Published online Nov 28, 2014. doi: 10.3748/wjg.v20.i44.16443
Revised: August 8, 2014
Accepted: September 29, 2014
Published online: November 28, 2014
Chronic abuse of alcohol leads to various histological abnormalities in the liver. These are conditions collectively known as alcoholic liver disease (ALD). Currently, ALD is considered to be one of the major causes of death worldwide. An impaired intestinal barrier with related endotoxemia is among the various pathogenetic factors. This is mainly characterized by circulating levels of lipopolysaccharide (LPS), considered critical for the onset of intra-hepatic inflammation. This in turn promotes hepatocellular damage and fibrosis in ALD. Elevated levels of LPS exert their effects by binding to Toll-like receptors (TLRs) which are expressed by all liver-resident cells. The activation of TLR signaling triggers an overproduction and release of some cytokines, which promote an autocatalytic cascade of other pro-inflammatory signals. In this review, we provide an overview of the mechanisms that sustain LPS-mediated activation of TLR signaling, reporting current experimental and clinical evidence of its role during inflammation in ALD.
Core tip: Alcoholic liver disease (ALD) pathogenesis is quite complex and requires the activation/inhibition of several molecular pathways. The inflammatory storm caused by alcohol abuse on the gut-liver axis and consequent activation of Toll-like receptor (TLR) signaling is topical for researchers and physicians, both for understanding ALD pathophysiology and for translating novel clues into clinical practice. Here, we focus on the current evidence of TLR involvement in inflammation during ALD in experimental models and humans, offering readers with no first-hand knowledge of this topic a valuable tool to start novel studies.
- Citation: Ceccarelli S, Nobili V, Alisi A. Toll-like receptor-mediated signaling cascade as a regulator of the inflammation network during alcoholic liver disease. World J Gastroenterol 2014; 20(44): 16443-16451
- URL: https://www.wjgnet.com/1007-9327/full/v20/i44/16443.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i44.16443
Toll-like receptors (TLRs) belong to the family of pattern recognition receptors and are crucial sensors of the innate immune system committed to the recognition of both pathogen and damage-associated molecular patterns (PAMPs and DAMPs, respectively)[1-4]. TLRs are key molecules of the innate immune system which play a major role in the control of the inflammation process, promoting the production of several circulating inflammatory molecules, including cytokines, chemokines and other molecules that may participate in tissue repair or exacerbate tissue damage in several diseases[5]. It is noteworthy that TLRs have also been implicated in both liver physiology and in the pathophysiology of several liver diseases as they are diffusely expressed in all types of liver cells[6-8].
The integrity of the intestinal barrier and appropriate gut permeability are crucial for maintaining the equilibrium of commensal and pathogenic microorganisms and for avoiding their translocation from the gut. Normally, only bacterial traces can pass the intestinal mucosa and reach the liver through the portal circulation where their clearance is accomplished. A large amount of the literature in animal models and humans has reported that excessive alcohol consumption increases intestinal permeability, disrupting the intestinal barrier and leading to a strong increase of portal and systemic levels of the most studied PAMP, lipopolysaccharide (LPS)[9-12]. However, only recently through novel metagenomic and metaproteomic approaches, the ability of acute and chronic ingestion of alcohol to alter gut microbiota composition by increased bacterial overgrowth and contributing to liver damage and inflammation has emerged[13-15].
Over the past decade, despite numerous prevention campaigns, alcohol consumption is still at alarming levels, particularly in industrialized countries[16]. Therefore, alcohol abuse is currently considered to be one of the major causes of chronic liver disease in Western countries, particularly in Europe, southern Europe and the United Kingdom[17]. Prevalently, heavy drinkers are susceptible to develop alcoholic liver disease (ALD) which may be characterized by different histological abnormalities, including steatosis, steatohepatitis and fibrosis, and evolving into more severe forms of liver injury, such as cirrhosis and hepatocellular carcinoma (HCC)[17,18].
During the last decade, the importance of research and clinical studies of the underlying molecular mechanisms that link TLRs and ALD have received increasing interest, particularly because of their therapeutic inference.
In the present review, we focus on the implication of TLRs and their role in inflammation in ALD pathogenesis and we provide an overview of their possible clinical impact in prevention and therapy.
TLRs are regulators of innate immune response and sensors of both the pathogen signature bacteria, fungi and virus PAMPs and the endogenous components, DAMPs. They are highly conserved type I transmembrane proteins which comprise an extracellular leucine-rich ligand binding domain and an intracellular domain, Toll/interleukin (IL)-1 receptor (TIR) domain, responsible for their intracellular signal transduction[19]. TLRs have been classified based on their ligand specificity and selectivity, accounting for more than 13 members in mammals, of which 11 are expressed in humans. However, each human TLR exhibits differential activities, depending on its tissue expression and ligand specificity[20].
In the liver, TLRs are expressed in Kupffer cells (KCs), hepatocytes and hepatic stellate cells (HSCs) and they have been extensively studied in various chronic liver diseases[21]. In more detail, TLR2 and TLR4 expression is shared by hepatocytes, KCs, HSCs and biliary epithelial cells, while TLR4 is also expressed by sinusoidal endothelial cells. Moreover, KCs also express both TLR3 as biliary epithelial cells and TLR9, similar to HSCs and sinusoidal endothelial cells[22].
LPS, a component of Gram-negative bacteria walls, composed of a carbohydrate (O-antigen), an oligosaccharide region and a lipid part (called Lipid A), is the ligand of TLR4. TLR4 cannot bind directly as the LPS molecule requires a complex assembly composed by the CD14 co-receptor which facilitates the transfer of LPS to TLR4 complex and MD-2, an adapter molecule that modulates the LPS recognition. Another cofactor is LPS-binding protein (LBP) that shuttles LPS to the CD14 molecule. The association of these auxiliary molecules triggers the signal, resulting in the homodimerization of TLR4 molecules and consequent signaling[23,24].
Once TLRs have bound their specific ligands on the cell membrane, they transduce the downstream signal by means of the myeloid differentiation factor 88 (MyD88), a common molecule adaptor for all TLRs except TLR3[25]. Actually, the TLR downstream signaling can be distinguished as MyD88-dependent or MyD88-independent pathways with the alternative adapter molecule as TIR-domain-containing adapter-inducing interferon-β (TRIF). As a final effect, MyD88-dependent cascade leads to the activation of nuclear factor-κB and activating protein-1 (AP-1) by means of IκB kinase (IKK) complex and mitogen-activated protein kinases (MAPKs) respectively. NF-κB and AP-1, in turn, conduct the production of specific pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-6 and IL-1β[25]. In the liver, the MyD88 pathway also promotes the activation of the LPS-induced TNF-α factor (LITAF), another transcription factor that regulates the transcription of pro-inflammatory cytokines[26]. Conversely, MyD88-independent cascade predominantly induces the expression of Type I interferons by the activation of IRF-3 through the critical regulators TANK-binding kinase 1 (TBK1) and IKKs[27].
KC-dependent production and release of pro-inflammatory cytokines, such as TNF-α and IL-1β, will further increase production and release of other pro-inflammatory cytokines (i.e., IL-6 and IL-8) and chemoattractant factors in a vicious cycle, leading to the activation of other immune cells, including neutrophils, monocytes and lymphocytes. These immune cells in turn also respond to TLR activation in KCs, generating reactive oxygen species (ROS), increasing phagocytosis and secreting anti-microbial peptides and a cascade of additional pro-inflammatory molecules[28].
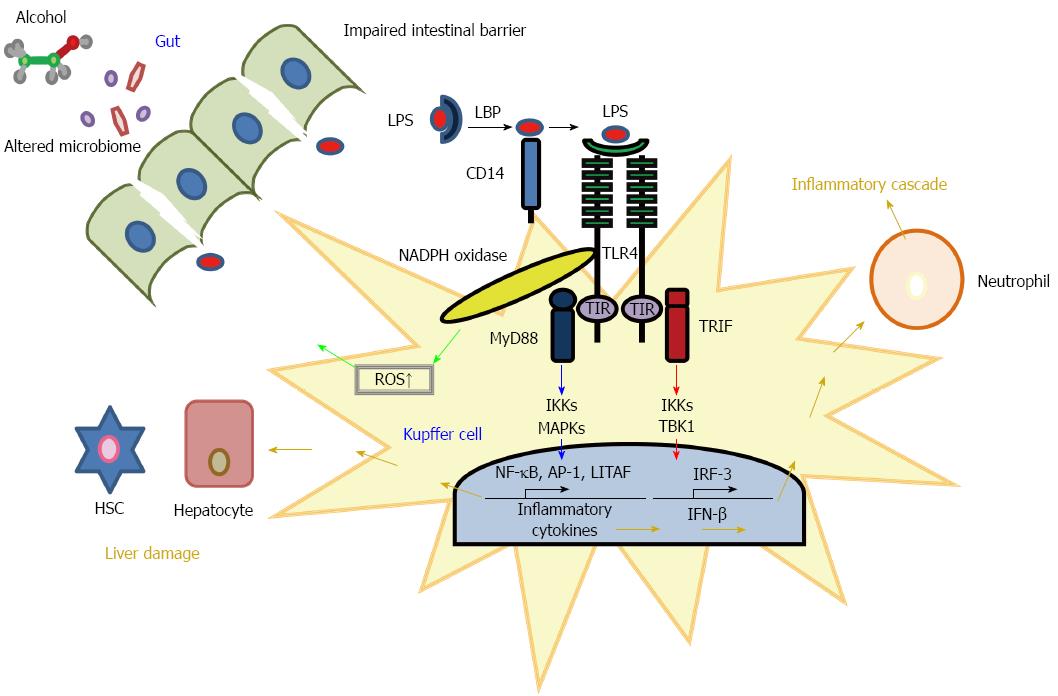
In Figure 1 we have schematized the LPS-mediated TLR4 signaling pathways leading to intra-hepatic inflammation.
ALD is considered to be one of the major causes of death worldwide, accounting for half of alcohol-related fatalities[17]. Excessive alcohol consumption may lead to several liver tissue abnormalities. Therefore, histological features in ALD include steatosis, steatohepatitis and fibrosis, which may progress to cirrhosis and ultimately to HCC[29]. Although research interests in ALD pathogenetic mechanisms have increased during the last decades, some relevant pathways have only recently been characterized[30]. Studies on the direct effect of alcohol on intestinal permeability have shown that acetaldehyde, the most toxic metabolite of ethanol metabolism, has a major role in the increment of tight junction tyrosine phosphorylation, conducting altered localization of both tight junction (occludin and ZO1) and adherens junction (E-cadherin and β-catenin) molecules[31,32].
Intra-hepatic lipid accumulation and steatosis in people who drink a toxic alcohol amount (40-80 g/d for men and 20-40 g/d for women for 10 years) is characterized by the impairment of the major pathways involved in alcohol metabolism, including its conversion to acetate by the activity of specific dehydrogenases which completes the conversion to acetate and mitochondrial fatty acid oxidation[33]. Furthermore, development of the progressive severe forms of ALD-related liver damage, such as steatohepatitis and fibrosis, results from a complex interplay between the oxidative stress due to release of ROS and the activation of several components of the innate immune system due to endotoxins such as LPS[34]. Besides, several studies suggested that LPS increases cytokine levels by NAPDH oxidase and ROS production in macrophages via the NF-κB pathway[35]. Although the complex upstream and downstream events and molecules that may explain the LPS-mediated mechanisms in ALD have been a subject of deep investigation for decades, they are only recently becoming clear[21]. Alcohol-induced LPS accumulation and consequent endotoxemia may occur by the alteration of gut-microbiome composition, which leads to endotoxin over-production and consequent disruption of intestinal barrier integrity. Therefore, the increased alcohol-mediated endotoxemia may reduce the detoxifying ability of KCs and the activation of the TLR4-mediated inflammatory response that promotes hepatocellular injury. Experimental and clinical evidence of this complex network of TLR4-associated upstream and downstream factors involved in ALD-associated inflammation are reported in the next paragraphs.
The involvement of gut-derived microbial products, such as LPS, which can pass the systemic and portal circulation due to the disruption of the intestinal barrier caused by ethanol was suggested several years ago[36]. Furthermore, Keshavarzian et al[37] demonstrated that intestinal barrier dysfunction and endotoxemia are early events preceding ethanol-dependent hepatic injury in rats. Particularly, serum LPS has been found to be increased in animal models resembling human ALD[38].
The relevance of TLR4-dependent KC activation followed by the initiation of the inflammatory cascade in the pathogenesis of alcohol-induced liver damage has been also proved in models of ALD[39,40]. Furthermore, studies performed on mice that have a functional mutation in the TLR4 gene showed an impaired response to bacterial endotoxins[40]. Moreover, LBP knockout mice were significantly reduced in the pathological parameters characterizing ethanol-fed mice, such as endotoxin levels, steatosis, inflammation and liver injury[41]. Further, CD14 knockout mice were protected from alcohol-caused severe liver injury and from ethanol-induced NF-κB, transforming growth factor (TGF)-β and TNF-α increase[42]. As previously described, TLR4 signaling encompasses two distinct cascades: the MyD88-dependent and the TRIF-dependent (MyD88-independent).
These downstream pathways have been widely studied in animal models in order to clarify their respective involvement in ALD pathogenesis. Experimental evidence established that in ALD, the TLR4 down-stream signaling is principally regulated by the MyD88-independent cascade. In fact, while TLR4-knockout (KO) mice were protected from alcohol-induced liver damage, ROS production and inflammation, MyD88-KO mice were not, both being exposed to the Lieber-De-Carli diet[43]. Moreover, the disruption of MyD88-independent signaling, in studying TRIF-deficient mice, reported protection from alcohol-induced liver disease[44]. Additionally, the lack of IRF-3, a transcription factor downstream to TLR4/TRIF, resulted in preserving IRF-3-KO mice from alcohol-induced liver injury, steatosis and inflammation[45]. This evidence demonstrates the dispensable role of MyD88 adapter in TLR4-mediated liver injury.
Recently, the involvement of the protein kinase C (PKC) activity in the increase of intestinal permeability related to alcohol consumption has been described[46]. Specifically, both in vitro and in vivo evidence showed that alcohol, in a dose-dependent manner, increases TLR4 expression, which in turn results in augmented PKC activity. The consequent reduction of occludin phosphorylation alters the intercellular junctions, leading to the intestinal permeability increment[46].
KCs are the primary cells that respond to LPS via TLR4-dependent pro-inflammatory cytokines (as TNF-α, IL-1β, IL-8, IL-6) and leading to liver inflammation. In addition, in animal models of ethanol exposure, hepatocytes can be driven to accumulate lipid and augment TLR4 levels, altering their TLR4 sensitivity and LPS hepatotoxicity[47,48]. Moreover, damaged hepatocytes can release high-mobility group box 1 (HMGB1), an endogenous ligand that can be recognized by TLR4 and participate with a mechanism similar to that of non-alcoholic fatty liver disease in the promotion of ALD[7]. In fact, Wang et al[49] found that HMGB1 serum levels increased in chronic alcohol feeding of mice and its balance with milk fat globule-EGF factor 8 may control macrophage efferocytosis.
Interestingly, a recent study in TLR4 deficient mice transplanted with bone marrow (BM)-derived cells (KCs) or non-BM-derived cells (HSCs included) demonstrated that in both liver cell lineages, TLR4 is essential for the progression of alcohol-induced liver steatosis, inflammation, injury and fibrosis[50]. Besides, ROS production has been implicated in the alcohol-induced sensitization to LPS. In more detail, it has been shown that the direct interaction between TLR4 and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase isozyme (Nox)-4 leads to NF-κB activation and LPS-mediated ROS generation in vitro[51]. In a chronic alcohol-fed rat model, the inhibition of NADPH oxidase through diphenyleneiodonium (DPI) diminished the LPS-induced ERK1/2 phosphorylation and both ROS and TNF-α levels in KCs[52]. Furthermore, in C57Bl6/J mice fed a modified Lieber-De Carli diet, DPI protected from steatosis and liver inflammation induced by the usage of diverse bacterial components specific for different TLRs[48]. Previous evidence also showed that mice p47 phox -/-, depleted of the main cytosolic part of NADPH oxidase, were preserved from alcohol-induced liver injury, corroborating the crucial role of oxidative stress in TLR signaling along the ALD condition[53].
By the way, TLR4 is an important player in the development of fatty liver, although how it can influence lipid metabolism has not been completely clarified. Experimental studies on TLR4 mutant animal models have documented that TLR4 is involved in lipid accumulation and lipid peroxidation imbalance[40,54].
It is established that KC activation due to alcohol intake can lead to an increment in the production of prostaglandin (PG) E(2) which, interacting with prostanoid receptors and augmenting cAMP, can build up triglyceride in the liver. Furthermore, hepatic fat accumulation can be induced by the augmentation of the NADH/NAD(+) ratio, the sterol regulatory element-binding protein-1 (SREBP-1) activity and the decrease of peroxisome proliferator-activated receptor-alpha (PPAR-alpha) activity[55,56].
Also, microRNAs (miRNAs) have been implicated in the inflammation process in ALD and its complications. MiRNAs are conserved single-strand small noncoding RNAs which have a post-transcriptional gene-expression regulation function. They act on the 3’-untranslated mRNA target with whom miRNAs pair with different specificity. This match influences RNA stability and degradation or the translation and finally leads to protein repression[57]. Specifically, miR-155 is a ruler of inflammation and TLR signaling. It regulates TNF-α mRNA stability, as demonstrated in in vitro experiments performed on alcohol-treated macrophages[58]. Such evidence has been confirmed in a mouse model of ALD in which isolated KCs exhibited increased levels of both miRNA-155 and TNF-α. The same author showed that in alcohol-fed mice there are augmented serum/plasma miR-155 levels and an increased quantity of TNF-α in the liver[59]. In the same model, the authors evidenced an increase of serum/plasma miR-122 which is known to exert multiple functions in hepatocytes as it is important in the regulation of cholesterol metabolism[59,60]. Furthermore, the increase of both miR-155 and miR-122 was absent in TLR4-deficient mice that were preserved from ALD[59]. It is also noteworthy that treatment with TLR9 ligands (that is CpG) leads to plasma increase of both miR-155 and miR-122 and increased levels of TLR9 and serum endotoxin have been reported in alcohol-fed mice[48,59]. Actually, to date, diverse TLRs have been extensively studied and found to be involved in ALD pathogenesis. In the Lieber-DeCarli chronic alcohol feeding model, expression of TLR1, 2, 4, 6, 7, 8 and 9 liver mRNA resulted from increased enteral administration concomitantly to steatosis induction. It has been also demonstrated that the alcohol treatment sensitizes hepatic inflammation and damage since the triggering of the TLR1, 2, 4, 6, 7, 8 and 9 with their specific ligands resulted in TNF-α augmentation. Moreover, TLR expression remained unaffected after antibiotic treatment which, however, ameliorated the alcoholic fatty liver condition[48]. Recently, Byun et al[61] studied the TLR3 involvement in alcohol liver injury both in HSCs and KCs in vivo. The authors demonstrated, by means of TLR3-KO and IL-10-KO mice fed a high-fat diet with added ethanol, that TLR3 is protective for alcoholic liver injury and exerts this function by stimulating IL-10 production. In more detail, polyinosinic-polycytidylic acid (poly I:C, that is TLR3 ligand) administration ameliorated alcoholic liver damage and diminished the amount of TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1) in control mice. The preservative action of poly I:C was abrogated in TLR3-KO and IL-10-KO murine models. Coherently, HSCs and KCs isolated from poly I:C-treated animals had higher levels of IL-10 than the controls. IL-10 was also over-expressed in vitro both in HSCs and KCs in the presence of poly I:C[61].
The most relevant experimental studies on the involvement of TLRs in inflammation during ALD are summarized in Table 1.
| Ref. | Experimental model | Upstream factor | Involved TLRs | Effects |
| Enomoto et al[39], 2000 | Ethanol-fed rats | LPS | TLR4 | Kupffer cell activation and consequent inflammation |
| Uesugi et al[40], 2001 | TLR4 non-functional mice fed ethanol | LPS | TLR4 | Decreased: |
| Steatosis | ||||
| Inflammation | ||||
| Focal necrosis | ||||
| Park et al[51], 2004 | HEK293T cells | Nox4 | TLR4 | NF-κB activation |
| LPS-mediated ROS generation | ||||
| Gustot et al[48], 2006 | Mice fed a modified Lieber-DeCarli diet | Increased: | ||
| LTA, PGN | TLR2 | Steatosis | ||
| LPS | TLR4 | Liver weight -aminotransferase levels | ||
| flagellin | TLR5 | Liver mRNA expression of different TLRs. | ||
| Addition of multiple bacterial products | Increased: | |||
| loxoribine | TLR7 | TNF-α mRNA expression | ||
| CpG | TLR9 | Liver inflammatory infiltrate | ||
| Hritz et al[43], 2008 | TLR4-(KO) mice feed Lieber-De-Carli diet | TLR4 | Protection from: | |
| Alcohol-induced liver damage | ||||
| ROS production | ||||
| Inflammation | ||||
| Bala et al[58], 2011 | ALD mouse model | LPS | TLR4 | MiRNA-155 increase and TNF-α levels in isolated KCs |
| Bala et al[59], 2012 | ALD mouse model | Plasma increase of: | ||
| administered with CpG + LPS | CpG | TLR9 | miR-155, miR-122 and miR-146a | |
| TLR4-KO | Protection from: | |||
| LPS | TLR4 | Alcohol-induced liver disease | ||
| Increase of both miR-155 and miR-122 | ||||
| Byun et al[61], 2013 | Mice feed high-fat diet plus binge ethanol | poly I:C | TLR3 | Protection from alcoholic liver damage |
| TLR3 (KO) mice | Preservative action of poly I:C abrogated |
The role of gut dysbiosis and increased gut permeability and endotoxemia into the portal circulation has also been extensively explored in patients with ALD[9,62]. Consequently, the critical role exerted by TLR signaling in human ALD has been investigated. Schäfer et al[63] found that circulating CD14 levels were higher in serum from patients with severe alcoholic hepatitis than in those from healthy controls, suggesting the crucial role of this TLR4 co-receptor in ALD. Furthermore, it has been reported that the CD14-159C>T polymorphism in patients with ALD may significantly correlate with the severity of disease and be associated with the risk for alcoholic cirrhosis[64,65]. However, no correlation has been found between TLR4, TNF-α and IL-1β gene variants and ALD in different populations to date[66,67].
Clinical evidence of the TLR involvement in intra-hepatic inflammatory response in ALD is still scarce but reinforces the experimental data reported above. The role of TLRs on neutrophils in patients with ALD was reported by Stadlbauer et al[68]. These authors demonstrated that LPS-induced over-expression of TLR2, 4 and 9 may play a pivotal role in the neutrophil dysfunction observed in patients with alcoholic hepatitis and suggested that the use of TLR antagonists was unable to prevent this dysfunction, while the use of an endotoxin scavenger might reduce inflammatory response and improve clinical outcome[69]. Interestingly, other authors found an impairment of TLR2 but not TLR4-mediated innate immune response in peripheral blood monocytes from patients with stable chronic ALD, explaining their susceptibility to immunodeficiency and disease worsening[70].
Interestingly, it has been reported that TLR-dependent priming of B cells might explain the increased circulating levels of immunoglobulins in patients with alcoholic liver cirrhosis[71].
As mentioned above, most experimental studies reported that NF-κB transcriptional activity is crucial for the TLR signal and Stärkel et al[72] demonstrated that a persistent activation of this transcription factor, an up-regulation of TLR3 and TLR7 expression and high pro-inflammatory cytokine levels were associated with end-stage ALD and involved in disease progression in humans.
Additional proof of the activation of the TLR-dependent inflammatory network in ALD was reported by the analysis of circulating levels of cytokines and chemokines. In fact, several authors found high levels of pro-inflammatory cytokines, including TNF-α and IL-6, in the serum of actively drinking patients with ALD, predicting their outcome and long-term survival[73,74]. Interestingly, fibroblast growth factor-inducible 14 (Fn14), a member of the TNF receptor superfamily (member 12A), was found to be over-expressed in the liver, primarily by hepatic progenitors, in patients with alcoholic hepatitis and it correlated with disease severity[75]. In addition, it has been reported that several chemokines, including IL-8, correlated with a worse prognosis in patients with alcoholic hepatitis[76].
Finally, a recent study demonstrated that increased serum levels of chemokine-ligand-1 (CXCL1), an inflammatory chemokine mainly expressed by mononuclear cells in response to LPS-dependent TLR activation, coupled with a polymorphism in the gene encoding for this protein are risk factors for alcoholic cirrhosis[77].
ALD remains one of the major causes of hepatic-associated morbidity and mortality worldwide. Furthermore, the current therapeutic options for patients with ALD are alcohol abstinence and liver transplantation for end-stage liver disease. The liver inflammatory response, which is generated early in ALD, is probably triggered by bacteria and their products, particularly LPS. Due to increased intestinal permeability, they translocate into circulation and activate TLR-dependent production and secretion of pro-inflammatory cytokines and chemokines. There is a body of experimental evidence of the crucial role of TLR-mediated inflammatory network in ALD development and progression. However, to date, these findings have little translational proof in patients. More studies in humans are urgently needed to advance the field and to translate experimental/informative findings into novel therapies.
Furthermore, as qualitative and quantitative changes of the gut microbiome play a major role in the TLR-dependent liver inflammatory response and ALD pathogenesis, further metagenomic, transcriptomic and metabolomic studies may help, not only to explain the “gut-liver axis’’ in ALD, but also to identify novel potential therapeutic targets. In fact, according to previous studies, treatment with probiotics inhibits alcohol-induced TLR4 and TLR5 activation of TNF-α production, reducing hepatic inflammation in experimental models, and restores neutrophil phagocytic ability in patients with alcoholic cirrhosis, probably by changing TLR4 expression and IL-10 secretion[78,79].
P- Reviewer: Abraham P, Apte MV, Corrales FJ, Gong ZG, Lee MK S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wang CH
| 1. | Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1144] [Cited by in F6Publishing: 1148] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 2. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5843] [Cited by in F6Publishing: 6175] [Article Influence: 441.1] [Reference Citation Analysis (0)] |
| 3. | Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5434] [Cited by in F6Publishing: 5953] [Article Influence: 425.2] [Reference Citation Analysis (0)] |
| 4. | Sasai M, Yamamoto M. Pathogen recognition receptors: ligands and signaling pathways by Toll-like receptors. Int Rev Immunol. 2013;32:116-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Lin Q, Li M, Fang D, Fang J, Su SB. The essential roles of Toll-like receptor signaling pathways in sterile inflammatory diseases. Int Immunopharmacol. 2011;11:1422-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 260] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 7. | Alisi A, Carsetti R, Nobili V. Pathogen- or damage-associated molecular patterns during nonalcoholic fatty liver disease development. Hepatology. 2011;54:1500-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28 Suppl 1:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 201] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 9. | Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Kirpich IA, Feng W, Wang Y, Liu Y, Beier JI, Arteel GE, Falkner KC, Barve SS, McClain CJ. Ethanol and dietary unsaturated fat (corn oil/linoleic acid enriched) cause intestinal inflammation and impaired intestinal barrier defense in mice chronically fed alcohol. Alcohol. 2013;47:257-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Elamin EE, Masclee AA, Dekker J, Jonkers DM. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev. 2013;71:483-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Yan AW, Schnabl B. Bacterial translocation and changes in the intestinal microbiome associated with alcoholic liver disease. World J Hepatol. 2012;4:110-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 549] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 16. | World Health Organization. Global status report on alcohol and health 2014. last access October. 2013; Available from: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/index.html. [Cited in This Article: ] |
| 17. | Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis. 2012;16:659-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 795] [Article Influence: 56.8] [Reference Citation Analysis (2)] |
| 19. | Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4232] [Cited by in F6Publishing: 4067] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 20. | Hopkins PA, Sriskandan S. Mammalian Toll-like receptors: to immunity and beyond. Clin Exp Immunol. 2005;140:395-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Petrasek J, Csak T, Szabo G. Toll-like receptors in liver disease. Adv Clin Chem. 2013;59:155-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Yang L, Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol. 2012;3:138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1860] [Cited by in F6Publishing: 2136] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 24. | Pålsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 847] [Cited by in F6Publishing: 889] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 25. | Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6034] [Cited by in F6Publishing: 6115] [Article Influence: 305.8] [Reference Citation Analysis (0)] |
| 26. | Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: Evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci USA. 2006;103:13777-13782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 548] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 28. | Voican CS, Perlemuter G, Naveau S. Mechanisms of the inflammatory reaction implicated in alcoholic hepatitis: 2011 update. Clin Res Hepatol Gastroenterol. 2011;35:465-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Crawford JM. Histologic findings in alcoholic liver disease. Clin Liver Dis. 2012;16:699-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Neuman MG, French SW, Casey CA, Kharbanda KK, Nanau RM, Rasineni K, McVicker BL, Kong V, Donohue TM. Changes in the pathogenesis of alcohol-induced liver disease -- preclinical studies. Exp Mol Pathol. 2013;95:376-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280-G1288. [PubMed] [Cited in This Article: ] |
| 32. | Sheth P, Delos Santos N, Seth A, LaRusso NF, Rao RK. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2007;293:G308-G318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol. 2014;20:2136-2142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 89] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 273] [Cited by in F6Publishing: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 35. | Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 650] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 36. | Hartmann P, Chen WC, Schnabl B. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Front Physiol. 2012;3:402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 38. | Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 39. | Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15 Suppl:D20-D25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 41. | Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gäbele E, Isayama F, Thurman RG. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963-2969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737-4742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 43. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 44. | Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049-3056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Petrasek J, Dolganiuc A, Csak T, Nath B, Hritz I, Kodys K, Catalano D, Kurt-Jones E, Mandrekar P, Szabo G. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53:649-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Li X, Wang C, Nie J, Lv D, Wang T, Xu Y. Toll-like receptor 4 increases intestinal permeability through up-regulation of membrane PKC activity in alcoholic steatohepatitis. Alcohol. 2013;47:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | von Montfort C, Beier JI, Guo L, Kaiser JP, Arteel GE. Contribution of the sympathetic hormone epinephrine to the sensitizing effect of ethanol on LPS-induced liver damage in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1227-G1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Devière J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 49. | Wang X, Bu HF, Zhong W, Asai A, Zhou Z, Tan XD. MFG-E8 and HMGB1 are involved in the mechanism underlying alcohol-induced impairment of macrophage efferocytosis. Mol Med. 2013;19:170-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509-1518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 51. | Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589-3593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 52. | Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 53. | Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 403] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 54. | Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 55. | Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 221] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 56. | Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Schemmer P, Bradford BU, Rivera CA. Kupffer cell-derived prostaglandin E(2) is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G100-G106. [PubMed] [Cited in This Article: ] |
| 57. | Ceccarelli S, Panera N, Gnani D, Nobili V. Dual Role of MicroRNAs in NAFLD. Int J Mol Sci. 2013;14:8437-8455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436-1444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 59. | Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946-1957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 480] [Cited by in F6Publishing: 494] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 60. | Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1612] [Cited by in F6Publishing: 1588] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 61. | Byun JS, Suh YG, Yi HS, Lee YS, Jeong WI. Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J Hepatol. 2013;58:342-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 339] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Schäfer C, Parlesak A, Schütt C, Bode JC, Bode C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcohol. 2002;37:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Järveläinen HA, Orpana A, Perola M, Savolainen VT, Karhunen PJ, Lindros KO. Promoter polymorphism of the CD14 endotoxin receptor gene as a risk factor for alcoholic liver disease. Hepatology. 2001;33:1148-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 65. | Zeng T, Zhang CL, Han XY, Zhao S, Xie KQ. Association between CD14-159C& gt; T polymorphisms and the risk for alcoholic liver disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:1183-1189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Nezi V, Deutsch M, Gazouli M, Alexopoulou A, Paparrigopoulos T, Liappas IA, Dourakis SP. Polymorphisms of the CD14 genes are associated with susceptibility to alcoholic liver disease in Greek patients. Alcohol Clin Exp Res. 2013;37:244-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Petrásek J, Hubácek JA, Stickel F, Sperl J, Berg T, Ruf E, Wichmann HE, Pfeufer A, Meitinger T, Trunecka P. Do common genetic variants in endotoxin signaling pathway contribute to predisposition to alcoholic liver cirrhosis? Clin Chem Lab Med. 2009;47:398-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jürgens G, Hallström S, Jalan R. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G15-G22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 70. | Pimentel-Nunes P, Roncon-Albuquerque R, Gonçalves N, Fernandes-Cerqueira C, Cardoso H, Bastos RP, Marques M, Marques C, Alexandre Sarmento J, Costa-Santos C. Attenuation of toll-like receptor 2-mediated innate immune response in patients with alcoholic chronic liver disease. Liver Int. 2010;30:1003-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Massonnet B, Delwail A, Ayrault JM, Chagneau-Derrode C, Lecron JC, Silvain C. Increased immunoglobulin A in alcoholic liver cirrhosis: exploring the response of B cells to Toll-like receptor 9 activation. Clin Exp Immunol. 2009;158:115-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Stärkel P, De Saeger C, Strain AJ, Leclercq I, Horsmans Y. NFkappaB, cytokines, TLR 3 and 7 expression in human end-stage HCV and alcoholic liver disease. Eur J Clin Invest. 2010;40:575-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267-276. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Felver ME, Mezey E, McGuire M, Mitchell MC, Herlong HF, Veech GA, Veech RL. Plasma tumor necrosis factor alpha predicts decreased long-term survival in severe alcoholic hepatitis. Alcohol Clin Exp Res. 1990;14:255-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 230] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 75. | Affò S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millán C, Loaeza-del-Castillo A, Altamirano J. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2013;62:452-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 76. | Dominguez M, Miquel R, Colmenero J, Moreno M, García-Pagán JC, Bosch J, Arroyo V, Ginès P, Caballería J, Bataller R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 77. | Nischalke HD, Berger C, Lutz P, Langhans B, Wolter F, Eisenhardt M, Krämer B, Kokordelis P, Glässner A, Müller T. Influence of the CXCL1 rs4074 A allele on alcohol induced cirrhosis and HCC in patients of European descent. PLoS One. 2013;8:e80848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, Suttles J, McClain C, Feng W. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem. 2013;24:1609-1615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 79. | Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |