Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15310
Revised: June 21, 2014
Accepted: July 16, 2014
Published online: November 7, 2014
AIM: To evaluate the therapeutic effect of hydroxynaphthoquinone mixture (HM) on dextran sulfate sodium (DSS)-induced colitis and explore the underlying mechanisms.
METHODS: BALB/c mice received 3.5% DSS for 6 d to induce ulcerative colitis. Groups of mice were orally administered HM 3.5, 7 and 14 mg/kg and mesalazine 200 mg/kg per day for 7 d. During the experiment, clinical signs and body weight, stool consistency and visible fecal blood were monitored and recorded daily. A disease activity index score was calculated for each animal. At the conclusion of the experiment, the colonic histopathological lesions were evaluated. Myeloperoxidase (MPO) activity and tumor necrosis factor-α (TNF-α) levels were determined. Protein expression levels of TNF-α, nuclear factor-κB (NF-κB) p65, inhibitor of κB (IκB) and phosphorylation of IκB (p-IκB) were analyzed by Western blot analysis.
RESULTS: Administration of 3.5% DSS for 6 d successfully induced acute colitis associated with soft stool, diarrhea, rectal bleeding, and colon shortening, as well as a loss of body weight. Administration of HM effectively attenuated the severity of colonic mucosa injury. For histopathological analysis, HM treatment improved histological alterations and lowered pathological scores compared with the DSS only group. This manifested as a reduction in the extent of colon injury and inflammatory cell infiltration, as well as the degree of mucosal destruction. In addition, HM at doses of 7 and 14 mg/kg significantly decreased MPO activity in colonic tissue (0.98 ± 0.22 U/g vs 1.32 ± 0.24 U/g, 0.89 ± 0.37 U/g vs 1.32 ± 0.24 U/g tissue, P < 0.05) and serum TNF-α levels (68.78 ± 7.34 ng/L vs 88.98 ± 17.79 ng/L, 64.13 ± 14.13 ng/L vs 88.98 ± 17.79 ng/L, P < 0.05). Furthermore, HM down-regulated the expression of TNF-α, NF-κB p65 and p-IκBα in colonic tissue while up-regulating IκBα protein expression. These results suggest that the significant anti-inflammatory effect of HM may be attributable to its inhibition of TNF-α production and NF-κB activation.
CONCLUSION: HM had a favorable therapeutic effect on DSS-induced ulcerative colitis, supporting its further development and clinical application in inflammatory bowel disease.
Core tip: There is an urgent need for effective and safe therapeutic approaches for the treatment of inflammatory bowel disease. We obtained a hydroxynaphthoquinone mixture (HM) from Zicao. Previously, HM demonstrated a favorable therapeutic effect in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. To exclude that the therapeutic effect was limited to TNBS-induced colitis, we evaluated the effect of HM in dextran sulfate sodium (DSS)-induced colitis. Similarly, we found that HM was beneficial in DSS-induced colitis. The underlying mechanism may be associated with the regulation of tumor necrosis factor-α level and nuclear factor-κB activity. These findings provide support for further development of HM for clinical applications.
- Citation: Zhang ZL, Fan HY, Yang MY, Zhang ZK, Liu K. Therapeutic effect of a hydroxynaphthoquinone fraction on dextran sulfate sodium-induced ulcerative colitis. World J Gastroenterol 2014; 20(41): 15310-15318
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15310
Inflammatory bowel disease (IBD), which encompasses Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic and recurrent inflammatory disorder of unknown etiology[1]. Its main clinical symptoms include abdominal pain, diarrhea, bloody mucopurulent stool and fistulization[1,2]. IBD has been identified as one of the most challenging health issues by the World Health Organization and is associated with a high risk of colon cancer if not treated in a timely manner. IBD is very common in developed countries, with the greatest incidences in northern Europe, the United Kingdom, and North America. In the West, the prevalence of UC and CD has increased to approximately 200/100000 persons. Recently, IBD cases have increased in China with the improvement of living conditions and the adoption of a western lifestyle[1]. IBD has become the main cause of digestive system disorders and chronic diarrhea in China, mostly affecting young people. IBD is gaining attention as it is a major threat to daily life and human health. To date there is no ideal treatment for IBD, and the primary goal of treatment is the mitigation of symptoms.
Although many studies suggest that genetic and environmental factors, infection, and immune system disorders are involved in the development of IBD, its cause and underlying mechanisms remain unclear. Molecular biology and immunology studies, the establishment of animal models, and recent studies of cytokines and adhesion molecules have led to a better understanding of the pathogenesis of IBD. It is widely accepted that activation of the nuclear factor-κB (NF-κB) signaling pathway and overexpression of associated cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-10, and interferon-γ (IFN-γ) play key roles in the development of IBD. NF-κB regulates the expression of multiple pro-inflammation genes and is thus a key player in maintaining immune system homeostasis[3,4]. Thus, inhibition of NF-κB and its associated molecules may be a novel therapeutic tool.
Zicao, the dried root of Arnebia euchroma (Royle) Johnst or Arnebia guttata Bunge, according to the Pharmacopeia of the People’s Republic of China (2010 ed), is a traditional Chinese herbal medicine. In China, Zicao has been extensively used for more than 1000 years in the treatment of burns, eczema, bedsores, hepatitis, allergic purpura and endocrine disorders. Naphthoquinones (also termed as hydroxynaphthoquinones in the Pharmacopoeia of China), including shikonin, alkannin and their derivatives, are the major active constituents of chemical components isolated from Zicao[5]. Shikonin derivatives have an R-configuration, and alkannin derivatives have an S-configuration; however, it has been reported that the biological activities of shikonin and alkannin do not differ significantly[5-7]. Pharmacological studies suggest that hydroxynaphthoquinones reduce inflammation, promote wound healing and are antibacterial[6]. Shikonin derivatives appear to exert their anti-inflammatory actions and modulate the immune system through inhibiting TNF-α, down-regulating NF-κB signaling and decreasing the production of other cytokines[8-11]. Furthermore, an ointment of alkannin and its derivatives has been used clinically in the treatment of traumatic ulcer and acute anal fissure[6].
Previously, we showed that a hydroxynaphthoquinone mixture (HM) obtained from the petroleum ether extract of Zicao showed favorable therapeutic action in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis[12]. To confirm that the therapeutic effect of HM is not limited to TNBS-induced colitis, we evaluated the effect of HM in an established model of acute ulcerative colitis induced with dextran sulfate sodium (DSS) and further explored its mechanism of action through investigation of the NF-κB signaling pathway.
Eight-week-old BALB/c mice were purchased from Vital River Laboratory Animal Technology Co., Ltd. (certificate No. 0247652). All animals were acclimated for at least 1 wk at a temperature of 24 °C ± 1 °C with 55% ± 5% humidity. The animals were maintained with free access to a standard diet and tap water. All experimental procedures were approved by the local Animal Care and Use Committee.
HM was provided by Wuxi Target Drug Research Co. Ltd. (Jiangsu, China), containing 89.43% total pigments of hydroxynaphthoquinone, calculated as shikonin. DSS was purchased from Beijing Bitab Biotechnology Co. Ltd. (Beijing, China). Mesalazine was purchased from Ethypharm Pharmaceutical Co., Ltd. (France). The myeloperoxidase (MPO) detection kit was purchased from Nanjing Jiancheng Bioengineering Institute. The mouse TNF-α enzyme-linked immunosorbent assay (ELISA) Kit, a product of R and D Systems (United States), was obtained from Shanghai Chuanxiang Biotechnology Co. Ltd. (Shanghai, China). The antibodies used in this study were anti-TNF-α (ab66579, Abcam), anti-NF-κB p65 (ab16502, Abcam), anti-inhibitor of NF-κB (IκB) α (ab32518, Abcam), and anti-phosphorylated IκBα (p-IκBα, Ser-36, ab133462, Abcam). Goat anti-rabbit IgG was purchased from Boster Biotechnology (Wuhan, Hubei province, China).
BALB/c mice were randomly assigned to the following groups (n = 8-10 per group): control, DSS alone (DSS), DSS plus mesalazine (200 mg/kg), DSS plus HM (3.5, 7, 14 mg/kg). Control mice were given filter-purified water, while the other groups were administered 3.5% DSS for six days to induce colitis. Mesalazine and HM were delivered by intragastric administration (0.2 mL/10 g) for seven days from the first day of induction.
The mice were checked daily for colitis based on body weight monitoring, gross rectal bleeding, and stool consistency. The overall disease severity was assessed by a clinical scoring system[13,14], and a disease activity index (DAI) score was calculated for each animal.
After 7 d of drug administration, blood was obtained from the inner canthus of the eye, and samples were centrifuged for 15 min at 3000 rpm, after which the supernatant was collected and stored at -20 °C until analysis. Mice were then sacrificed, and the colon and rectum were dissociated. After removal of the entire colon and rectum, the total length was measured, and the colon was then opened longitudinally, gently washed with ice-cold saline and blotted dry with filter paper. The colon tissue was then weighed and cut into several segments. A 1-cm colon segment 2 cm from the anus was fully expanded, affixed to filter paper, and then fixed in 10% neutral formalin. The tissues were then embedded in paraffin, stained with hematoxylin and eosin, and assessed under light microscopy. Colonic damage was scored as described previously[15]. The remaining colon tissue was stored at -80 °C for biochemical measurements and Western blot analysis.
MPO activity was measured using a detection kit according to the manufacturer’s instructions. MPO activity was determined using the O-dianisidine method and expressed as units per gram of wet tissue. TNF-α serum levels were assayed using a mouse TNF-α ELISA kit and expressed as ng/L.
Nuclear and cytoplasmic extracts were prepared using a nuclear and cytoplasmic protein extraction kit (Beyotime, China). Briefly, colon tissues (approximately 80 mg) were homogenized in cytoplasmic extraction buffer. Tissue homogenates were rapidly lysed by vortexing. The homogenate was centrifuged at 12000 g for 10 min at 4 °C and proteins were collected from the supernatant. The pellets were re-suspended in 50 μL of nuclear protein extraction buffer, lysed by ultrasound in an ice water bath, and then centrifuged to yield the nuclear fraction. Protein concentration was measured with a BCA protein assay kit. Protein samples (50-80 μg) were separated using 8%-12% SDS-PAGE gels and transferred onto PVDF membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween 20 for 2 h at room temperature and then incubated with primary antibodies to anti-TNF-α, anti-NF-κB p65, anti-IκBα, or anti-p-IκBα at a 1:1000-1:10000 dilution overnight at 4 °C. Then, the membrane was washed 3 times for 10 min each and incubated with a horseradish peroxidase- conjugated anti-rabbit IgG antibody. Protein bands were detected using an enhanced chemiluminescence detection kit (Beyotime Institute of Biotechnology) and visualized by exposure to photographic film.
Statistical analyses were performed using SPSS software 11.5 for Windows. All results are expressed as mean ± SD. Quantitative data were tested for homogeneity of variance. If the variance was homogeneous, one-way ANOVA followed by the Bonferroni post hoc test was used. Comparisons between groups of nonparametric data were made using the Kruskal-Wallis test followed by the Mann-Whitney U test. P < 0.05 was considered significant.
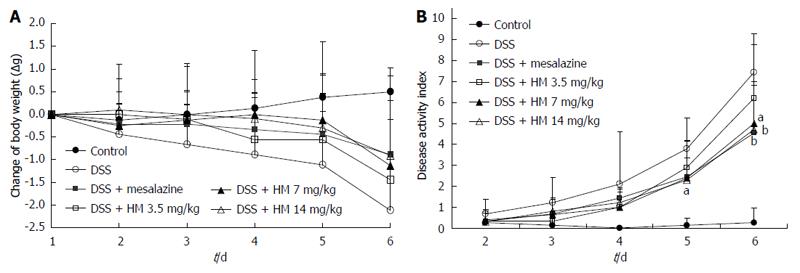
Animals treated with 3.5% DSS for 6 d developed symptoms of acute colitis with an incidence rate of 100%. Acute colitis manifested as diarrhea and bloody feces accompanied by a notable loss of body weight. Control animals showed a steady increase in body weight. In comparison with control mice, the DSS alone treatment group had a significantly increased DAI score. HM treatment suppressed DSS-induced colitis in a dose-dependent manner, ameliorated diarrhea and rectal bleeding, and reduced the loss of body weight. Similarly, mesalazine treatment also produced a significant reduction in the disease index and the severity of lesions caused by DSS treatment (Figure 1). On the sixth day of the experiment, the DAI score in the DSS alone group was 7.4 ± 1.3 mg/kg, while in the HM 3.5, 7, and 14 mg/kg treatment groups, it was 6.2 ± 3.1 , 5.0 ± 2.0 and 4.7 ± 2.2 mg/kg, respectively.
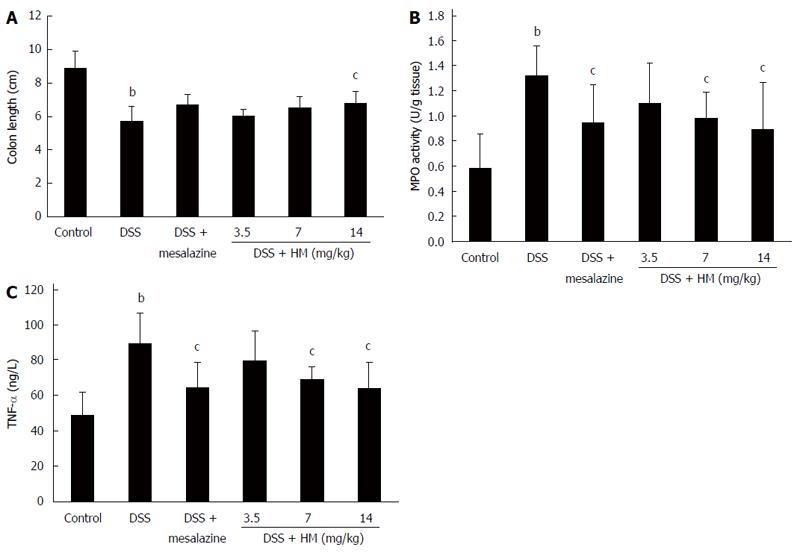
Our results showed that following 6 d of DSS treatment, colon length was shortened in all animals. Additionally, congestion and swelling in the colon and a slight increase in colon weight was observed. The mean colon length was 5.71 ± 0.87 cm for DSS-only mice, showing a significant reduction compared with normal mice (8.91 ± 0.97 cm) (P < 0.01). Treatment with different doses of HM prevented colon shortening. The mean colon length was 6.02 ± 0.37 cm for HM 3.5 mg/kg, 6.51 ± 0.66 cm for HM 7 mg/kg, and 6.80 ± 0.70 cm for HM 14 mg/kg. A significant effect was observed at a dose of 14 mg/kg. Mesalazine also inhibited colon shortening to some extent (Figure 2A).
MPO activity in colonic tissue showed a significant increase to 1.32 ± 0.24 U/g tissue after DSS administration (Figure 2B), suggesting the induction of a severe inflammatory response. Following HM (3.5, 7 and 14 mg/kg) and mesalazine treatment, MPO activity was reduced to 1.10 ± 0.31, 0.98 ± 0.22, 0.89 ± 0.37 and 0.95 ± 0.30 U/g tissue, respectively.
As shown in Figure 2C, the TNF-α level in the DSS-only group significantly increased in contrast to controls (88.98 ± 17.79 ng/L vs 48.92 ± 12.92 ng/L, P < 0.01). HM 3.5 mg/kg showed a tendency to reduce the TNF-α level, but the reduction was not statistically significant. Mesalazine and HM 7 and 14 mg/kg significantly reduced TNF-α levels (P < 0.05). The mean serum TNF-α levels were determined to be 64.33 ± 14.28 ng/L for mesalazine, 79.75 ± 16.45 ng/L for HM 3.5 mg/kg, 68.78 ± 7.34 ng/L for HM 7 mg/kg and 64.13 ± 14.13 ng/L for HM 14 mg/kg.
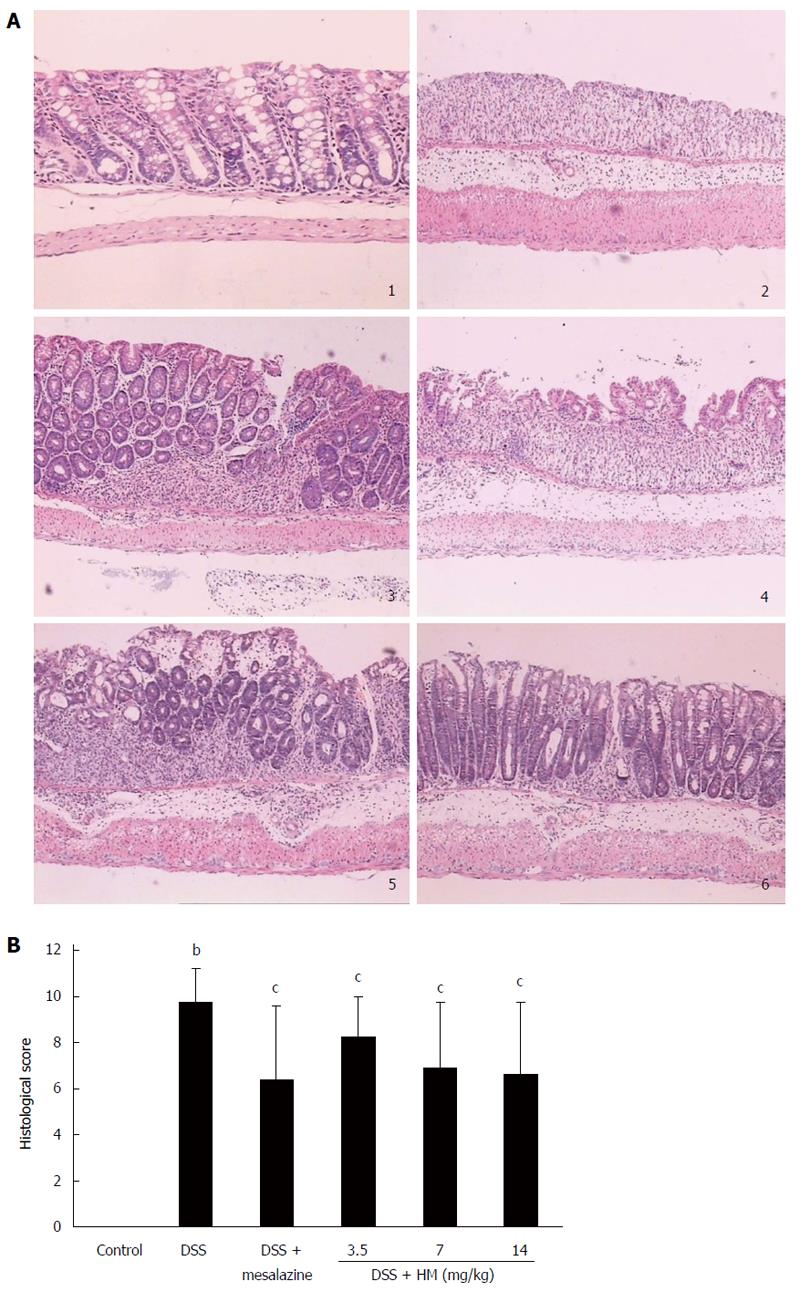
On histological examination, colonic epithelial cells and crypt structures were intact, with no reduction of goblet cells in control mice. In DSS-treated mouse mucosa, severe lesions, with a complete loss of colonic epithelial cells, crypt structure and goblet cells were present in most colonic samples. In addition, the mucosa and submucosa were infiltrated with inflammatory cells in areas of focal lesions, and associated congestion and edema were observed. Histopathological injury scores were significantly elevated. Treatment of animals with HM showed significant effects at all dose levels. Treatment with HM 3.5 mg/kg reduced the area of injury and the extent of congestion or edema compared with that in the DSS-only group (P < 0.05). Although there was still widespread destruction of the crypt structure, the intact epithelial mucosa was preserved. Likewise, HM 7 mg/kg significantly reduced the extent of colon injury, congestion and edema (P < 0.05). In mice treated with HM 14 mg/kg, the colonic mucosa showed slight pathological changes including a reduced extent of colon damage and reduced infiltration of inflammatory cells. The structure of the crypt epithelial cells remained intact, and the histopathological injury scores were significantly decreased (P < 0.05). A similar efficacy was observed for mesalazine (Figure 3).
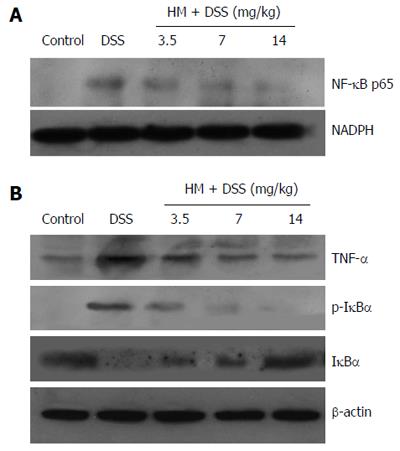
Our results showed that the expression levels of p-IκBα and NF-κB p65 were increased in the colonic tissue, while IκBα expression was decreased compared with those in controls. This indicates that NF-κB activity was increased. The administration of HM down-regulated the expression of p-IκBα and NF-κB p65 while up-regulating the expression of IκBα. Moreover, HM inhibited TNF-α expression. These results suggest that the anti-inflammatory effect of HM may be associated with inhibition of TNF-α production and NF-κB activation (Figure 4).
The establishment of animal models of bowel tract inflammation has provided effective approaches for investigating the pathogenesis of IBD and for screening new drugs. The sulfated polysaccharide DSS can disrupt the epithelial cell barrier. DSS may induce colitis by affecting DNA replication, inhibiting the overgrowth of epithelial cells, inducing macrophage activation, increasing the release of cytokines and breaking the balance of gut microflora[16]. The T cell response in DSS-induced colitis consists of a Th1 response in the acute phase with a mixed Th1/Th2 response in later and chronic phases of inflammation. In either case, DSS elicits the secretion of large amounts of TNF-α and IL-6, which are primarily responsible for the tissue damage associated with the disease[17]. The pathological changes in the DSS-induced IBD model are similar to human UC, and this simple experimental process is easily reproduced[16,17]. Thus, it is an ideal model that has been widely used to study the mechanism of UC and for screening potential drugs.
In the current study, we successfully established a murine IBD model by treating BALB/c mice with 3.5% DSS for six days. Beginning on the third day of DSS administration, mice began to show reduced activity, loose stools and decreased body weight. During later phases, mice had serious bloody stools, and a few mice died. Colon hyperemia, edema and bloody contents were also observed. The intestinal tract wall became thick and stiff. Shortening of the colon was found in all DSS-treated animals. Histopathological examination showed that the crypt structure in the colon tissue disappeared, and epithelial and goblet cells decreased, while inflammatory cell infiltration occurred in the mucosa and submucosa. Treatment with HM decreased the lesion severity, reduced the extent of colon injury and alleviated the infiltration of inflammatory cells. This confirms that HM has a beneficial effect on UC. In addition, decreased MPO activity illustrated the benefits of HM treatment in the mouse model of DSS-induced colitis. MPO is an enzyme that is mainly expressed in neutrophil granulocytes. Increased MPO activity can produce extra oxidizing agents that can cause tissue damage. MPO activity is positively associated with the number of neutrophil granulocytes; thus, the measurement of MPO activity can be used as a quantitative assay for acute intestinal inflammation[18]. Our results showed that MPO activity was significantly inhibited after HM treatment, suggesting that HM has a stronger anti-inflammatory activity.
The mechanism of IBD progression is very complex. In addition to genetic and environmental factors, the mucosal immune system plays a key role in disease progression. In a normal intestinal tract, the mucosal immune system can maintain the balance between proinflammatory and anti-inflammatory cytokines, thus avoiding injury caused by microflora. In chronic mucosal inflammation occurring with IBD, macrophages and T cells can be widely activated and produce high concentrations of proinflammatory cytokines such as TNF-α, IL-6 and IFN-γ, causing lesions in colon tissue[3]. The main pathological changes of UC are regional intestinal tract inflammatory responses that are characterized by increased numbers of immune cells and epithelial cell disruption. Previous studies showed that in UC patients, the mRNA expression levels of several cytokines, including IFN-γ, TNF-α, IL-1β, IL-6 and IL-10 increased and were associated with UC severity[19-21]. Among these cytokines, the overexpression of TNF-α is critical in intestinal mucosal lesions[3]. In the clinic, TNF-α blockers such as infliximab, adalimumab and certolizumab pegol have been successfully used for the treatment of IBD patients[22-26]. Our results showed that HM treatment decreased serum and colon tissue levels of TNF-α in DSS-induced mouse colitis, suggesting that the protective effect of HM against colonic injury is related to the regulation of TNF-α levels.
Pro-inflammatory cytokines are well known to be linked to the pathogenesis of IBD[27]. With the development of molecular medicine, studies of nuclear transcription factors such as NF-κB have garnered more attention. Pro-inflammatory cytokines such as TNF-α and IL-1 interact physically with the NF-κB signaling pathway. These triggering substances activate the NF-κB signaling pathway, which in turn promotes and controls the expression of TNF-α and other cytokines. The constant activation of NF-κB maintains and increases the inflammatory response in IBD; thus, NF-κB may act as a key regulator[4]. Most clinical studies suggest that the expression level of NF-κB p65 and NF-κB binding activity significantly increase in patients with ulcerative colitis[28-31]. Clinical and pre-clinical evidence suggests that the application of NF-κB p65 oligonucleotides significantly decreases the expression of NF-κB p65 and cytokines in mucosa and decreases the severity of clinical symptoms[13,32,33], indicating that NF-κB may be a therapeutic target. To determine whether HM affected NF-κB activity, we planned to investigate the key molecules regulating NF-κB activation. The activation of NF-κB is regulated by IκB and IκB kinase (IKK). When inactive, the NF-κB dimer associates with IκB proteins, the best-studied and most important of which is IκBα[34]. The NF-κB signaling pathway can be activated by several stimuli including lipopolysaccharide, pro-inflammatory cytokines, and viruses[35]. These stimuli can activate the IKK complex. IKKs phosphorylate the inhibitory IκBα protein, resulting in the dissociation of IκBα from NF-κB. The uncovered nuclear localization signals will then cause the activation of NF-κB proteins[34]. High NF-κB activity is accompanied by the increased expression levels of several cytokines (IL-1, IL-6 and TNF-α) and adhesion molecules[29,31]. Because NF-κB is the key regulator in the pathogenesis of IBD, it has become an attractive target for the treatment of IBD. Most anti-inflammatory drugs, including cortical hormones, salazosulfapyridine, aminosalicylic acids and TNF-α monoclonal antibodies, exert their anti-inflammatory function at least partially through the inhibition of NF-κB activation[30,31,36].
In our study, we examined whether HM regulates the activation of NF-κB in tissue affected by colitis. Our results showed that treatment with HM decreased the expression of NF-κB p65 and p-IκBα and increased the expression of IκBα. Thus, we hypothesize that the induction of IκB expression and the suppression of IκB degradation may be a mechanism by which HM inhibits NF-κB activation. Similarly, HM decreased the expression of TNF-α in colon tissue. Through these two pathways, HM could inhibit the increased inflammatory response and reduce the severity of colitis lesions.
In summary, our results suggest that HM can effectively reduce clinical symptoms in a mouse model of DSS-induced ulcerative colitis and may be a suitable candidate drug for the treatment of IBD. The underlying anti-inflammatory mechanism of HM acts through the inhibition of TNF-α production and NF-κB activation. These novel findings provide the pharmacological foundation for the application of HM in the treatment of IBD.
The incidence and prevalence of inflammatory bowel disease (IBD) are substantially rising worldwide and it is a major global health challenge. To date there are no ideal drugs for IBD treatment.
It is widely accepted that the activation of the nuclear factor-kappa B (NF-κB) signaling pathway and the overexpression of associated cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 play key roles in the development of IBD. Inhibition of NF-κB and its associated molecules can be used as a novel therapeutic approach. Studies have shown that many plant-derived extracts and other natural products possess favorable anti-IBD activity via inhibition of cytokine production or regulation of NF-κB activation. Thus, the identification of new agents from herbal or natural products is very promising for new treatment approaches.
This is the first study to report that hydroxynaphthoquinones possess a therapeutic effect on mucosal inflammation via regulation of TNF-α and NF-κB activation.
Elucidation of the effects and mechanisms of the action of a hydroxynaphthoquinone mixture in the treatment of inflammatory intestinal disease provides support for its further development and clinical application.
A solid paper describing the medicinal effects of hydroxynaphthoquinone in colitis. The topic is important, and the methods used are standard. Results are sound and well described, and discussion is adequate.
P- Reviewer: Chen JJ, Vetvicka V S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Ma S
| 1. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1390] [Cited by in F6Publishing: 1417] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 2. | Wang YF, Zhang H, Ouyang Q. Clinical manifestations of inflammatory bowel disease: East and West differences. J Dig Dis. 2007;8:121-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Biasi F, Leonarduzzi G, Oteiza PI, Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013;19:1711-1747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 536] [Cited by in F6Publishing: 581] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 5. | Huang ZS, Zhang M, Ma L, Gu LQ. A survery of chemical and pharmacologic studies on Zicao. Tianranchanwu Yanjiu Yu Kaifa. 1999;12:73-82. [Cited in This Article: ] |
| 6. | Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int Ed. 1999;38:270-300. [Cited in This Article: ] |
| 7. | Tanaka S, Tajima M, Tsukada M, Tabata M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J Nat Prod. 1986;49:466-469. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Dai Q, Fang J, Zhang FS. Dual role of shikonin in early and late stages of collagen type II arthritis. Mol Biol Rep. 2009;36:1597-1604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Staniforth V, Wang SY, Shyur LF, Yang NS. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J Biol Chem. 2004;279:5877-5885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Su PF, Staniforth V, Li CJ, Wang CY, Chiao MT, Wang SY, Shyur LF, Yang NS. Immunomodulatory effects of phytocompounds characterized by in vivo transgenic human GM-CSF promoter activity in skin tissues. J Biomed Sci. 2008;15:813-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Cheng YW, Chang CY, Lin KL, Hu CM, Lin CH, Kang JJ. Shikonin derivatives inhibited LPS-induced NOS in RAW 264.7 cells via downregulation of MAPK/NF-kappaB signaling. J Ethnopharmacol. 2008;120:264-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Fan HY, Zhang ZL, Liu K, Yang MY, Lv WH, Che X, Xu H, Song WW. Effectiveness of a hydroxynaphthoquinone fraction from Arnebia euchroma in rats with experimental colitis. World J Gastroenterol. 2013;19:9318-9327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 236] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328-G1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 401] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 15. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 747] [Cited by in F6Publishing: 858] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 16. | Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci. 2012;105:263-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1030] [Cited by in F6Publishing: 1009] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 18. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] [Cited in This Article: ] |
| 19. | Raddatz D, Bockemühl M, Ramadori G. Quantitative measurement of cytokine mRNA in inflammatory bowel disease: relation to clinical and endoscopic activity and outcome. Eur J Gastroenterol Hepatol. 2005;17:547-557. [PubMed] [Cited in This Article: ] |
| 20. | Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820-2828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Inoue S, Matsumoto T, Iida M, Mizuno M, Kuroki F, Hoshika K, Shimizu M. Characterization of cytokine expression in the rectal mucosa of ulcerative colitis: correlation with disease activity. Am J Gastroenterol. 1999;94:2441-2446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2744] [Cited by in F6Publishing: 2693] [Article Influence: 141.7] [Reference Citation Analysis (2)] |
| 23. | Caspersen S, Elkjaer M, Riis L, Pedersen N, Mortensen C, Jess T, Sarto P, Hansen TS, Wewer V, Bendtsen F. Infliximab for inflammatory bowel disease in Denmark 1999-2005: clinical outcome and follow-up evaluation of malignancy and mortality. Clin Gastroenterol Hepatol. 2008;6:1212-1217; quiz 1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Fiorino G, Szabò H, Fries W, Malesci A, Peyrin-Biroulet L, Danese S. Adalimumab in Crohn’s disease: tips and tricks after 5 years of clinical experience. Curr Med Chem. 2011;18:1230-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 693] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 26. | Löwenberg M, D’Haens G. Novel targets for inflammatory bowel disease therapeutics. Curr Gastroenterol Rep. 2013;15:311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 738] [Cited by in F6Publishing: 781] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 28. | Andresen L, Jørgensen VL, Perner A, Hansen A, Eugen-Olsen J, Rask-Madsen J. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut. 2005;54:503-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Neurath MF, Fuss I, Schürmann G, Pettersson S, Arnold K, Müller-Lobeck H, Strober W, Herfarth C, Büschenfelde KH. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann N Y Acad Sci. 1998;859:149-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Gan H, Ouyang Q, Chen Y, Xia Q. [Activation of nuclear factor-kappaB and effects of anti-inflammatory treatment thereon in intestinal mucosa of patients with ulcerative colitis]. Zhonghua Yi Xue Zazhi. 2002;82:384-388. [PubMed] [Cited in This Article: ] |
| 31. | Chen Y, Gan H, Ouyang Q, Xu D, Pan Y, A Z. [The effects of anti-inflammatory on activation of nuclear factor-kappaB and expression of cell adhesion molecules in patients with ulcerative colitis]. Shengwu Yixue Gongchenxue Zazhi. 2004;21:732-736. [PubMed] [Cited in This Article: ] |
| 32. | Li Z, Zhang de K, Yi WQ, Ouyang Q, Chen YQ, Gan HT. NF-kappaB p65 antisense oligonucleotides may serve as a novel molecular approach for the treatment of patients with ulcerative colitis. Arch Med Res. 2008;39:729-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides. J Clin Invest. 2005;115:3057-3071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3011] [Cited by in F6Publishing: 2989] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 35. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2227] [Cited by in F6Publishing: 2298] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 36. | Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol. 2000;95:3452-3457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |