting cell death signaling in colorectal cancer. The complex signaling network of apoptosis will be demonstrated and the “druggability” of targets will be identified. In detail, proteins regulating mitochondrial cell death in colorectal cancer, such as Bcl-2 and survivin, will be discussed with respect to potential therapeutic exploitation. Death receptor signaling and targeting in colorectal cancer will be outlined. Encouraging clinical trials including cell death based targeted therapies for colorectal cancer are under way and will be demonstrated. Our conceptual understanding of cell death in cancer is rapidly emerging and new types of controlled cellular death have been identified. To meet this progress in cell death research, the implication of autophagy and necroptosis for colorectal carcinogenesis and therapeutic approaches will also be depicted. The main focus of this topic highlight will be on the revelation of the complex cell death concepts in colorectal cancer and the bridging from basic research to clinical use.
Core tip: This review highlights current strategies targeting cell death signaling in colorectal cancer. The role of apoptosis, autophagy and necroptosis in the normal colon mucosa as well as in colorectal cancer onset and therapy is defined. Relevant small molecule compounds as well as antisense based approaches for the treatment of colorectal cancer are illustrated. Furthermore, clinical trials investigating new cell death based compounds are discussed. Finally, future directions in translational cell death research are discussed.
- Citation: Koehler BC, Jäger D, Schulze-Bergkamen H. Targeting cell death signaling in colorectal cancer: Current strategies and future perspectives. World J Gastroenterol 2014; 20(8): 1923-1934
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/1923.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.1923
The crypts of the colorectal mucosa are organized in a polarized fashion. Very few stem cells at the base of a crypt comprise the pool of the regenerative epithelium in which cells travel from bottom to top of the crypt. On the apical edge of the mucosa, about 1010 cells per day die by apoptosis and are subsequently shed in the lumen[1]. This fact illustrates the essential need of a proper regulated cell death for the homeostasis of a normal colorectal mucosa. However, defective signaling or dysbalanced regulation of apoptosis is a very likely cause for the initiation and progression of an adenoma to carcinoma sequence ending up in colorectal cancer (CRC). Of note, proteins relevant for apoptosis (e.g., Bak or Bcl-2) are not equally expressed in all parts of the colorectal mucosa pointing on distinct regulation of death in the intestine[2,3].
In addition to apoptosis as the classical form of programmed cell death, autophagy, a controlled process of cellular self digestion of great importance in situations of cellular stress or upon energy deprivation, has been shown to be active and relevant in colorectal glands. In contrast to apoptosis, the autophagic flux intensity decreases in the crypt from bottom to top[4]. This has been indicated by high expression levels of proautophagic protein Beclin-1 and the conversion of LC3-I to LC3-II in lower crypt cells. On their way to the apex of a crypt the epithelial cells lose Beclin-1 expression and accumulate high levels of SQSTM1/p62, which is an ubiquitin-associated adaptor protein maintaining autophagic flux[4].
In summary, the integrity of the complex interplay of cell death signaling is fundamental for mucosal development and homeostasis in the colorectum. Defective or dysbalanced cell death signaling is involved in the pathogenesis of a variety of colorectal diseases from chronic bowel diseases (Crohn’s disease as well as ulcerative colitis) to colorectal carcinoma.
Colorectal carcinoma can occur sporadically, the most common situation, on the base of defined mutations and also as a final consequence of chronic inflammatory diseases of the intestine[5,6]. The intriguing field of cancer related to chronic inflammation will not be in the focus of this review and the reader might refer to comprehensive literature by others addressing this issue[7-11].
During the development of CRCs from benign polyps through adenomas and finally adenocarcinomas, cell death plays a fundamental role. Key regulating proteins of an appropriate mucosal cell death undergo changes in expression during the transition of an adenoma-carcinoma-sequence[12-14]. For instance, antiapoptogenic Bcl-2 gets lost during the development from adenoma to carcinoma[14]. However, especially the value of cell death related proteins as biomarkers for prognosis and prediction of CRC is of great interest, but the available literature is inconsistent and controversial[15-17]. In summary, apoptosis signaling proteins are in the context of biomarkers either ill defined or need further validation[18]. The reason for these contradictory reports might be due to the extraordinary heterogeneity of CRCs and the broad variety of the carcinogenesis driving mutations[5,19,20]. The aim of this review is to identify possible targets in the cell death signaling network and discuss the compounds available to foster killing of colorectal cancer cells.
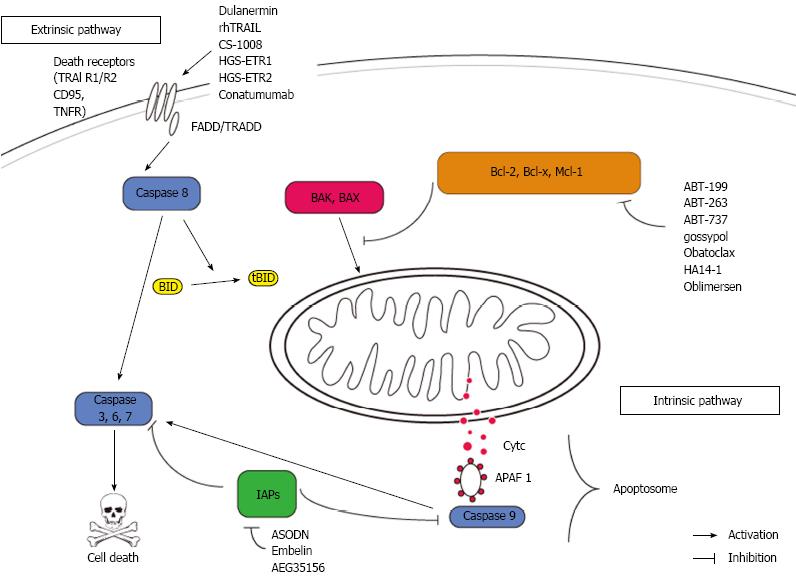
Defects in apoptosis signaling are common in colorectal cancers. An acquired resistance towards cell death may be a key feature of both, carcinogenesis and therapy resistance[21]. However, proteins within the apoptosis signaling pathways have been evaluated for their value as predictive and or prognostic markers as well as targets for therapeutic approaches[18]. Figure 1 shows a synopsis of apoptosis signaling and indicates relevant targets and compounds.
Mitochondria are in the very centre of the intrinsic pathway of apoptosis. The mitochondrial membrane integrity is regulated by the Bcl-2 family of proteins. A tight balance of pro- and antiapoptotic Bcl-2 proteins governs cell’s fate at the mitochondrial surface. In response to several unfavorable conditions (e.g., growth factor withdrawal, DNA damage), this balance shifts towards death. In this case, the proapoptotic proteins (e.g., BAX and BAK) are released by their antiapoptotic relatives (Bcl-2, Bcl-xL, Mcl-1, Bcl-w and A1)[22]. The proapoptotic proteins finally lead to mitochondrial outer membrane permeabilisation and the immediate release of cytochrome C (cytC) into the cytosol. Together with APAF-1 and Caspase 9, cytC forms a death inducing protein platform called apoptosome which in turn leads to activation of caspase 3 as the central downstream event of cell death execution[23].
Within the intrinsic pathway of apoptosis, the antiapoptotic Bcl-2 proteins have been extensively studied as “druggable” targets. Various small molecules targeting the antiapoptotic proteins by binding to their BH3 cleft. This mechanism of action causes a release of multidomain proapoptotic Bcl-2 proteins (e.g., Bim, Bak or/and Bax) which in turn promote cell death. ABT-737 and its orally available derivate ABT-263 (navitoclax) are potent inhibitors of Bcl-2, Bcl-w and Bcl-xL. ABT-263 has recently been shown to induce cell death in colorectal cancer cells in vitro synergistically with the inhibition of the prosurvival kinase MAP kinase/ERK kinase 1/2[24]. This mechanism of death induction by ABT-263 was completely dependent on Bax and Bim. Several phase I trials in solid cancers have proven the safety of ABT263 in combination with established therapy regimes (http://http://www.clinicaltrials.gov). ABT-737 has been shown to act synergistically with oxaliplatin on CRC cells in vitro[25]. An ex vivo evaluation of ABT-737 in samples of ovarian tumors is under way (http://http://www.clinicaltrials.gov). In addition, ABT-737 enhanced apoptosis in CRC cells induced by cyclo-oxygenase-2 inhibitor celecoxib[26]. Importantly, the sensitivity of cancer cells towards ABT-737 is dictated by the expression of NOXA and its control by Mcl-1, which is not targeted by ABT-737[27,28]. Interestingly, Mcl-1 sparing BH-3 mimetics such as ABT-737, ABT-199 and ABT-263, have been shown to effectively induce apoptosis in hypoxic regions of human colorectal tumor spheres. Hypoxia led to a profound downregulation of Mcl-1 which is responsible for ABT-737 resistance in many settings[29]. This work is of great interest since few normal tissues are exposed to hypoxia, but it is a common challenge for growing tumors[30]. HA14-1 is a highly selective small molecule targeting Bcl-2 only. HA 14-1 has been shown to overcome TRAIL resistance in CRC cells by counteracting Bcl-2 overexpression[31,32].
Obatoclax is a first-in-class BH-3 mimetic with an inhibitory profile including Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1 (pan-Bcl-2-inhibitor)[33]. Given the crucial role of Mcl-1 for resistance towards BH-3 mimetics, obatoclax is a promising new agent targeting the complete antiapoptoic Bcl-2 protein family members at once. Few studies investigated the potency of obatoclax for colorectal cancer treatment. It has been recently shown that cell death induction through inhibition of the proproliferative protein Notch by gamma secretase inhibitors is fostered by obatoclax[34].
Oblimersen is an antisense oligonucleotide targeting the first six codons of Bcl-2. Antisense technology represents a highly specific approach for downregulation of antiapoptotic proteins without off-target effects[35]. A phase I trial has shown the safety of oblimersen in combination with irinotecan when intravenously administered in patients with metastatic CRC[36].
In summary, Bcl-2 proteins are context-sensitive targets in colorectal cancer treatment alongside established chemotherapy or radiation. Future studies are urgently warranted to reveal the potential of BH-3 mimetics in colorectal cancer in the clinical setting.
The inhibitor of apoptosis (IAP) family acts by blocking caspase activity (primarily caspase 3). IAPs are found to be overexpressed in several cancer entities including CRC and are able to protect cancer cells from various death stimuli[37,38]. Several compounds inhibit IAPs (primarily XIAP and Survivin). AEG35156 is a second generation antisense oligonucleotide targeting XIAP. Preclinical and early clinical data revealed a promising death-inducing potential of AEG35156 in several solid tumor entities including CRC[39-42]. Survivin is a second promising target among the IAP family overexpressed in CRC. Survivin antisense oligonucleotides strikingly cleared the way for death induction in CRC cells in vitro[43]. Embelin, a naturally occurring benoquinone, has been proven effective in various tumor entities by targeting survivin and other antiapoptotic proteins (Bcl-2 and Bcl-xL)[44]. In the colon, Embelin was able to sufficiently attenuate colitis and carcinoma development in rodents[45,46]. Finally, a double edged approach targeting survivin and XIAP might be a very promising approach for CRC treatment[47].
Second mitochondria activator of caspases (SMAC)/ Diablo is a mitochondria derived, proapoptotic protein acting by blocking IAPs thereby promoting caspase dependent cell death[48]. SMAC mimetics have been shown to strongly sensitize CRC cells towards NSAID induced apoptosis through a feedback amplification resulting in the activation of caspase 3[49]. In TRAIL-induced apoptosis in CRC cells, SMAC/Diablo release from the mitochondria plays a pivotal and role and is Bax dependent[50,51]. Further studies are warranted to clarify the exact role of SMAC for colon carcinogenesis and CRC therapy.
The extrinsic pathway of apoptosis becomes activated in case of binding of a specific ligand to its surface death receptor. Most engaged receptors belong to the tumor necrosis factor receptor family (TNFR, CD95/FAS, TRAIL) and share broad similarity in structure and action[52,53].
In response to ligand binding, the receptor homotrimerises and an adaptor molecule (FADD, TRADD) containing a death domain (DD) is recruited to the cytosolic DD of the receptor. Procaspase 8 is hereafter recruited and catalytically activated in its active form. Finally, caspase 8 leads to an activation of caspase 3 where extrinsic and intrinsic pathways of apoptosis converge[54]. In addition to this direct road to death via caspase 8 and caspase 3, there is a possible detour integrating mitochondria to enhance the death signal. The BH3 only protein Bid is a direct target of Caspase 8 and after cleavage of Bid truncated Bid (tBid) is able to activate mitochondria herewith involving intrinsic apoptosis[55,56].
The receptors involved in extrinsic cell death signaling have been shown to be promising targets. Various compounds and approaches aim to induce apoptosis via direct receptor activation.
Recombinant tumor necrosis factor-α (TNF-α) has been approved for regional treatment of melanoma and soft tissue sarcoma in Europe. The use of TNF-α as a systemic approach is hampered by severe toxicity and adverse side effects such as hypotension, organ failure and cachexia[57]. The efficacy of TNF-α for CRC treatment remains to be clarified, but might be restricted due to TNF-α’s nature as a proinflammatory cytokine. TNFerade® is an adenoviral delivered, intratumoral therapy with a proven safety in rectal cancer patients[58,59]. In advanced pancreatic cancer, TNFerade® was safe but did not prolong survival of patients[60]. The final investigation of TNFerade® for CRC treatment remains elusive. Furthermore, human monoclonal antibody-cytokine fusion protein L19-TNF has been shown to be safe in solid tumors and effective in sarcomas[61,62]. Again, more studies addressing the efficacy for CRC treatment are needed.
CD95 and its ligand have a highly complex role in the colorectal mucosa as well as in onset and progression of CRC. In CRC tissue, CD95 has been shown to be expressed at higher levels compared to adjacent healthy mucosa[63]. Tumor stromal cells and infiltrating immune cells should be considered as bystander targets of CD95 triggering[64,65]. There is some evidence for a metastasis promoting function of CD95 signaling in colorectal cancer via induction of epithelial to mesenchymal transition[66]. As response to hypoxia and radiation, CD95 becomes activated on CRC cells and induces local invasion and promotes liver metastasis in mice[67,68]. In addition, invasive properties of CRC cells have been linked to CD95 signaling[69,70]. At least in vitro, CD95 participates in the activity of PEG-liposomal oxaliplatin induced death in CRC[71]. The anti-Fas monoclonal antibody CH-11 showed antitumor activity in CRC cells with high expression levels of CD95. This death inducing effect was effectively prevented by overexpression of Bcl-2 pointing on a pivotal role of mitochondria for CD95 signaling in CRC[72]. Moreover, there is evidence for a regulatory effect of other antitumor drugs [5-fluorouracil (5-FU), mitomycin (MM), cisplatin (CP) and all-trans retinoic acid] on CD95 expression of CRC cells. Here, MM and CP were able to increase CD95-induced apoptosis. By contrast, 5-FU led to a receptor downregulation causing immune escape of CRC cells[73]. In summary, CD95’s value as a therapeutic target in CRC is complex and might be limited due to the multifaceted role of CD95 in immune-mediated tumor surveillance[74]. As for TRAIL detailed below, several ways of resistance to CD95-induced death further complicate CD95-based therapeutic approaches[75-77].
Tumor Necrosis factor inducing ligand (TRAIL) receptors have been considered as extraordinary promising antitumor targets, since activation preferably kills tumor cells while sparing healthy cells[54]. However, normal colon mucosa epithelium is resistant to TRAIL-induced death[78]. TRAIL directly targets death receptor 4 (DR4) and death receptor 5 (DR5). The recombinant, soluble ligand rhApo2L/TRAIL as well as several antibodies targeting DR4 and/or DR5 have been developed and tested for clinical use.
The agonistic DR4 antibody HGSETR1 (Mapatumumab) and the agonistic DR5 antibody HGSETR2 (Lexatumumab) induced apoptosis in vitro as well as in xenograft bearing nude mice when combined with radiation[79]. In addition, both agonistic antibodies have strong synergistic effects with the mitosis disrupting agent paclitaxel in CRC cells in vitro and in vivo. This sensitizing effect is due to an upregulation of the cognate receptors[80]. Several other antibodies targeting DR4 or DR5 have been shown to have strong antitumor potential on CRC cells[81-85]. Dulanermin (rhApo2L/TRAIL), an optimized and soluble form of TRAIL, has been successfully evaluated in early clinical trials[86]. A clinical trial with Dulanermin in combination with a chemotherapy backbone (FOLFIRI) for patients with metastatic CRC has been completed recently and data from this trial should be available soon (http://www.clinicaltrials.gov).
It is important to have in mind that several CRC cells show intrinsic or acquired resistance towards TRAIL-induced apoptosis. Several proteins have been shown to counteract TRAIL-induced apoptosis. For instance, two decoy receptors within the TRAIL system can counteract DR4 and DR5 activation[87]. Moreover, the interference of antiapoptotic Bcl-2 proteins with TRAIL-receptor-mediated apoptosis has been reported[54,88]. Again at the mitochondrial level, Bax is apparently mandatory for TRAIL’s efficiency to kill CRC cells, since Bax deficiency completely abrogates TRAIL-induced death[89]. Furthermore, high levels of XIAP block TRAIL-induced mitochondrial activation[90]. At the receptor level, mutations of caspase 8 have been reported to cause TRAIL resistance[91]. Moreover, high expression levels of FLIP counteract the interaction between the adaptor FADD and Caspase 8 in CRC cells[92,93]. Pennarun and coworkers presented proof of concept of a combined approach: Downregulation of Mcl-1 and FLIP by multikinase inhibitor sorafenib and NSAID aspirin resensitized cells towards TRAIL[94]. These data are indicative for the feasibility of a combination approach of TRAIL receptor targeting and mitochondrial activation, e.g., by BH3-mimetics.
Taken together, a final and clinical proof of concept for individualized TRAIL tailored therapy for CRC is still elusive and large cohort prospective trials addressing this issue are needed. Table 1 provides an overview of strategies and trials targeting TRAIL receptors in CRC. The awaited results from the Dulanermin trial in metastatic CRC might gain important information for further study designs using TRAIL based therapy.
| Drug | Target | Clinical1 | Ref. |
| Smac mimetics | IAPs | Phase I (NCT01573780) | [49,139] |
| Survivin peptide vaccine | survivin | Phase I-II (NCT00108875) | [140,141] |
| Oblimersen | Bcl-2 | Phase I (NCT00004870) | [142,143] |
| Dulanermin | DR4/5 dual | Phase Ib (NCT00671372) | [86] |
| Tigatuzumab | DR5 | Phase I | [144] |
| CS-1008 | DR5 | Phase I (NCT01220999) | [145] |
| HGS-ETR1 | DR4 | Preclinical in vivo | [79] |
| HGS-ETR2 | DR5 | Phase I (NCT00428272) | [79,146] |
| rhApo2L/TRAIL | DR4/DR 5 | Phase I-II | [147] |
| (NCT00819169) | |||
| Conatumumab | DR5 | Phase II (NCT01327612) | [148] |
| ABT-263 | Bcl-2/Bcl-xl | Phase I (NCT00891605, NCT01009073) | [24] |
| ABT-737 | Bcl-2/Bcl-xl | Preclinical in vivo | [25,26,30] |
| Gossypol | Pan-Bcl2 | Preclinical in vivo | [149] |
The conceptual understanding of cell death is under constant expansion and various subtypes of cellular death have been defined[1,95,96]. Among the emerging cell death concepts, this work will deeper discuss necroptosis and autophagy in order to dissect the current knowledge concerning colorectal carcinogenesis and CRC treatment.
Necrosis has long been considered as a passive, mainly accidental and uncontrolled form of cellular death. To date there is a growing body of literature implicating a tight regulation of necrotic processes similar to apoptosis[97]. Therefore, a programmed form of necrosis, termed necroptosis, has been defined. The signaling events responsible for initiation and execution of necroptosis have been studied best in the context of TNFR signaling. Necroptosis is crucially mediated by receptor-interacting protein 1 (RIP 1) along with its cognate kinase RIP3. Upon TNF induction, a multimeric complex containing FADD, caspase 3, RIP 1 and RIP 3 assembles[98]. This complex is termed complex IIb or necrosome. The determination of cells’ fate is complicated by the observation that the ubiquitination status of the engaged proteins (e.g., RIP) appears to be the master switch between apoptosis and necroptosis[99]. Necroptosis has also been demonstrated after activation of TRAIL receptors on hepatocytes and colorectal cancer cells[100]. Mechanistically, there are various central proteins involved in both, apoptosis and necroptosis. Which form of cell death prevails, is cell type and stimulus dependent[101-103]. Necroptosis and its role in various diseases, including CRC and inflammatory bowel disease, are currently under investigation[104-107]. There is evidence for a central role of caspase 8 as a key switch from apoptosis to necroptosis in carcinoma related inflammatory bowel disease[104].
The relevance of necroptotic cell death for colorectal cancer cells has been evaluated preclinically in the context of azathioprine plus buthionine sulfoximine treatment in CRC and HCC[108]. This work shows a necroptosis phenotype with mitochondrial dependency illustrating the interplay between necroptosis and apoptosis. Another study investigated the role of hypoxia for necroptotic death in colorectal cancer cells. In this study, RIP-dependent necroptosis can be conferred by pyruvate scavenging of mitochondria derived radicals[109]. Finally, targeted approaches to induce necroptotic cell death in cancer cells are still missing due to the absence of appropriate compounds for clinical usage so far. It has been shown that TRAIL receptor ligation causes necroptosis in an acidic extracellular milieu. Necrostatin-1, a chemical inhibitor of RIPK1, sufficiently blocked TRAIL-induced necroptosis in this experimental setting[100]. An indirect or secondary activation of necroptosis has been reported after treatment of CRC cells with TRAIL or inhibition of the multifaceted kinase GSK3-β[100,110].
Autophagy is an evolutionary conserved process by which cells collect proteins and organelles, deliver them to the lysosomal compartment where the cargo is finally degraded for recycling[111]. The implications of autophagy for cell physiology as well as for onset and progression of various diseases including cancer are rapidly emerging[112,113]. A disruption of autophagic flux leads to an intracellular accumulation of organelles, protein aggregates and lipid droplets. These accumulations may lead to the production of reactive oxygen species and cause metabolic insufficiency. Especially in stressful situation and in conditions of energy deprivation, a disruption of autophagic flux can promote carcinogenesis. For instance, the allelic loss of the essential autophagy protein Beclin 1 (also known as Atg6) causes HCC in mice[114,115].
By contrast, autophagy is essential for the survival of cancer cells and cancer cells show an extraordinary high level of autophagy. However, autophagy induction promotes survival under conditions of hypoxia and growth factor withdrawal[116]. Autophagosome formation is most prominent in tumors growing in a hypoxic environment. With regard to these findings, drugs inhibiting autophagy are promising anticancer agents. The anti-malaria drug Chloroquine is a known inhibitor of autophagy and is currently being under investigation in several clinical trials (http://www.clinicaltrials.gov, Table 2)[117]. Various other compounds or drugs are known regulators of autophagy and have been evaluated preclinically as treatment options for CRC[118-121]. In vitro, Chloroquine has been effective in overcoming 5-FU resistance in CRC cells[122,123]. Intriguingly, the approved chimeric anti-EGFR antibody cetuximab exerts its antitumor effect at least partly via autophagy-induced cell death[123].
| Drug | Target | Clinical1 | Ref. |
| Hydroxychloroquine | Autophagosome | Phase I (NCT01206530) Phase II (NCT01006369) | [122,150] |
| Everolimus/rapamycin | mTOR | Phase II (NCT00419159, NCT01387880) | [126,127,151] |
Counterintuitive, drugs directly inducing autophagy are under clinical investigation as therapeutic approaches in CRC, too. Mammalian target of rapamycin is a prominent target to induce lethal autophagy in colorectal cancer cells[124]. The Rapamycin derivate Everolimus has recently been established for the treatment of colorectal neuroendocrine tumors[125]. A Phase II study with Everolimus showed appropriate tolerability, but failed to show meaningful efficacy in heavily pretreated patients with metastatic CRC[126]. Another trial using a combination of vascular endothelial growth factor receptor tyrosine kinase inhibitor tivozanib with everolimus resulted in stable disease of 50 % of all patients with metastatic cancer enrolled[127,128]. These partly contradictory findings highlight the important implication of autophagy in colorectal carcinogenesis.
Importantly, there is a broad overlap of the apoptosis and autophagy signaling network. Most prominently, Bcl-2 proteins function as both, inhibitors of apoptosis and autophagy by binding proautophagic Beclin1. Therefore, it has been shown that BH3-mimetics induce apoptosis and autophagy. For instance, ABT-737 can synergistically induce cell death with the COX2 inhibitor celecoxib in CRC cells by facilitating autophagy and apoptosis[26,129].
The past decade of cell death research has shown that necrosis, apoptosis and autophagy are regulated by similar pathways engaging the same proteins. It might be worthwhile targeting the apoptotic and autophagic machinery in a combined approach, since a massive induction of autophagy is able to drive cancer cells in apoptotic death. Recently, various efforts in this direction have been made in order to overcome cell death resistance in colorectal cancer. For instance, silibin, a plant derived natural compound, is able to induce both, apoptosis and autophagy[130]. In line with these observations, compound C, a small molecule inhibitor of AMP-activated protein kinase, is able to sufficiently suppress colorectal cancer cell growth by inducing apoptosis and autophagy[131]. The capability of such a double-edged approach has been successfully proven in vivo in a model of hepatic metastasis in mice[132]. Future studies are needed to further exploit combinatorial approaches for cell death induction in colorectal cancer.
From an oncological point of view, it is of outstanding importance to further increase research efforts aiming at more effective and individualized therapies. The effectiveness of monotherapeutic systemic approaches in colorectal cancer treatment is limited. However, combined therapy regimes are now state of the art. Manipulation of cell death represents a promising tool to further amplify response to chemotherapy. In addition to direct cell death induction in cancer cells, triggering cell death via cancer-directed immunotherapy or immunomodulation with the aim to overcome major mechanisms of immune resistance, is a newly arising field[133]. For example, recent reports on long-term results from first-in-human clinical trials using anti-PD1 antibody-based immunotherapy are encouraging[134]. Future trials are warranted to identify the best combinatorial approach yielding at cell death induction in cancer cells.
On the way to personalized oncology, it will be mandatory to broaden our knowledge concerning the selection of patients for a specific therapeutic setting. Having in mind that cell death relevant proteins vary in their expression in different subsets and stages of CRC, a stratification of patients to identify those who benefit most of a manipulation of apoptosis requires further research.
Finally, the question whether and how cell death could be measured to monitor therapy in patients needs further attention. There are some elegant and encouraging studies evaluating liquid biopsy markers for cell death in cancer[135,136]. In addition, imaging of cell death on routine basis for non-invasive monitoring of tumor biology and therapeutic response might open new windows for therapy surveillance and outcome prediction in colorectal cancer[137,138].
BCK holds a Postdoctoral-Fellowship from the Medical Faculty of the University of Heidelberg, Germany. HSB receives grants from the German Research Foundation (DFG SCHU 1443/4-1). All authors are members of the colorectal cancer clinical research unit at the University Hospital Heidelberg, Germany (KFO227).
P- Reviewers: Bujanda L, Qin SF, Wasim F S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 828] [Cited by in F6Publishing: 852] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 2. | Liu LU, Holt PR, Krivosheyev V, Moss SF. Human right and left colon differ in epithelial cell apoptosis and in expression of Bak, a pro-apoptotic Bcl-2 homologue. Gut. 1999;45:45-50. [PubMed] [Cited in This Article: ] |
| 3. | Watson AJ. Apoptosis and colorectal cancer. Gut. 2004;53:1701-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Groulx JF, Khalfaoui T, Benoit YD, Bernatchez G, Carrier JC, Basora N, Beaulieu JF. Autophagy is active in normal colon mucosa. Autophagy. 2012;8:893-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4:769-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Harpaz N, Ward SC, Mescoli C, Itzkowitz SH, Polydorides AD. Precancerous lesions in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2013;27:257-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Soetikno R, Subramanian V, Kaltenbach T, Rouse RV, Sanduleanu S, Suzuki N, Tanaka S, McQuaid K. The detection of nonpolypoid (flat and depressed) colorectal neoplasms in patients with inflammatory bowel disease. Gastroenterology. 2013;144:1349-152, 1349-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Matkowskyj KA, Chen ZE, Rao MS, Yang GY. Dysplastic lesions in inflammatory bowel disease: molecular pathogenesis to morphology. Arch Pathol Lab Med. 2013;137:338-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Foersch S, Waldner MJ, Neurath MF. Colitis and colorectal cancer. Dig Dis. 2012;30:469-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Neumann H, Vieth M, Langner C, Neurath MF, Mudter J. Cancer risk in IBD: how to diagnose and how to manage DALM and ALM. World J Gastroenterol. 2011;17:3184-3191. [PubMed] [Cited in This Article: ] |
| 12. | Manne U, Shanmugam C, Katkoori VR, Bumpers HL, Grizzle WE. Development and progression of colorectal neoplasia. Cancer Biomark. 2010;9:235-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Laurent-Puig P, Blons H, Cugnenc PH. Sequence of molecular genetic events in colorectal tumorigenesis. Eur J Cancer Prev. 1999;8 Suppl 1:S39-S47. [PubMed] [Cited in This Article: ] |
| 14. | Hao XP, Ilyas M, Talbot IC. Expression of Bcl-2 and p53 in the colorectal adenoma-carcinoma sequence. Pathobiology. 1997;65:140-145. [PubMed] [Cited in This Article: ] |
| 15. | Baretton GB, Diebold J, Christoforis G, Vogt M, Müller C, Dopfer K, Schneiderbanger K, Schmidt M, Löhrs U. Apoptosis and immunohistochemical bcl-2 expression in colorectal adenomas and carcinomas. Aspects of carcinogenesis and prognostic significance. Cancer. 1996;77:255-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Manne U, Myers RB, Moron C, Poczatek RB, Dillard S, Weiss H, Brown D, Srivastava S, Grizzle WE. Prognostic significance of Bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int J Cancer. 1997;74:346-358. [PubMed] [Cited in This Article: ] |
| 17. | Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422-2427. [PubMed] [Cited in This Article: ] |
| 18. | Hector S, Prehn JH. Apoptosis signaling proteins as prognostic biomarkers in colorectal cancer: a review. Biochim Biophys Acta. 2009;1795:117-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Sieber OM, Heinimann K, Tomlinson IP. Genomic instability--the engine of tumorigenesis? Nat Rev Cancer. 2003;3:701-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Tenesa A, Dunlop MG. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet. 2009;10:353-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 21. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39812] [Cited by in F6Publishing: 42985] [Article Influence: 3306.5] [Reference Citation Analysis (4)] |
| 22. | Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398-6406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 613] [Cited by in F6Publishing: 644] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 23. | Fadeel B, Ottosson A, Pervaiz S. Big wheel keeps on turning: apoptosome regulation and its role in chemoresistance. Cell Death Differ. 2008;15:443-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Sale MJ, Cook SJ. The BH3 mimetic ABT-263 synergizes with the MEK1/2 inhibitor selumetinib/AZD6244 to promote BIM-dependent tumour cell death and inhibit acquired resistance. Biochem J. 2013;450:285-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Raats DA, de Bruijn MT, Steller EJ, Emmink BL, Borel-Rinkes IH, Kranenburg O. Synergistic killing of colorectal cancer cells by oxaliplatin and ABT-737. Cell Oncol (Dordr). 2011;34:307-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Huang S, Sinicrope FA. Celecoxib-induced apoptosis is enhanced by ABT-737 and by inhibition of autophagy in human colorectal cancer cells. Autophagy. 2010;6:256-269. [PubMed] [Cited in This Article: ] |
| 27. | Okumura K, Huang S, Sinicrope FA. Induction of Noxa sensitizes human colorectal cancer cells expressing Mcl-1 to the small-molecule Bcl-2/Bcl-xL inhibitor, ABT-737. Clin Cancer Res. 2008;14:8132-8142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Mazumder S, Choudhary GS, Al-Harbi S, Almasan A. Mcl-1 Phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Res. 2012;72:3069-3079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67:2908-2911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Harrison LR, Micha D, Brandenburg M, Simpson KL, Morrow CJ, Denneny O, Hodgkinson C, Yunus Z, Dempsey C, Roberts D. Hypoxic human cancer cells are sensitized to BH-3 mimetic–induced apoptosis via downregulation of the Bcl-2 protein Mcl-1. J Clin Invest. 2011;121:1075-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res. 2004;10:8284-8292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124-7129. [PubMed] [Cited in This Article: ] |
| 33. | Joudeh J, Claxton D. Obatoclax mesylate: pharmacology and potential for therapy of hematological neoplasms. Expert Opin Investig Drugs. 2012;21:363-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Timme CR, Gruidl M, Yeatman TJ. Gamma-secretase inhibition attenuates oxaliplatin-induced apoptosis through increased Mcl-1 and/or Bcl-xL in human colon cancer cells. Apoptosis. 2013;18:1163-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Oblimersen: Augmerosen, BCL-2 antisense oligonucleotide - Genta, G 3139, GC 3139, oblimersen sodium. Drugs R D. 2007;8:321-334. [PubMed] [Cited in This Article: ] |
| 36. | Mita MM, Ochoa L, Rowinsky EK, Kuhn J, Schwartz G, Hammond LA, Patnaik A, Yeh IT, Izbicka E, Berg K. A phase I, pharmacokinetic and biologic correlative study of oblimersen sodium (Genasense, G3139) and irinotecan in patients with metastatic colorectal cancer. Ann Oncol. 2006;17:313-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247-3259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 755] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 38. | Krieg A, Werner TA, Verde PE, Stoecklein NH, Knoefel WT. Prognostic and clinicopathological significance of survivin in colorectal cancer: a meta-analysis. PLoS One. 2013;8:e65338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Cummings J, Ranson M, Lacasse E, Ganganagari JR, St-Jean M, Jayson G, Durkin J, Dive C. Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound (AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2006;95:42-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | LaCasse EC, Cherton-Horvat GG, Hewitt KE, Jerome LJ, Morris SJ, Kandimalla ER, Yu D, Wang H, Wang W, Zhang R. Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res. 2006;12:5231-5241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Dean E, Jodrell D, Connolly K, Danson S, Jolivet J, Durkin J, Morris S, Jowle D, Ward T, Cummings J. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J Clin Oncol. 2009;27:1660-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Miura K, Fujibuchi W, Ishida K, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C. Inhibitor of apoptosis protein family as diagnostic markers and therapeutic targets of colorectal cancer. Surg Today. 2011;41:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Yao H, Huang ZH, Li Z, Su GQ, He R, Gao F, Cui DX. [Specific killing effects of combination of recombinant adenovirus containing double suicide gene driven by KDR promoter and survivin antisense oligonucleotide on colorectal cancer cells and vascular endothelial cells]. Zhonghua Wei Chang Wai Ke Zazhi. 2008;11:61-66. [PubMed] [Cited in This Article: ] |
| 44. | Kim SW, Kim SM, Bae H, Nam D, Lee JH, Lee SG, Shim BS, Kim SH, Ahn KS, Choi SH. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate. 2013;73:296-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Kumar G K, Dhamotharan R NM, Honnegowda S. Embelin ameliorates dextran sodium sulfate-induced colitis in mice. Int Immunopharmacol. 2011;11:724-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Thippeswamy BS, Mahendran S, Biradar MI, Raj P, Srivastava K, Badami S, Veerapur VP. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur J Pharmacol. 2011;654:100-105. [PubMed] [Cited in This Article: ] |
| 47. | Hehlgans S, Petraki C, Reichert S, Cordes N, Rödel C, Rödel F. Double targeting of Survivin and XIAP radiosensitizes 3D grown human colorectal tumor cells and decreases migration. Radiother Oncol. 2013;108:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Anguiano-Hernandez YM, Chartier A, Huerta S. Smac/DIABLO and colon cancer. Anticancer Agents Med Chem. 2007;7:467-473. [PubMed] [Cited in This Article: ] |
| 49. | Bank A, Wang P, Du C, Yu J, Zhang L. SMAC mimetics sensitize nonsteroidal anti-inflammatory drug-induced apoptosis by promoting caspase-3-mediated cytochrome c release. Cancer Res. 2008;68:276-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Mohr A, Büneker C, Gough RP, Zwacka RM. MnSOD protects colorectal cancer cells from TRAIL-induced apoptosis by inhibition of Smac/DIABLO release. Oncogene. 2008;27:763-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 373] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 52. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [PubMed] [Cited in This Article: ] |
| 53. | Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 960] [Cited by in F6Publishing: 911] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 54. | Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 645] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 55. | Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;19:42-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 56. | Gross A. BID as a double agent in cell life and death. Cell Cycle. 2006;5:582-584. [PubMed] [Cited in This Article: ] |
| 57. | Lejeune FJ, Rüegg C. Recombinant human tumor necrosis factor: an efficient agent for cancer treatment. Bull Cancer. 2006;93:E90-100. [PubMed] [Cited in This Article: ] |
| 58. | Call JA, Eckhardt SG, Camidge DR. Targeted manipulation of apoptosis in cancer treatment. Lancet Oncol. 2008;9:1002-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Citrin D, Camphausen K, Wood BJ, Quezado M, Denobile J, Pingpank JF, Royal RE, Alexander HR, Seidel G, Steinberg SM. A pilot feasibility study of TNFerade™ biologic with capecitabine and radiation therapy followed by surgical resection for the treatment of rectal cancer. Oncology. 2010;79:382-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, Donehower RC, Pawlik TM, Ziegler MA, Cai H. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. 2013;31:886-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 61. | Spitaleri G, Berardi R, Pierantoni C, De Pas T, Noberasco C, Libbra C, González-Iglesias R, Giovannoni L, Tasciotti A, Neri D. Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19-TNF in patients with advanced solid tumours. J Cancer Res Clin Oncol. 2013;139:447-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Hemmerle T, Probst P, Giovannoni L, Green AJ, Meyer T, Neri D. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br J Cancer. 2013;109:1206-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Zhu Q, Liu JY, Xu HW, Yang CM, Zhang AZ, Cui Y, Wang HB. Mechanism of counterattack of colorectal cancer cell by Fas/Fas ligand system. World J Gastroenterol. 2005;11:6125-6129. [PubMed] [Cited in This Article: ] |
| 64. | Hoogwater FJ, Steller EJ, Westendorp BF, Borel Rinkes IH, Kranenburg O. CD95 signaling in colorectal cancer. Biochim Biophys Acta. 2012;1826:189-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Sugita J, Ohtani H, Mizoi T, Saito K, Shiiba K, Sasaki I, Matsuno S, Yagita H, Miyazawa M, Nagura H. Close association between Fas ligand (FasL; CD95L)-positive tumor-associated macrophages and apoptotic cancer cells along invasive margin of colorectal carcinoma: a proposal on tumor-host interactions. Jpn J Cancer Res. 2002;93:320-328. [PubMed] [Cited in This Article: ] |
| 66. | Zheng HX, Cai YD, Wang YD, Cui XB, Xie TT, Li WJ, Peng L, Zhang Y, Wang ZQ, Wang J. Fas signaling promotes motility and metastasis through epithelial-mesenchymal transition in gastrointestinal cancer. Oncogene. 2013;32:1183-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Nijkamp MW, Hoogwater FJ, Steller EJ, Westendorp BF, van der Meulen TA, Leenders MW, Borel Rinkes IH, Kranenburg O. CD95 is a key mediator of invasion and accelerated outgrowth of mouse colorectal liver metastases following radiofrequency ablation. J Hepatol. 2010;53:1069-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Sheard MA, Vojtesek B, Janakova L, Kovarik J, Zaloudik J. Up-regulation of Fas (CD95) in human p53wild-type cancer cells treated with ionizing radiation. Int J Cancer. 1997;73:757-762. [PubMed] [Cited in This Article: ] |
| 69. | Steller EJ, Ritsma L, Raats DA, Hoogwater FJ, Emmink BL, Govaert KM, Laoukili J, Rinkes IH, van Rheenen J, Kranenburg O. The death receptor CD95 activates the cofilin pathway to stimulate tumour cell invasion. EMBO Rep. 2011;12:931-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Kasper HU, Konze E, Kern M, Stippel DL. CD95 and TNFα-induced apoptosis in liver metastases of colorectal carcinoma. In Vivo. 2010;24:653-657. [PubMed] [Cited in This Article: ] |
| 71. | Yang C, Liu HZ, Fu ZX. PEG-liposomal oxaliplatin induces apoptosis in human colorectal cancer cells via Fas/FasL and caspase-8. Cell Biol Int. 2012;36:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Houghton JA, Harwood FG, Gibson AA, Tillman DM. The fas signaling pathway is functional in colon carcinoma cells and induces apoptosis. Clin Cancer Res. 1997;3:2205-2209. [PubMed] [Cited in This Article: ] |
| 73. | Young NL, Saudek CD, Walters L, Lapeyrolerie J, Chang V. Preventing hyperphagia normalizes 3-hydroxy-3-methylglutaryl-CoA reductase activity in small intestine and liver of diabetic rats. J Lipid Res. 1982;23:831-838. [PubMed] [Cited in This Article: ] |
| 74. | Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1217] [Cited by in F6Publishing: 1140] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 75. | Tillman DM, Harwood FG, Gibson AA, Houghton JA. Expression of genes that regulate Fas signalling and Fas-mediated apoptosis in colon carcinoma cells. Cell Death Differ. 1998;5:450-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Villa-Morales M, Fernández-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:85-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 77. | Wang WS, Chen PM, Wang HS, Liang WY, Su Y. Matrix metalloproteinase-7 increases resistance to Fas-mediated apoptosis and is a poor prognostic factor of patients with colorectal carcinoma. Carcinogenesis. 2006;27:1113-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Sträter J, Walczak H, Pukrop T, Von Müller L, Hasel C, Kornmann M, Mertens T, Möller P. TRAIL and its receptors in the colonic epithelium: a putative role in the defense of viral infections. Gastroenterology. 2002;122:659-666. [PubMed] [Cited in This Article: ] |
| 79. | Marini P, Denzinger S, Schiller D, Kauder S, Welz S, Humphreys R, Daniel PT, Jendrossek V, Budach W, Belka C. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene. 2006;25:5145-5154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Gong J, Yang D, Kohanim S, Humphreys R, Broemeling L, Kurzrock R. Novel in vivo imaging shows up-regulation of death receptors by paclitaxel and correlates with enhanced antitumor effects of receptor agonist antibodies. Mol Cancer Ther. 2006;5:2991-3000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Motoki K, Mori E, Matsumoto A, Thomas M, Tomura T, Humphreys R, Albert V, Muto M, Yoshida H, Aoki M. Enhanced apoptosis and tumor regression induced by a direct agonist antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2. Clin Cancer Res. 2005;11:3126-3135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Qiu Y, Zhang Z, Shi J, Liu S, Liu Y, Zheng D. A novel anti-DR5 chimeric antibody and epirubicin synergistically suppress tumor growth. IUBMB Life. 2012;64:757-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Li M, Wu Y, Qiu Y, Yao Z, Liu S, Liu Y, Shi J, Zheng D. 2A peptide-based, lentivirus-mediated anti-death receptor 5 chimeric antibody expression prevents tumor growth in nude mice. Mol Ther. 2012;20:46-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Rosevear HM, Lightfoot AJ, Griffith TS. Conatumumab, a fully human mAb against death receptor 5 for the treatment of cancer. Curr Opin Investig Drugs. 2010;11:688-698. [PubMed] [Cited in This Article: ] |
| 85. | Siegemund M, Pollak N, Seifert O, Wahl K, Hanak K, Vogel A, Nussler AK, Göttsch D, Münkel S, Bantel H. Superior antitumoral activity of dimerized targeted single-chain TRAIL fusion proteins under retention of tumor selectivity. Cell Death Dis. 2012;3:e295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 86. | Pan Y, Xu R, Peach M, Huang CP, Branstetter D, Novotny W, Herbst RS, Eckhardt SG, Holland PM. Evaluation of pharmacodynamic biomarkers in a Phase 1a trial of dulanermin (rhApo2L/TRAIL) in patients with advanced tumours. Br J Cancer. 2011;105:1830-1838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Manzo F, Nebbioso A, Miceli M, Conte M, De Bellis F, Carafa V, Franci G, Tambaro FP, Altucci L. TNF-related apoptosis-inducing ligand: signalling of a ‘smart’ molecule. Int J Biochem Cell Biol. 2009;41:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Koehler BC, Urbanik T, Vick B, Boger RJ, Heeger S, Galle PR, Schuchmann M, Schulze-Bergkamen H. TRAIL-induced apoptosis of hepatocellular carcinoma cells is augmented by targeted therapies. World J Gastroenterol. 2009;15:5924-5935. [PubMed] [Cited in This Article: ] |
| 89. | LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 408] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 90. | Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004;64:3006-3008. [PubMed] [Cited in This Article: ] |
| 91. | Kim HS, Lee JW, Soung YH, Park WS, Kim SY, Lee JH, Park JY, Cho YG, Kim CJ, Jeong SW. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology. 2003;125:708-715. [PubMed] [Cited in This Article: ] |
| 92. | Hernandez A, Thomas R, Smith F, Sandberg J, Kim S, Chung DH, Evers BM. Butyrate sensitizes human colon cancer cells to TRAIL-mediated apoptosis. Surgery. 2001;130:265-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Hernandez A, Wang QD, Schwartz SA, Evers BM. Sensitization of human colon cancer cells to TRAIL-mediated apoptosis. J Gastrointest Surg. 2001;5:56-65. [PubMed] [Cited in This Article: ] |
| 94. | Pennarun B, Kleibeuker JH, Boersma-van Ek W, Kruyt FA, Hollema H, de Vries EG, de Jong S. Targeting FLIP and Mcl-1 using a combination of aspirin and sorafenib sensitizes colon cancer cells to TRAIL. J Pathol. 2013;229:410-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 95. | Kreuzaler P, Watson CJ. Killing a cancer: what are the alternatives? Nat Rev Cancer. 2012;12:411-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 96. | Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 938] [Cited by in F6Publishing: 968] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 97. | Fulda S. The mechanism of necroptosis in normal and cancer cells. Cancer Biol Ther. 2013;14:999-1004. [PubMed] [Cited in This Article: ] |
| 98. | Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1583] [Cited by in F6Publishing: 1769] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 99. | Zhou Z, Han V, Han J. New components of the necroptotic pathway. Protein Cell. 2012;3:811-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D, Vandenabeele P. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003-2014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 101. | Jain MV, Paczulla AM, Klonisch T, Dimgba FN, Rao SB, Roberg K, Schweizer F, Lengerke C, Davoodpour P, Palicharla VR. Interconnections between apoptotic, autophagic and necrotic pathways: implications for cancer therapy development. J Cell Mol Med. 2013;17:12-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 102. | Gong J, Kumar SA, Graham G, Kumar AP. FLIP: Molecular Switch Between Apoptosis and Necroptosis. Mol Carcinog. 2013;Apr 26; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 103. | Lee EW, Seo J, Jeong M, Lee S, Song J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012;45:496-508. [PubMed] [Cited in This Article: ] |
| 104. | Becker C, Watson AJ, Neurath MF. Complex roles of caspases in the pathogenesis of inflammatory bowel disease. Gastroenterology. 2013;144:283-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 105. | Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 608] [Cited by in F6Publishing: 739] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 106. | Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernández-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 555] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 107. | Yu X, Deng Q, Bode AM, Dong Z, Cao Y. The role of necroptosis, an alternative form of cell death, in cancer therapy. Expert Rev Anticancer Ther. 2013;13:883-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Hernández-Breijo B, Monserrat J, Ramírez-Rubio S, Cuevas EP, Vara D, Díaz-Laviada I, Fernández-Moreno MD, Román ID, Gisbert JP, Guijarro LG. Preclinical evaluation of azathioprine plus buthionine sulfoximine in the treatment of human hepatocarcinoma and colon carcinoma. World J Gastroenterol. 2011;17:3899-3911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 109. | Huang CY, Kuo WT, Huang YC, Lee TC, Yu LC. Resistance to hypoxia-induced necroptosis is conferred by glycolytic pyruvate scavenging of mitochondrial superoxide in colorectal cancer cells. Cell Death Dis. 2013;4:e622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 110. | Grassilli E, Narloch R, Federzoni E, Ianzano L, Pisano F, Giovannoni R, Romano G, Masiero L, Leone BE, Bonin S. Inhibition of GSK3B bypass drug resistance of p53-null colon carcinomas by enabling necroptosis in response to chemotherapy. Clin Cancer Res. 2013;19:3820-3831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 111. | Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651-662. [PubMed] [Cited in This Article: ] |
| 112. | White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1226] [Cited by in F6Publishing: 1292] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 113. | Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 618] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 114. | Ding WX, Ni HM, Gao W, Chen X, Kang JH, Stolz DB, Liu J, Yin XM. Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy. Mol Cancer Ther. 2009;8:2036-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 115. | Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077-15082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1534] [Cited by in F6Publishing: 1637] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 116. | Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1454] [Cited by in F6Publishing: 1514] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 117. | Sotelo J, Briceño E, López-González MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337-343. [PubMed] [Cited in This Article: ] |
| 118. | Tai CJ, Wang CK, Tai CJ, Lin YF, Lin CS, Jian JY, Chang YJ, Chang CC. Aqueous Extract of Solanum nigrum Leaves Induces Autophagy and Enhances Cytotoxicity of Cisplatin, Doxorubicin, Docetaxel, and 5-Fluorouracil in Human Colorectal Carcinoma Cells. Evid Based Complement Alternat Med. 2013;2013:514719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 119. | Basile V, Belluti S, Ferrari E, Gozzoli C, Ganassi S, Quaglino D, Saladini M, Imbriano C. bis-Dehydroxy-Curcumin triggers mitochondrial-associated cell death in human colon cancer cells through ER-stress induced autophagy. PLoS One. 2013;8:e53664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 120. | Biggers JW, Nguyen T, Di X, Gupton JT, Henderson SC, Emery SM, Alotaibi M, White KL, Brown R, Almenara J. Autophagy, cell death and sustained senescence arrest in B16/F10 melanoma cells and HCT-116 colon carcinoma cells in response to the novel microtubule poison, JG-03-14. Cancer Chemother Pharmacol. 2013;71:441-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Greene LM, O’Boyle NM, Nolan DP, Meegan MJ, Zisterer DM. The vascular targeting agent Combretastatin-A4 directly induces autophagy in adenocarcinoma-derived colon cancer cells. Biochem Pharmacol. 2012;84:612-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Sasaki K, Tsuno NH, Sunami E, Kawai K, Hongo K, Hiyoshi M, Kaneko M, Murono K, Tada N, Nirei T. Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study. Anticancer Drugs. 2012;23:675-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 123. | Sasaki K, Tsuno NH, Sunami E, Tsurita G, Kawai K, Okaji Y, Nishikawa T, Shuno Y, Hongo K, Hiyoshi M. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 124. | Altomare I, Hurwitz H. Everolimus in colorectal cancer. Expert Opin Pharmacother. 2013;14:505-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Castellano D, Bajetta E, Panneerselvam A, Saletan S, Kocha W, O’Dorisio T, Anthony LB, Hobday T. Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-2 study. Oncologist. 2013;18:46-53. [PubMed] [Cited in This Article: ] |
| 126. | Ng K, Tabernero J, Hwang J, Bajetta E, Sharma S, Del Prete SA, Arrowsmith ER, Ryan DP, Sedova M, Jin J. Phase II study of everolimus in patients with metastatic colorectal adenocarcinoma previously treated with bevacizumab-, fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens. Clin Cancer Res. 2013;19:3987-3995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 127. | Wolpin BM, Ng K, Zhu AX, Abrams T, Enzinger PC, McCleary NJ, Schrag D, Kwak EL, Allen JN, Bhargava P. Multicenter phase II study of tivozanib (AV-951) and everolimus (RAD001) for patients with refractory, metastatic colorectal cancer. Oncologist. 2013;18:377-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 128. | Altomare I, Bendell JC, Bullock KE, Uronis HE, Morse MA, Hsu SD, Zafar SY, Blobe GC, Pang H, Honeycutt W. A phase II trial of bevacizumab plus everolimus for patients with refractory metastatic colorectal cancer. Oncologist. 2011;16:1131-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 129. | Jendrossek V. Targeting apoptosis pathways by Celecoxib in cancer. Cancer Lett. 2013;332:313-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 130. | Raina K, Agarwal C, Wadhwa R, Serkova NJ, Agarwal R. Energy deprivation by silibinin in colorectal cancer cells: a double-edged sword targeting both apoptotic and autophagic machineries. Autophagy. 2013;9:697-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 131. | Yang WL, Perillo W, Liou D, Marambaud P, Wang P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J Surg Oncol. 2012;106:680-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 132. | Tian M, Wan Y, Tang J, Li H, Yu G, Zhu J, Ji S, Guo H, Zhang N, Li W. Depletion of tissue factor suppresses hepatic metastasis and tumor growth in colorectal cancer via the downregulation of MMPs and the induction of autophagy and apoptosis. Cancer Biol Ther. 2011;12:896-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 133. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8693] [Cited by in F6Publishing: 9310] [Article Influence: 775.8] [Reference Citation Analysis (0)] |
| 134. | Lipson EJ. Re-orienting the immune system: Durable tumor regression and successful re-induction therapy using anti-PD1 antibodies. Oncoimmunology. 2013;2:e23661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 135. | Linder S, Olofsson MH, Herrmann R, Ulukaya E. Utilization of cytokeratin-based biomarkers for pharmacodynamic studies. Expert Rev Mol Diagn. 2010;10:353-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 136. | Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004;214:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | De Saint-Hubert M, Bauwens M, Verbruggen A, Mottaghy FM. Apoptosis imaging to monitor cancer therapy: the road to fast treatment evaluation? Curr Pharm Biotechnol. 2012;13:571-583. [PubMed] [Cited in This Article: ] |
| 138. | Krause BJ, Schwarzenböck S, Schwaiger M. [Tracers in oncology - preclinical and clinical evaluation]. Nuklearmedizin. 2010;49 Suppl 1:S41-S45. [PubMed] [Cited in This Article: ] |
| 139. | Huerta S, Gao X, Livingston EH, Kapur P, Sun H, Anthony T. In vitro and in vivo radiosensitization of colorectal cancer HT-29 cells by the smac mimetic JP-1201. Surgery. 2010;148:346-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 140. | Kameshima H, Tsuruma T, Torigoe T, Takahashi A, Hirohashi Y, Tamura Y, Tsukahara T, Ichimiya S, Kanaseki T, Iwayama Y. Immunogenic enhancement and clinical effect by type-I interferon of anti-apoptotic protein, survivin-derived peptide vaccine, in advanced colorectal cancer patients. Cancer Sci. 2011;102:1181-1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 141. | Kim HS, Kim CH, Park MY, Park JS, Park HM, Sohn HJ, Kim HJ, Kim SG, Oh ST, Kim TG. Efficient co-transduction of adenoviral vectors encoding carcinoembryonic antigen and survivin into dendritic cells by the CAR-TAT adaptor molecule enhance anti-tumor immunity in a murine colorectal cancer model. Immunol Lett. 2010;131:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 142. | Moreira JN, Santos A, Simões S. Bcl-2-targeted antisense therapy (Oblimersen sodium): towards clinical reality. Rev Recent Clin Trials. 2006;1:217-235. [PubMed] [Cited in This Article: ] |
| 143. | Wiedenmann N, Koto M, Raju U, Milas L, Mason KA. Modulation of tumor radiation response with G3139, a bcl-2 antisense oligonucleotide. Invest New Drugs. 2007;25:411-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 144. | Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C, Luo FR, Wojtowicz-Praga S, Percent I, Saleh M. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5). Cancer Biother Radiopharm. 2010;25:13-19. [PubMed] [Cited in This Article: ] |
| 145. | Yada A, Yazawa M, Ishida S, Yoshida H, Ichikawa K, Kurakata S, Fujiwara K. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 146. | Merchant MS, Geller JI, Baird K, Chou AJ, Galli S, Charles A, Amaoko M, Rhee EH, Price A, Wexler LH. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J Clin Oncol. 2012;30:4141-4147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 147. | Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31-38. [PubMed] [Cited in This Article: ] |
| 148. | Cohn AL, Tabernero J, Maurel J, Nowara E, Sastre J, Chuah BY, Kopp MV, Sakaeva DD, Mitchell EP, Dubey S. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann Oncol. 2013;24:1777-1785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 149. | Ko CH, Shen SC, Yang LY, Lin CW, Chen YC. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int J Cancer. 2007;121:1670-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 150. | Choi JH, Yoon JS, Won YW, Park BB, Lee YY. Chloroquine enhances the chemotherapeutic activity of 5-fluorouracil in a colon cancer cell line via cell cycle alteration. APMIS. 2012;120:597-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 151. | Chu C, Noël-Hudson MS, Boige V, Goéré D, Marion S, Polrot M, Bigot L, Gonin P, Farinotti R, Bonhomme-Faivre L. Therapeutic efficiency of everolimus and lapatinib in xenograft model of human colorectal carcinoma with KRAS mutation. Fundam Clin Pharmacol. 2013;27:434-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |