Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17483
Revised: July 5, 2014
Accepted: September 12, 2014
Published online: December 14, 2014
AIM: To analyze prognostic factors for survival after transarterial chemoembolization (TACE) combined with microwave ablation (MWA) for hepatocellular carcinoma (HCC).
METHODS: Clinical data of 86 patients who underwent TACE combined with MWA between January 2006 and December 2013 were retrospectively analyzed in this study. Survival curves were detected using log-rank test. Univariate analysis was performed using log-rank test with respect to 13 prognostic factors affecting survival. All statistically significant prognostic factors identified by univariate analysis were entered into a Cox proportion hazards regression model to identify independent predictors of survival. P values were two-sided and P < 0.05 was considered statistically significant.
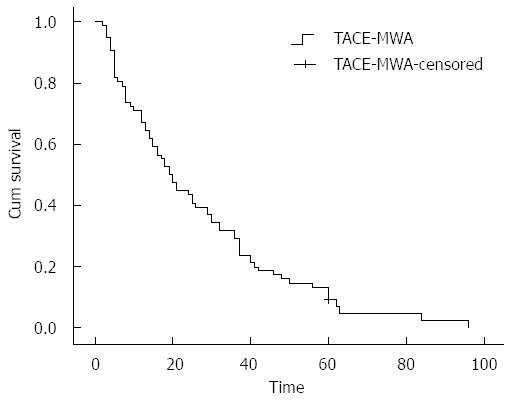
RESULTS: Median follow-up time was 47.6 mo, and median survival time of enrolled patients was 21.5 mo. The 1-, 2-, 3- and 5-year overall survival rates were 72.1%, 44.1%, 31.4% and 13.9%, respectively. Tumor size(χ2 = 14.999, P = 0.000), Barcelona Clinic Liver Cancer (BCLC) stage (χ2 = 29.765, P = 0.000), Child-Pugh class (χ2 = 51.820, P = 0.000), portal vein tumor thrombus (PVTT) (χ2 = 43.086, P = 0.000), arterio-venous fistula (χ2 = 29.791, P = 0.000), MWA therapy times (χ2 = 12.920, P = 0.002), Eastern Cooperative Oncology Group (ECOG) score (χ2 = 28.660, P = 0.000) and targeted drug usage (χ2 = 10.901, P = 0.001) were found to be significantly associated with overall survival by univariate analysis. Multivariate analysis identified that tumor size (95%CI: 1.608-4.962, P = 0.000), BCLC stage (95%CI: 1.016-2.208, P = 0.020), PVTT (95%CI: 2.062-9.068, P = 0.000), MWA therapy times (95%CI: 0.402-0.745, P = 0.000), ECOG score (95%CI: 1.012-3.053, P = 0.045) and targeted drug usage (95%CI: 1.335-3.143, P = 0.001) were independent prognostic factors associated with overall survival.
CONCLUSION: Superior performance status, MWA treatment and targeted drug were favorable factors, and large HCC, PVTT and advanced BCLC stage were risk factors for survival after TACE-MWA for HCC.
Core tip: Transarterial chemoembolization (TACE) combined with microwave ablation (MWA) has been used more and more widely for treatment of patients with hepatocellular carcinoma (HCC). However, there has been no study designed to analyze prognostic factors for survival after TACE combined with MWA for HCC. In this study, we retrospectively collected clinicopathologic data of 86 patients who were treated by TACE sequentially combined with MWA, and to analyze prognostic factors for survival after the combinational therapy. We hope that our finding could serve as significant information for clinicians and patients in the decision for selecting treatment strategies.
- Citation: Ni JY, Sun HL, Chen YT, Luo JH, Chen D, Jiang XY, Xu LF. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol 2014; 20(46): 17483-17490
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17483.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17483
Hepatocellular carcinoma (HCC) is a kind of highly aggressive malignant tumor, and it is one of leading causes of cancer death. HCC ranks fifth in incidence for men and eighth for women and accounts for more than 660000 new cases worldwide annually[1,2]. Surgical resection is still considered the preferred treatment choice for patients with early-stage HCC. However, the majority of HCC patients were diagnosed at intermediate or advanced stage of tumor growth and accompanied with poor hepatic function which was caused by hepatitis, alcoholic liver disease or cirrhosis, and made surgical resection impossible[3-5]. Liver transplantation also offers a treatment option for HCC patients, but the shortage of organ donors limits its application. In recent years, transarterial chemoembolization (TACE) as a palliative treatment has been accepted as the firstly considerable treatment for patients with surgically unresectable HCC[3,6,7]. However, the long-term outcomes of TACE were not satisfying. The complete necrosis rate of tumor tissue after TACE was just about 10%-20%[8-10]. Hence, in order to improve clinical effectiveness of TACE and provide better prognosis for patients with HCC, alternative treatment strategies are being explored. One such strategy is TACE sequentially combined with microwave ablation (MWA). MWA as a thermal in situ destruction treatment has been proved to be a safe and effective treatment[11,12].
TACE sequentially combined with MWA provides a new treatment choice for HCC. Previous studies had reported that clinical efficacy of combination of TACE and MWA was much better than that of TACE or MWA monotherapy in the treatment of HCC[13,14]. However, to the best of our knowledge, there has been no study designed to analyze prognostic factors for survival after TACE combined with MWA for HCC. In this study, we collected clinicopathologic data of patients who underwent TACE sequentially combined with MWA and to analyze prognostic factors for survival after the combinational therapy. We hope that our finding could serve as significant information for clinicians and patients in the decision for selecting treatment strategies.
Over a 5-year follow-up period between January 2006 and December 2013, we studied 86 patients with a formal diagnosis of HCC (according to criteria of the American Association for the Study of Liver Diseases). All included patients were treated by TACE sequentially combined with MWA in the Department of Interventional Radiology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, China. There were 76 males and 10 females, and their age ranged from 15 to 78 years (mean age, 54.9 years). Exclusion criteria were listed as following aspects: (1) patients without a definite diagnosis of HCC; (2) patients with diffuse-type HCC; (3) HCC after surgical treatment or liver transplantation; (4) patients with poor baseline hepatic function or general condition, who were not able to tolerate the treatments; (5) TACE combined with any other interventional procedure besides MWA, such as 125I seed implantation, radiofrequency ablation, and percutaneous ethanol injection; and (6) patients without regularly clinical data for evaluating overall survival and prognostic factors.
Main equipment used in this study was listed as follows: digital subtraction angiography (DSA) system (Philips Allura Xper FD20, Amsterdam, Netherlands), Siemens 64-slice spiral computed tomography (CT) system (Somatom 64 Sensation, Muenchen, Germany), ECO-100 water-cooled microwave apparatus and monopole microwave antenna (16G) (Nanjing Eco Medical Equipment Co., Ltd, Nanjing, China).
All 86 patients were initially treated by TACE. TACE was performed using DSA. Hepatic artery angiography was performed using the Seldinger technique. Femoral arterial catheterization was conducted through the common hepatic artery or proper hepatic artery. The location, number, size and blood supply of tumors were evaluated. If necessary, micro-catheter was super-selectively inserted into hepatic lobe or hepatic segmental artery. Chemoembolization therapy was then performed via the targeted artery using iodized oil emulsion (lipiodol mixed with chemotherapy drugs), and gelatin sponge embolization was used in the treatment of tumors with rich blood supply. Chemotherapeutics were performed using 5-FU/FUDR (0.5-1.0 g), THP-ADM (10-40 mg), hydroxycamptothecin (5-10 mg) or mitomycin (2-8 mg), regularly. The amount of iodized oil emulsion, gelatin sponge and medical chemotherapy drugs used in TACE treatment was based on tumor size, lesion extension and tolerance of patients.
After TACE treatment, a sequential CT-guided MWA procedure was performed based on the response of tumors. If residual tumors belong to stable situation or partial response such as focal lipiodol-defect region, progression of focal lesion and/or residual lesion with insufficient blood supply or occlusion of the feeding artery, the MWA procedure was applied sequentially with TACE.
During the MWA procedure, a non-enhanced CT scan was first performed to determine puncture pathway. The puncture site was then anesthetized with 2% lidocaine, and a 16G guided needle was inserted into focus of tumor via the puncture pathway. CT scan was performed again to ensure the location of the guided needle. MWA electrode probe was then inserted along the path of the puncture needle to reach the opposite edge of tumor through its center. The microwave power was set at 60-70 Watt and the procedure lasted for 10-20 min. Vital signs such as heart rate, blood pressure and oxygen saturation were monitored during the procedure. After ablation, the electrode probe and puncture needle were pulled out and CT scan was performed to reexamine coagulation area of MWA treatment.
In this study, follow-up was done by telephone or clinical visits at monthly interval. Physical examination, hepatic function test, alpha fetal protein (AFP) level and CT/magnetic resonance imaging (MRI) scan were reviewed. According to the result of post-ablation CT scan, incomplete ablation of tumor was reassessed by our multidisciplinary team of radiologists and oncologists in terms of tumor response and hepatic function. Complete response of tumor after further ablation treatment was defined as complete disappearance based on CT or MRI imaging.
Student’s t test and Fischer’s exact test were used to compare quantitative variables. The results are expressed as mean ± SD. Cumulative survival curve was calculated using the Kaplan-Meier method. The χ2 test was used to compare qualitative variables. The differences in the survival curves of different groups were detected using log-rank test. Univariate analysis was performed using log-rank test with respect to 13 prognostic factors affecting survival, including age, gender, BCLC stage, tumor size, portal vein tumor thrombus (PVTT), Child-Pugh class, Eastern Cooperative Oncology Group score, AFP, serum hepatitis B surface antigen, arterio-venous fistula (AVF), MWA therapy times, TACE therapy times and targeted drug usage. All statistically significant prognostic factors identified by univariate analysis were entered into a Cox proportion hazards regression model to identify independent predictors of survival. For all analyses, P-values were two-sided and P < 0.05 was considered statistically significant. All statistical analyses and graphics were performed using SPSS software package (version 19.0, SPSS Inc., Chicago, IL).
A total of 86 patients with surgically unresectable HCC were included in this study. Mean age of the included patients was 54.9 years (range: 15-78 years). There were 13 (15.1%), 32 (37.2%), 11 (12.8%) and 30 (34.9%) patients with small, nodular-type, massive-type and huge-type HCC, respectively. Branch and trunk portal vein tumor thrombi were detected in 10 (11.6%) and 9 (10.5%) patients, respectively. Serum hepatitis B surface antigen and AFP were positive in 41 (47.7%) and 55 (64.0%) patients, and no case had anti-HCV antibody detected. There were 18 (21.0%) patients with arterio-venous fistula, which was detected by digital subtraction angiography during TACE treatment. Sixty-four (74.4%) patients underwent treatment with targeted drugs such as sorafenib to control progression of tumor. Liver function was evaluated using Child-Pugh standard, and 62 (72.0%) cases were considered class A and 24 (28.0%) considered class B. More details of clinicopathological characteristics of the included patients are shown in Table 1.
| Characteristic | Patients |
| Age (yr) | |
| ≤ 40 | 17 (19.8) |
| 41-50 | 16 (18.6) |
| 51-60 | 29 (33.7) |
| > 60 | 24 (27.9) |
| Gender | |
| Male | 76 (88.3) |
| Female | 10 (11.7) |
| BCLC stage | |
| A | 4 (4.7) |
| B | 60 (69.7) |
| C | 22 (25.6) |
| Tumor size (TS) | |
| Small HCC (TS ≤ 3 cm) | 13 (15.1) |
| Nodular type (3 cm < TS ≤ 5 cm) | 32 (37.2) |
| Massive type (5 cm < TS ≤ 10 cm) | 11 (12.8) |
| Huge type (10 cm < TS) | 30 (34.9) |
| PVTT | |
| None | 67 (77.9) |
| Branch type | 10 (11.6) |
| Trunk type | 9 (10.5) |
| Child-Pugh class | |
| A | 62 (72.0) |
| B | 24 (28.0) |
| ECOG score | |
| 0 | 11 (12.8) |
| 1 | 45 (52.3) |
| ≥ 2 | 30 (34.9) |
| AFP | |
| Negative ( ≤ 20 μg/L) | 31 (36.0) |
| Positive (> 20 μg/L) | 55 (64.0) |
| HBsAg | |
| Without | 45 (52.3) |
| With | 41 (47.7) |
| AVF | |
| Without | 68 (79.0) |
| With | 18 (21.0) |
| MWA times | |
| Once | 35 (40.1) |
| Twice | 24 (27.9) |
| ≥ Triple | 27 (32.0) |
| TACE times | |
| Once | 19 (22.1) |
| Twice | 16 (18.6) |
| Triple | 21 (24.4) |
| ≥ Triple | 30 (34.9) |
| Targeted drug | |
| Without | 22 (25.6) |
| With | 64 (74.4) |
Median follow-up time in this study was 47.6 mo. Of 86 patients with unresectable HCC, 5 survived and 81 died. Median survival time was 21.5 mo (range: 2-96 mo), and the 1-, 2-, 3- and 5-year overall survival rates were 72.1% (62/86), 44.1% (38/86), 31.4% (27/86) and 13.9% (12/86), respectively. Kaplan-Meier survival curve of 86 patients who were treated by combined TACE-MWA is shown in Figure 1.
Univariate analysis revealed that tumor size (χ2 = 14.999, P = 0.000), BCLC stage (χ2 = 29.765, P = 0.000), Child-Pugh class (χ2 = 51.820, P = 0.000), PVTT (χ2 = 43.086, P = 0.000), AVF (χ2 = 29.791, P = 0.000), MWA therapy times (χ2 = 12.920, P = 0.002), ECOG score (χ2 = 28.660, P = 0.000) and targeted drug usage (χ2 = 10.901, P = 0.001) were significantly associated with overall survival of patients who underwent TACE sequentially combined with MWA (Table 2).
| Variable | Patients (n) | Median survival time (mo) | χ2 | P value |
| BCLC stage | ||||
| A | 4 | 36 (range: 25-60) | ||
| B | 60 | 25 (range: 3-96) | ||
| C | 22 | 5 (range: 2-40) | 29.765 | 0 |
| Tumor size | ||||
| Small HCC | 13 | 50 (range: 26-63) | ||
| Nodular type | 32 | 29 (range: 13-96) | ||
| Massive type | 11 | 12 (range: 4-60) | ||
| Huge type | 30 | 8 (range: 2-43) | 14.999 | 0 |
| PVTT | ||||
| None | 67 | 25 (range: 3-96) | ||
| Branch type | 10 | 5 (range: 2-40) | ||
| Trunk type | 9 | 4 (range: 3-6) | 43.086 | 0 |
| Child-Pugh class | ||||
| A | 62 | 30 (range: 4-96) | ||
| B | 24 | 7 (range: 2-19) | 51.820 | 0 |
| ECOG score | ||||
| 0 | 11 | 50 (range: 30-96) | ||
| 1 | 45 | 29 (range: 5-84) | ||
| ≥ 2 | 30 | 8 (range: 2-32) | 28.660 | 0 |
| AVF | ||||
| Without | 68 | 25 (range: 3-96) | ||
| With | 18 | 5 (range: 2-40) | 29.791 | 0 |
| MWA times | ||||
| Once | 35 | 14 (range: 2-60) | ||
| Twice | 24 | 20 (range: 3-60) | ||
| ≥ Triple | 27 | 32 (range: 8-96) | 12.920 | 0.002 |
| Targeted drug | ||||
| Without | 22 | 6 (range: 2-60) | ||
| With | 64 | 24 (range: 5-96) | 10.901 | 0.001 |
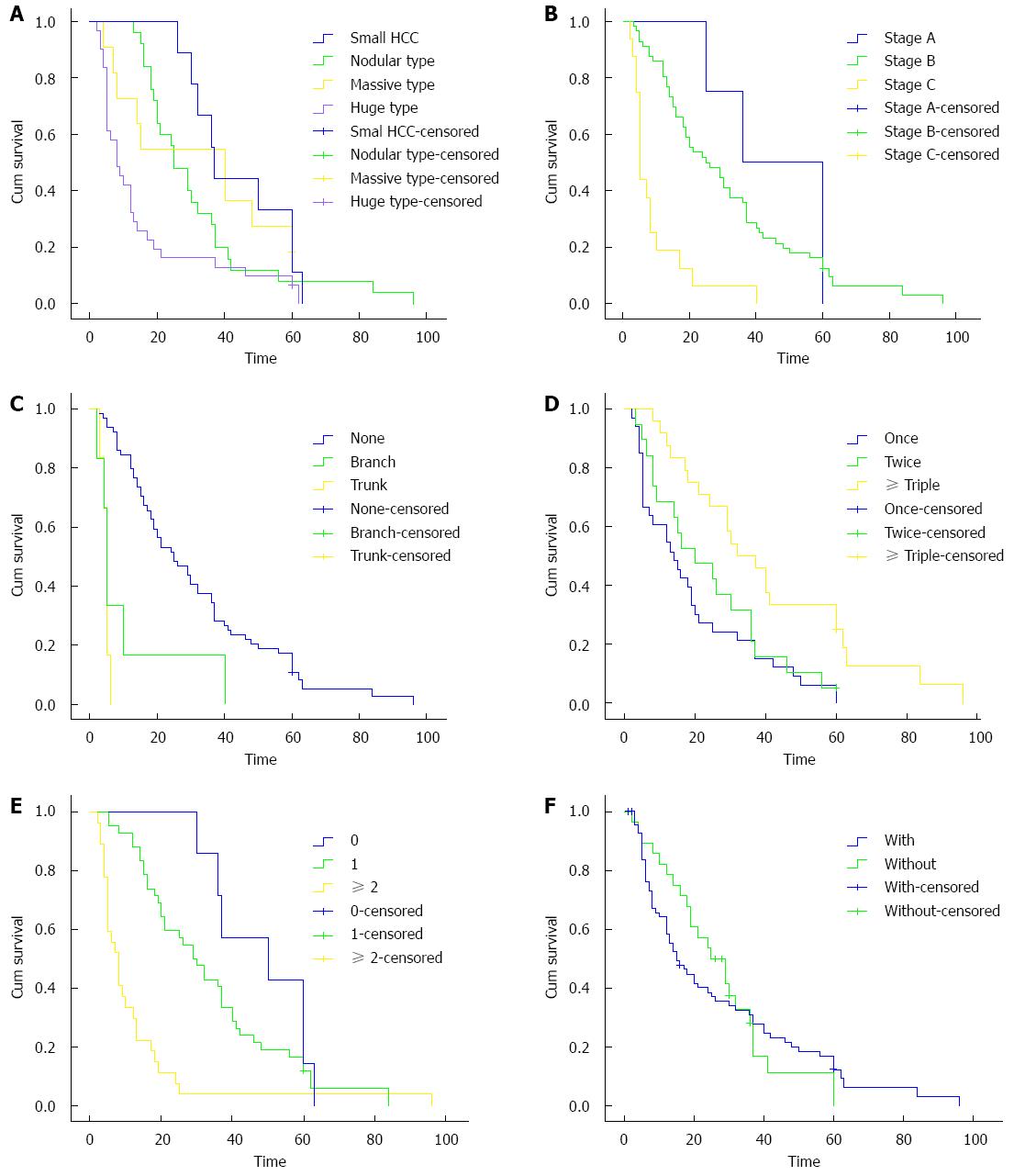
All statistically significant prognostic factors evaluated by univariate analysis were entered into a Cox proportion hazards regression model. The results of multivariate analysis identified that tumor size (95%CI: 1.608-4.962, P = 0.000), BCLC stage (95%CI: 1.016-2.208, P = 0.020), PVTT (95%CI: 2.062-9.068, P = 0.000), MWA therapy times (95%CI: 0.402-0.745, P = 0.000), ECOG score (95%CI: 1.012-3.053, P = 0.045) and targeted drug usage (95%CI: 1.335-3.143, P = 0.001) were independent prognostic factors associated with overall survival (Table 3). Survival curves of 86 patients with different characteristics of tumor size, BCLC stage, PVTT, MWA therapy times, ECOG score and targeted drug usage were shown in Figure 2A-F.
| Variable | B | SE | Sig | Exp(B) | Wald | 95%CI for Exp(B) | |
| Lower | Upper | ||||||
| Tumor size | 1.038 | 0.288 | 0 | 2.824 | 13.039 | 1.608 | 4.962 |
| PVTT | 1.464 | 0.378 | 0 | 4.324 | 15.011 | 2.062 | 9.068 |
| BCLC stage | 0.383 | 0.165 | 0.020 | 1.467 | 5.3830 | 1.016 | 2.028 |
| ECOG score | 0.564 | 0.282 | 0.045 | 1.757 | 4.0030 | 1.012 | 3.053 |
| MWA | -0.602 | 0.157 | 0 | 0.547 | 14.689 | 0.402 | 0.745 |
| TD | 0.670 | 0.213 | 0.001 | 2.107 | 10.203 | 1.335 | 3.143 |
Both TACE and MWA are minimally invasive treatments. There were no procedure-related mortalities or serious complications. The most common adverse effect was post-embolization syndrome, such as right upper quadrant abdominal pain, vomiting, nausea and fever. Abdominal pain and fever were found in patients who underwent MWA as well. Hepatic dysfunction was the second most common complication, which could be found by biochemical tests and clinical manifestations, including ascites, jaundice or bleeding during the period of follow-up. Symptomatic treatments were necessary for patients with hepatic dysfunction. No other complications emerged after TACE or MWA treatment.
Nowadays, TACE sequentially combined with MWA has been widely accepted as an important treatment option for surgically unresectable HCC. To the best of our knowledge, there has been no study designed to analyze prognostic factors for survival after combined TACE-MWA for HCC. In this study, we collected the clinical data of 86 patients who underwent TACE combined with MWA. Overall survival and prognostic factors were analyzed. We found that patients who were treated by TACE combined with MWA had relatively satisfying overall survival. In addition, our study revealed that tumor size, BCLC stage, PVTT, MWA therapy times, ECOG score and targeted drug usage were significantly independent factors effecting overall survival.
Although there was no comparative data in this study, our previous study had reported that combined TACE-MWA treatment was associated with better effectiveness in comparison with TACE monotherapy in HCC[14]. During previous clinical practice and experimental research, some synergistic effects between TACE and MWA could be observed. The advantages of combined TACE-MWA may be explained as following aspects: (1) embolization of tumor vessel by TACE was expected to enlarge thermal coagulation area of MWA by reducing the “cooling effect” of hepatic blood flow[13-15]; (2) edematous change in tumor tissues after TACE was expected to increase thermal effects of MWA; (3) some hypovascular HCCs, regenerated tumor feeding vessel or mimicking vessel after TACE made it difficult to perform TACE procedure, while imaging-guided MWA can destroy targeted tumors precisely and directly[16-18]; and (4) TACE sequentially combined with MWA can effectively decrease the liver function impairment caused by TACE monotherapy, and improve the prognosis of patients. All the above aspects indicated that combination of TACE and MWA had advantages in improving overall survival of patients with HCC[13,14].
Referring to prognostic factors for survival after combined TACE-MWA for unresectable HCC, we found that tumor size, BCLC stage, PVTT, MWA therapy times, ECOG score and targeted drug usage were independent prognostic factors associated with overall survival of patients.
Complete necrosis rate of tumor tissue after TACE was about 10%, while for large HCC it was much lower in clinical practice[19,20]. In the treatment of large HCC, it was difficult to reach complete necrosis for MWA. A previous study had reported that 1- and 3-year overall survival rates of patients who underwent MWA treatment were 92% and 72%, respectively[21]. In this study, we found that the effectiveness of combined TACE-MWA in small- and nodular-type HCC was superior to that in massive-type and huge-type HCC. Peng et al[22] had reported that the overall survival of patients with small HCC, who were treated by combination of TACE and radiofrequency ablation, was much better than that of patients with large-size HCC. Hence, our study revealed that large tumor lesion was associated with an increased risk for poor prognosis of patients with HCC.
In clinical practice, the BCLC staging system has been widely accepted as an important staging system that comprehensively considers tumor size, hepatic function and performance status of patients with HCC[23]. Our analysis showed that overall survival of patients with BCLC stage A HCC was much longer in comparison with patients with BCLC stages B and C. Our study suggested that BCLC stage was an independent prognostic factor for survival after combined TACE-MWA for HCC.
In this study, 10 and 9 of all enrolled patients had branch- and trunk-type PVTT, respectively, which was detected by DSA during TACE treatment. It was obvious that PVTT led to a high risk of intrahepatic and/or extrahepatic metastasis of cancer cells. Additionally, advanced tumor thrombus usually occluded the portal vein, and resulted in portal hypertension and further damage of hepatic function[24,25]. PVTT was also associated with hepatic arterio-portal fistula[26]. Ngan et al[27] had reported that hepatic arterio-portal fistula played a critic role in distant metastasis of tumor cells. In analysis of the effect of PVTT on overall survival, we found that PVTT significantly affected median survival time of patients with HCC. The survival time of patients with branch- and trunk-type PVTT was much shorter than that of patients without PVTT. Our study suggested that PVTT was an independent prognostic factor associated with survival.
Progression and metastasis of residual tumor cells after TACE or MWA seriously affected long-term survival of patients with HCC. TACE sequentially combined with MWA can significantly enhance the necrosis rate of tumor tissue[13,14]. Our analysis suggested that MWA treatment times was an independent prognostic factor associated with overall survival.
Good performance status is very important for patients to tolerate interventional therapy and chemotherapeutics. Sorafenib tosylate as a targeted drug has been used more and more widely to control progression of tumor[28-30]. We found that the prognosis of patients who were treated with targeted drugs was much better in comparison with patients without usage of targeted drugs.
In conclusion, the data of our study indicated that superior performance status, MWA treatment and targeted drug usage were favorable factors, and large HCC, PVTT and advanced BCLC stage were risk factors for survival after TACE-MWA for HCC.
Transarterial chemoembolization (TACE) combined with microwave ablation (MWA) has been used more and more widely in the treatment for hepatocellular carcinoma (HCC). Previous studies had reported that combined TACE-MWA was associated with better effectiveness in comparison with TACE or MWA monotherapy. However, there has been no study designed to analyze prognostic factors for survival after combined TACE and MWA for HCC.
In the current report, authors designed an analysis to study prognostic factors for survival after combined TACE-MWA, and the study is the first one to analyze prognostic factors for survival after TACE combined with MWA for HCC.
In this study, 13 prognostic factors were enrolled. In univariate analysis, authors found that tumor size, Barcelona Clinic Liver Cancer (BCLC) stage, Child-Pugh class, portal vein tumor thrombus (PVTT), arterio-venous fistula, MWA therapy times, Eastern Cooperative Oncology Group (ECOG) score and targeted drug usage were significantly associated with survival. Multivariate analysis showed that tumor size, BCLC stage, PVTT, MWA therapy times, ECOG score and targeted drug usage were independent prognostic factors associated with overall survival of patients who underwent TACE sequentially combined with MWA
In this study, authors found that tumor size, BCLC stage, PVTT, MWA therapy times, ECOG score and targeted drug usage independently affected the survival of patients who were treated by combined TACE-MWA. They believe that their finding could serve as significant information for clinicians and patients in the decision for selecting treatment strategies.
TACE is one of the most widely performed treatments for unresectable HCC, which is a type of interventional radiological treatment. MWA is a thermal in situ destruction technique, and it has been proved to be a safe and effective treatment. MWA has been accepted as one of the best treatment options for early-stage HCC.
This is an interesting manuscript describing the various factors that impact survival in HCC patients undergoing TACE and MWA. Being a retrospective analysis, it has its inherent drawbacks. However, the results support the outcomes of others where radiofrequency ablation/MWA has been combined with TACE. As expected the outcomes correlate with liver function, tumor size and PVTT. The main limit of the paper is the use of inferential analyses in a small cohort study.
P- Reviewer: Kapoor S, Lai Q S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Ma S
| 1. | Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 626] [Cited by in F6Publishing: 702] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 2. | Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA. World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): a global perspective. J Gastrointestin Liver Dis. 2010;19:311-317. [PubMed] [Cited in This Article: ] |
| 3. | Padhya KT, Marrero JA, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2013;29:285-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 118] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | McCurdy HM. Improving outcomes for patients receiving transarterial chemoembolization for hepatocellular carcinoma. Gastroenterol Nurs. 2013;36:114-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Kishi Y, Saiura A, Yamamoto J, Koga R, Seki M, Morimura R, Yoshioka R, Kokudo N, Yamaguchi T. Preoperative transarterial chemoembolization for hepatocellular carcinoma. Hepatogastroenterology. 2012;59:2295-2299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 8. | Marelli L, Shusang V, Buscombe JR, Cholongitas E, Stigliano R, Davies N, Tibballs J, Patch D, Meyer T, Burroughs AK. Transarterial injection of (131)I-lipiodol, compared with chemoembolization, in the treatment of unresectable hepatocellular cancer. J Nucl Med. 2009;50:871-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, Lim YS, Lee HC, Chung YH, Lee YS. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109-114. [PubMed] [Cited in This Article: ] |
| 11. | Tombesi P, Di Vece F, Sartori S. Resection vs thermal ablation of small hepatocellular carcinoma: What’s the first choice? World J Radiol. 2013;5:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Minami Y, Kudo M. Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4952-4959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Liu C, Liang P, Liu F, Wang Y, Li X, Han Z, Liu C. MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperthermia. 2011;27:654-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Xu LF, Sun HL, Chen YT, Ni JY, Chen D, Luo JH, Zhou JX, Hu RM, Tan QY. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28:456-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Fong ZV, Palazzo F, Needleman L, Brown DB, Eschelman DJ, Chojnacki KA, Yeo CJ, Rosato EL. Combined hepatic arterial embolization and hepatic ablation for unresectable colorectal metastases to the liver. Am Surg. 2012;78:1243-1248. [PubMed] [Cited in This Article: ] |
| 16. | Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK, Sung KB, Kim PN. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012;81:e189-e193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Yang W, Chen MH, Wang MQ, Cui M, Gao W, Wu W, Wu JY, Dai Y, Yan K. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res. 2009;39:231-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Shen SQ, Xiang JJ, Xiong CL, Wu SM, Zhu SS. Intraoperative radiofrequency thermal ablation combined with portal vein infusion chemotherapy and transarterial chemoembolization for unresectable HCC. Hepatogastroenterology. 2005;52:1403-1407. [PubMed] [Cited in This Article: ] |
| 19. | Kawaguchi T, Ohkawa K, Imanaka K, Tamai C, Kawada N, Ikezawa K, Uehara H, Itou Y, Nakanishi K, Katayama K. Lipiodol accumulation and transarterial chemoembolization efficacy for HCC patients. Hepatogastroenterology. 2012;59:219-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 353] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 21. | Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299-307. [PubMed] [Cited in This Article: ] |
| 22. | Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 24. | Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561-7567. [PubMed] [Cited in This Article: ] |
| 25. | Aldrighetti L, Pulitanò C, Catena M, Arru M, Guzzetti E, Halliday J, Ferla G. Liver resection with portal vein thrombectomy for hepatocellular carcinoma with vascular invasion. Ann Surg Oncol. 2009;16:1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Nie L, Luo XF, Li X. Gastrointestinal bleeding caused by extrahepatic arterioportal fistula associated with portal vein thrombosis. World J Gastroenterol. 2012;18:6501-6503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997;52:36-40. [PubMed] [Cited in This Article: ] |
| 28. | Genco C, Cabibbo G, Maida M, Brancatelli G, Galia M, Alessi N, Butera G, Genova C, Romano P, Raineri M. Treatment of hepatocellular carcinoma: present and future. Expert Rev Anticancer Ther. 2013;13:469-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | D’Angelo S, Secondulfo M, De Cristofano R, Sorrentino P. Selection and management of hepatocellular carcinoma patients with sorafenib: recommendations and opinions from an Italian liver unit. Future Oncol. 2013;9:485-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Kim MS, Jin YJ, Lee JW, Lee JI, Kim YS, Lee SY, Chae MH. Complete remission of advanced hepatocellular carcinoma by sorafenib: A case report. World J Gastrointest Oncol. 2013;5:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |