Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14934
Revised: May 8, 2014
Accepted: June 12, 2014
Published online: October 28, 2014
AIM: To compare the utility of the Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) and Asia-Pacific Association for the Study of Liver (APASL) definitions of acute-on-chronic liver failure (ACLF) in predicting short-term prognosis of patients with ACLF.
METHODS: Consecutive patients of cirrhosis with acute decompensation were prospectively included. They were grouped into ACLF and no ACLF groups as per CLIF-SOFA and APASL criteria. Patients were followed up for 3 mo from inclusion or mortality whichever was earlier. Mortality at 28-d and 90-d was compared between no ACLF and ACLF groups as per both criteria. Mortality was also compared between different grades of ACLF as per CLIF-SOFA criteria. Prognostic scores like CLIF-SOFA, Acute Physiology and Chronic Health Evaluation (APACHE)-II, Child-Pugh and Model for End-Stage Liver Disease (MELD) scores were evaluated for their ability to predict 28-d mortality using area under receiver operating curves (AUROC).
RESULTS: Of 50 patients, 38 had ACLF as per CLIF-SOFA and 19 as per APASL criteria. Males (86%) were predominant, alcoholic liver disease (68%) was the most common etiology of cirrhosis, sepsis (66%) was the most common cause of acute decompensation while infection (66%) was the most common precipitant of acute decompensation. The 28-d mortality in no ACLF and ACLF groups was 8.3% and 47.4% (P = 0.018) as per CLIF-SOFA and 39% and 37% (P = 0.895) as per APASL criteria. The 28-d mortality in patients with no ACLF (n = 12), ACLF grade 1 (n = 11), ACLF grade 2 (n = 14) and ACLF grade 3 (n = 13) as per CLIF-SOFA criteria was 8.3%, 18.2%, 42.9% and 76.9% (χ2 for trend, P = 0.002) and 90-d mortality was 16.7%, 27.3%, 78.6% and 100% (χ2 for trend, P < 0.0001) respectively. Patients with prior decompensation had similar 28-d and 90-d mortality (39.3% and 53.6%) as patients without prior decompensation (36.4% and 63.6%) (P = NS). AUROCs for 28-d mortality were 0.795, 0.787, 0.739 and 0.710 for CLIF-SOFA, APACHE-II, Child-Pugh and MELD scores respectively. On multivariate analysis of these scores, CLIF-SOFA was the only significant independent predictor of mortality with an odds ratio 1.538 (95%CI: 1.078-2.194).
CONCLUSION: CLIF-SOFA criteria is better than APASL criteria to classify patients into ACLF based on their prognosis. CLIF-SOFA score is the best predictor of short-term mortality.
Core tip: The most common acute precipitant for acute-on-chronic liver failure (ACLF) is infection. The Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) criteria is better than Asia-Pacific Association for the Study of Liver criteria in defining ACLF as the CLIF-SOFA criteria identifies patients with a high likelihood of mortality who could benefit from liver transplantation or inclusion into trials of newer therapeutic modalities. Mortality increases with increasing grades of ACLF based on number of organ failures in Asian-Indian patients similar to the results seen in the European population. Also, the multi-organ failure CLIF-SOFA score is better than liver specific Model for End-Stage Liver Disease and Child-Pugh scores, suggesting that ACLF leads to multi-organ failure and is not limited to the liver.
- Citation: Dhiman RK, Agrawal S, Gupta T, Duseja A, Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol 2014; 20(40): 14934-14941
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14934
The definition of acute-on-chronic liver failure (ACLF) differs between the Asia-Pacific region and Europe and United States. While the Asia-Pacific Association for the Study of Liver (APASL) stresses on arbitrarily defined values of degree of liver dysfunction within a 4 wk time frame[1], the American Association for the Study of Liver Disease and the European Association for the Study of the Liver focus of organ dysfunction and increased 3-mo mortality[2]. Also, unlike the Western definitions, the APASL criteria do not recognize non-hepatitic insults like sepsis as acute precipitating events and excludes previously decompensated patients from the ambit of ACLF. Recently the chronic liver failure (clif) acute-on-chronic liver failure in cirrhosis (CANONIC) study proposed the Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) scoring system based on number and type of organ failure to define ACLF and stratify patients according to their 28-d mortality[3]. They demonstrated increasing mortality with increasing number of organ failures. Also, they found infections to be a major cause of ACLF. However, in an accompanying editorial, Bajaj[4] emphasized the need to validate this system in Asian patients as the etiology of acute and chronic components of ACLF may be different in them. It is important to have a universally applicable definition of ACLF that is predictive of prognosis so as to identify patients most at need of liver transplantation or newer treatment modalities, and also to have common inclusion criteria for further research into ACLF. Also, the utility of the APASL and CLIF-SOFA definitions have never been directly compared.
To address this issue, we for the first time compared the CLIF-SOFA and APASL definitions in Asian-Indian patients of acute decompensation of cirrhosis with regards to the short-term mortality and compared various prognostic scores to predict 28-d mortality.
The study was a prospective observational cohort study. The patients of cirrhosis admitted under Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh, India from July 2013 to December 2013 were screened for enrollment after ethical approval from the institute ethics committee. Consecutive patients with cirrhosis with acute decompensation were included. Both previously compensated and decompensated patients were enrolled in the study provided the previous decompensation had improved after treatment. All patients were followed up for 3 mo from inclusion into the study or until mortality, whichever was earlier.
The diagnosis of cirrhosis of liver was based on previous liver biopsy if available or based on clinical, imaging (heterogenous echotexture of liver with irregular outline, altered liver size, or portosystemic collaterals), laboratory (low serum albumin, aspartate aminotransferase/alanine aminotransferase ratio > 1) and endoscopic findings (≥ grade II oesophageal varices).
Acute decompensation of cirrhosis was defined as per the criteria of the CANONIC study[3]. Acute ascites was defined as development of grade 2 to 3 ascites, according to the International Ascites Club Classification[5] within a period of 2 wk, either as the first episode or recurrence in a previously controlled patient. Acute hepatic encephalopathy was defined as acute change in metal status in the absence of other acute neurological disease, either as first episode or recurrence in a previously treated patient. Worsening of chronic hepatic encephalopathy or uncontrolled ascites were not considered as acute decompensation. Acute gastrointestinal bleed was defined by upper or lower gastrointestinal hemorrhage of any etiology. Acute bacterial infections included spontaneous bacterial peritonitis, urinary tract infection, respiratory tract infections, cellulitis, bacteremia, or bacterial infections of any other site. Infections were diagnosed either based on culture positivity, or if procalcitonin was elevated in a patient with systemic inflammatory response with evidence of infection based on chest imaging for pneumonia, urine microscopy showing pus cells for urinary tract infection, ascitic fluid polymorphonuclear count more than 250 cells/mL for spontaneous bacterial peritonitis or clinical examination compatible with cellulitis. Active alcoholism was defined as alcohol consumption within the previous 3 mo.
ACLF as per the APASL criteria was defined as “acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 wk by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease.” As per the APASL criteria patients with prior decompensated cirrhosis were not considered to be having ACLF, and infections were not included as acute precipitating events[1].
Definition of ACLF as per CLIF-SOFA system and grading of severity of ACLF was based on number and type of organ failures as per the CANONIC study[3]. Organ failures were defined as per the CANONIC study criteria[3]. Diagnosis of liver failure was by a serum bilirubin level of ≥ 12.0 mg/dL. Kidney failure was defined if serum creatinine level was ≥ 2.0 mg/dL or the need for renal replacement therapy. Cerebral failure was defined by grade III or IV hepatic encephalopathy as per the West Haven classification[6]. Coagulation failure included an international normalized ratio of ≥ 2.5 and/or platelet count of ≤ 20000/cc. Circulatory failure was defined by need for the use of vasopressors like dopamine, dobutamine, or terlipressin at any dose. Respiratory failure was defined by a PaO2 to FiO2 ratio of ≤ 200 or a SpO2 to FiO2 ratio of ≤ 214.
Prognostic scores including CLIF-SOFA[3], Acute Physiology and Chronic Health Evaluation (APACHE)-II[7], Child-Pugh[8] and UNOS modified Model for End-Stage Liver Disease (MELD) (according to http://www.mayoclinic.org/meld/mayomodel6.html) scores were calculated as per previously published criteria. All scores and definitions were applied at the time of admission to this Institute.
All patients underwent detailed clinical evaluation including history and physical examination, and routine biochemical and imaging evaluation. Investigations for etiology of cirrhosis and cause of acute deterioration were performed as required on a case by case basis. Acute viral hepatitis was diagnosed based on compatible clinical presentation, liver function tests and a positive serology for hepatitis E virus (IgM anti-HEV) or hepatitis A virus (IgM anti-HAV) by ELISA). The diagnosis of hepatitis B virus (HBV) flare was based on the American Association for the Study of Liver Diseases (AASLD) practice guidelines[9], and the diagnosis of autoimmune hepatitis (AIH) was based on the simplified criteria for AIH[10].
Screening for infections including bacterial cultures of blood, urine, and ascitic fluid, blood fungal culture, galactomannan and procalcitonin assays was done at admission and subsequently as required according to the clinical suspicion of the treating physician. All patients were given intravenous antibiotics for gram negative cover empirically at admission and later modified according to culture and sensitivity patterns. Antibiotics were escalated and gram positive coverage was added after 48 h of treatment if there was evidence of infection and no improvement with the initial antibiotics. Antifungal therapy was added after 5 d of hospitalization if there was evidence of infection but bacterial cultures were sterile, procalcitonin was normal and patient had features of ongoing sepsis. Acute kidney injury was managed with intravenous 20% human albumin with vasoactive agents if needed and dialysis when required indicated. Patients with hepatic encephalopathy were treated with lactulose and rifaximin. Diuretics were given for ascites if no contraindications like renal failure or hepatic encephalopathy were present. Inotropes were given for hypotension and endotracheal intubation with mechanical ventilation was done for respiratory failure. Patients requiring inotropes, mechanical ventilation or having evidence of multi-organ failure were managed in the liver intensive care unit.
Specific therapy included pentoxifylline 400 mg three times per day per orally for alcoholic hepatitis and tenofovir 300 mg once daily (modified according to renal function) for hepatitis B infection. No patient underwent liver transplantation or any experimental therapy during the period of the study.
All data were presented as mean with SD and/or range for quantitative variables and as proportions with percentages for qualitative variables. Comparison between groups was done using χ2 and Fischer exact tests for categorical variables and student’s t-test and Mann-Whitney test for parametric and non-parametric variables respectively. The 28-d mortality was compared between various grades of ACLF using analysis of variance. Prediction of 28-d mortality by CLIF-SOFA, Child-Pugh, MELD and APACHE-II scores was evaluated using area under receiver operating curves (AUROC). Backward conditional logistic regression analysis was done to compare the utility of these scores. A P value of ≤ 0.05 was considered statistically significant.
Statistical analysis was done using SPSS software version 17.0 (SPSS Inc., Chicago, IL).
Out of 50 patients recruited, ACLF was present in 38 (76%) as per CLIF-SOFA criteria and 19 (38%) as per APASL criteria. Majority [43 (86%)] of the patients were male and mean age was 46 ± 13 years (range 22-78 years) (Table 1). Prior decompensation was present in 28 (56%) patients. Alcoholic liver disease (58%) was the most common etiology of cirrhosis followed by cryptogenic (14%), hepatitis C virus with alcohol (10%), autoimmune liver disease (6%), hepatitis B virus (6%), and Wilson disease (6%). Active alcoholism was present in 56% of the patients with alcohol related chronic liver disease. Precipitant for acute decompensation were bacterial infections (66%), active alcoholism (40%), flare of autoimmune hepatitis (4%), flare of hepatitis B virus (4%), acute gastrointestinal bleed (4%), acute HEV infection (2%), cytomegalovirus infection (2%) and unknown cause (8%). More than one acute precipitating cause was identified in 30% of patients, most commonly active alcoholism with infection. Infection (66%) was the most common form of acute decompensation followed by hepatic encephalopathy (34%), ascites (16%), and acute gastrointestinal bleeding (10%). More than one form of acute decompensation was seen in 30% of patients, most commonly infection with hepatic encephalopathy (Table 1).
| Characteristics | n = 50 |
| Age (yr) | 46 ± 131 |
| Male gender | 43 (86)2 |
| Cause of cirrhosis | |
| Alcohol | 29 (58) |
| Hepatitis C virus + alcohol | 5 (10) |
| Autoimmune | 3 (6) |
| Hepatitis B virus | 3 (6) |
| Wilson | 3 (6) |
| Cryptogenic | 7 (14) |
| Acute precipitating event | |
| Sepsis | 33 (66) |
| Active alcoholism | 20 (40) |
| Autoimmune flare | 2 (4) |
| Hepatitis B virus flare | 2 (4) |
| Upper gastrointestinal bleed | 2 (4) |
| Acute hepatitis E virus infection | 1 (2) |
| Cytomegalovirus infection | 1 (2) |
| Unknown | 4 (8) |
| More than one cause | 15 (30) |
| Type of acute decompensation | |
| Sepsis | 33 (66) |
| Ascites | 8 (16) |
| Hepatic encephalopathy | 17 (34) |
| Upper gastrointestinal bleed | 5 (10) |
| More than one decompensation | 13 (26) |
| Prior decompensation | 28 (56) |
| Bilirubin (mg/dL) | 13.2 (0.6-43.0)3 |
| Total leukocyte count (/mm3) | 10100 (2700-39000) |
| Platelet count (103/mm3) | 90.0 (14.4-444.0) |
| International normalized ratio | 2.5 ± 0.8 |
| Serum creatinine (mg/dL) | 1.6 (0.5-7.9) |
| Jaundice | 45 (90) |
| Ascites | 42 (84) |
| Hepatic encephalopathy | 17 (34) |
| Upper gastrointestinal bleed | 6 (12) |
| Organ failures | |
| Liver | 29 (58) |
| Kidney | 22 (44) |
| Cerebral | 5 (10) |
| Coagulation | 23 (46) |
| Respiratory | 2 (4) |
| Circulation | 2 (4) |
| ≥ 2 organ failures | 14 (28) |
| Prognostic scores | |
| Child-Pugh score | 11.4 ± 1.7 |
| MELD score | 29.2 ± 7.8 |
| APACHE II score | 13.0 (4.0-41.0) |
| CLIF-SOFA score | 9.9 ± 2.7 |
| Mortality | |
| 28-d mortality | 19 (38) |
| 90-d mortality | 29 (58) |
Compared to no ACLF patients, ACLF patients as per CLIF-SOFA criteria were younger and had significantly higher international normalized ratio (INR), serum creatinine and Child-Pugh, MELD, CLIF SOFA and APACHE II scores (P < 0.05). The 28-d mortality (8.3% vs 47.4% respectively, P = 0.018] and 90-d mortality (16.7% vs 71.1% respectively, P = 0.002) differed significantly between no ACLF and ACLF groups (Table 2). However, when the groups were defined as per APASL criteria, ACLF patients did not significantly differ from no ACLF patients except for higher bilirubin, INR and proportion of patients with ascites, younger age and higher Child-Pugh score in ACLF group. The 28-d mortality (38.7% vs 36.8% respectively, P = 0.895) and 90-d mortality (51.6% vs 68.4% respectively, P = 0.242) was not different between no ACLF and ACLF groups using the APASL definition (Table 2).
| Characteristics | CLIF-SOFA criteria | APASL criteria | ||||
| No ACLF (n = 12) | ACLF (n = 38) | P value | No ACLF (n = 31) | ACLF (n = 19) | P value | |
| Age (yr) | 53 ± 161 | 44 ± 12 | 0.048 | 50 ± 12 | 41 ± 14 | 0.02 |
| 52 (26-78)3 | 44 (22-70) | 48 (34-78) | 37 (22-70) | |||
| Male gender | 11 (92)2 | 32 (84) | 1.000 | 29 (94) | 14 (33) | 0.089 |
| Bilirubin (mg/dL) | 9.6 (2.1-30.6) | 15.5 (0.6-43.0) | 0.195 | 10.7 (0.6-43.0) | 18.0 (5.0-32.6) | 0.029 |
| Total leukocyte count (/mm3) | 9450 (4500-21500) | 10100 (2700-39000) | 1.000 | 9300 (3600-36400) | 11300 (2700-39000) | 0.460 |
| Platelet count (103/mm3) | 85.5 (14.4-151.0) | 91.5 (14.8-444.0) | 0.525 | 72.0 (14.4-444.0) | 110.0 (14.8-382.0) | 0.276 |
| International normalized ratio | 1.8 ± 0.3 | 2.7 ± 0.8 | < 0.001 | 2.2 ± 0.6 | 2.9 ± 0.9 | 0.005 |
| Serum creatinine (mg/dL) | 1.0 (0.6-1.9) | 2.1 (0.5-7.9) | 0.002 | 2.1 (0.5-7.9) | 1.5 (0.5-3.3) | 0.112 |
| Jaundice | 11 (92) | 34 (90) | 1.000 | 26 (84) | 19 (100) | 0.142 |
| Ascites | 8 (67) | 34 (90) | 0.082 | 23 (74) | 19 (100) | 0.018 |
| Hepatic encephalopathy | 3 (25) | 14 (37) | 0.510 | 13 (42) | 4 (21) | 0.218 |
| Acute gastrointestinal bleed | 2 (17) | 4 (11) | 0.621 | 5 (16) | 1 (5) | 0.387 |
| Organ failures | ||||||
| Liver | 4 (33) | 25 (66) | 0.091 | 14 (45) | 15 (79) | 0.037 |
| Kidney | 0 (0) | 22 (58) | < 0.001 | 16 (52) | 6 (32) | 0.166 |
| Cerebral | 0 (0) | 5 (13) | 0.319 | 4 (13) | 1 (5) | 0.637 |
| Coagulation | 0 (0) | 23 (61) | < 0.001 | 11 (36) | 12 (63) | 0.057 |
| Respiratory | 0 (0) | 2 (5) | 1.000 | 2 (7) | 0 (0) | 0.519 |
| Circulation | 1 (8) | 1 (3) | 0.426 | 2 (7) | 0 (0) | 0.519 |
| Prognostic scores | ||||||
| Child-Pugh score | 10.3 ± 1.6 | 11.7 ± 1.6 | 0.014 | 11.0 ± 1.9 | 12.0 ± 1.3 | 0.045 |
| MELD score | 21.8 ± 5.6 | 31.6 ± 6.9 | < 0.001 | 28.5 ± 8.3 | 30.4 ± 6.9 | 0.408 |
| APACHE-II score | 9.5 (4.0-21.0) | 14.0 (6.0-41.0) | 0.019 | 12.0 (4-41) | 14.0 (4-22) | 0.508 |
| CLIF-SOFA score | 7.7 ± 2.3 | 10.6 ± 2.5 | 0.002 | 9.6 ± 2.7 | 10.3 ± 2.7 | 0.356 |
| Mortality | ||||||
| 28-d mortality | 1 (8.3) | 18 (47.4) | 0.018 | 12 (38.7) | 7 (36.8) | 0.895 |
| 90-d mortality | 2 (16.7) | 27 (71.1) | 0.002 | 16 (51.6) | 13 (68.4) | 0.242 |
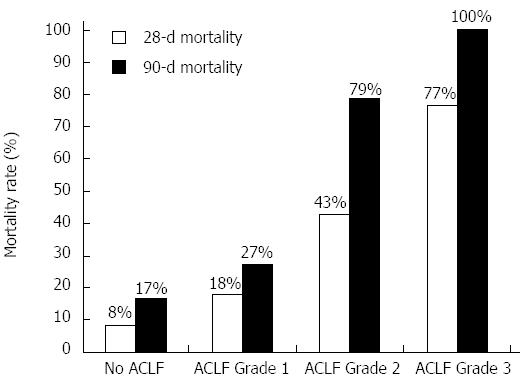
The 28-d mortality in patients with no ACLF (n = 12), grade 1 (n = 11), grade 2 (n = 14) and grade 3 ACLF (n = 13) as per CLIF-SOFA criteria was 8.3%, 18.2%, 42.9% and 76.9% (χ2 for trend, P = 0.002) respectively and 90-day mortality was 16.7%, 27.3%, 78.6% and 100% (χ2 for trend, P < 0.001) respectively (Figure 1).
Non-survivors irrespective of ACLF status had significantly higher incidence of hepatic encephalopathy (53% vs 23%, P = 0.037), cerebral failure (26% vs 0%, P = 0.005) and kidney failure (63% vs 32%, P = 0.033), and higher mean Child-Pugh (12.3 vs 10.8, P = 0.002), MELD (32.8 vs 27.1, P = 0.010), CLIF SOFA (11.6 vs 8.8, P < 0.001) and APACHE II (15.0 vs 10.0, P = 0.001) scores as compared to survivors (Table 3). Patients with prior decompensation had similar 28-d and 90-d mortality (39.3% and 53.6%, respectively) as patients without prior decompensation (36.4% vs 63.6%, respectively) (P = 1.000 and 0.569 respectively).
| Characteristics | 28-d mortality | ||
| Yes (n = 19) | No (n = 31) | P value | |
| Age (yr) | 45 ± 121 | 47 ± 14 | 0.649 |
| Male gender | 16 (84)2 | 27 (87) | 1.000 |
| Prior decompensation | 11 (57.9) | 17 (54.8) | 0.833 |
| Bilirubin (mg/dL) | 17.7 (1.2-37.6)3 | 10.7 (0.6-43.0) | 0.139 |
| Total leukocyte count (/mm3) | 11200 (2700-39000) | 9300 (3600-36400) | 0.873 |
| Platelet count (103/mm3) | 81.0 (14.8-382.0) | 90.0 (14.4-444.0) | 0.734 |
| International normalized ratio | 2.7 ± 0.7 | 2.3 ± 0.8 | 0.054 |
| Serum creatinine (mg/dL) | 2.2 (0.5-7.9) | 1.6 (0.6-6.3) | 0.509 |
| Jaundice | 18 (95) | 27 (87) | 0.637 |
| Ascites | 17 (90) | 25 (81) | 0.693 |
| Hepatic encephalopathy | 10 (53) | 7 (23) | 0.037 |
| Upper gastrointestinal bleed | 1 (5) | 5 (16) | 0.387 |
| Organ failures | |||
| Liver | 14 (74) | 15 (48) | 0.139 |
| Kidney | 12 (63) | 10 (32) | 0.033 |
| Cerebral | 5 (26) | 0 (0) | 0.005 |
| Coagulation | 12 (63) | 11 (36) | 0.057 |
| Respiratory | 0 (0) | 2 (11) | 0.140 |
| Circulation | 1 (5) | 1 (5) | 1.000 |
| Prognostic scores | |||
| Child-Pugh score | 12.3 ± 1.3 | 10.8 ± 1.7 | 0.002 |
| MELD score | 32.8 ± 7.1 | 27.1 ± 7.5 | 0.010 |
| APACHE II score | 15.0 (8-41) | 10.0 (4.0-25.0) | 0.001 |
| CLIF-SOFA score | 11.6 ± 2.5 | 8.8 ± 2.3 | < 0.001 |
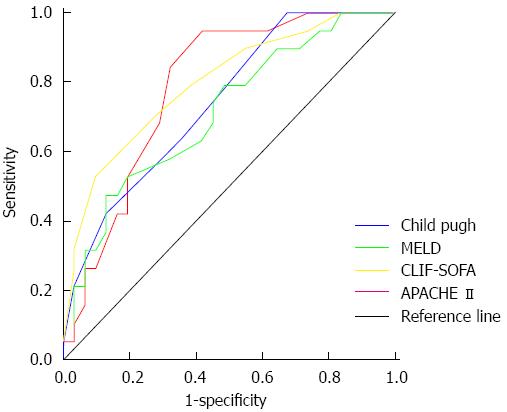
AUROC for 28-d mortality for CLIF-SOFA, APACHE-II, Child-Pugh and MELD scores was 0.795, 0.787, 0.739 and 0.710 respectively (Figure 2). A CLIF-SOFA score cut-off of 8 had a high sensitivity of 97% and with specificity of 38% while a cut-off of 12 had sensitivity of 38% with a high specificity of 95%. The best single value for prediction of 28-d mortality was 10 with a sensitivity and specificity of 72% and 71% respectively. On multivariate analysis of these scores CLIF-SOFA score was the only significant independent predictor of mortality with an odds ratio 1.538 (95%CI: 1.078-2.194).
This study demonstrated that the common causes of acute worsening in patients with ACLF were infections and active alcoholism, whereas the most common causes of chronic liver disease was alcohol. Infection was present in 66% of patients at admission while 56% of the patients had prior history of decompensation. ACLF was present in 76% by the CLIF-SOFA criteria and only 38% by the APASL criteria. Mortality was significantly higher in patients with ACLF as per CLIF-SOFA criteria but not with APASL criteria compared to patients without ACLF. Mortality significantly increased with increasing grades of ACLF. Mortality depended upon organ failures and higher values of various prognostic scores. Of all the prognostic scores tested the CLIF-SOFA score had the best accuracy for predicting 28-d mortality and was the only independent predictor of mortality.
The common causes of acute deterioration (bacterial infection and active alcoholism) and underlying chronic liver disease (alcohol related) in Asian-Indian patients was similar to that seen in the West[2,3,11]. This is similar to our earlier observation in Indian patients[12,13]. However, most studies from countries in eastern Asia like China have reported mainly hepatitis B virus related ACLF[14,15]. Conversely, a retrospective study from Bangladesh found hepatitis E infection and septicemia to be important acute precipitants for ACLF and did not find any case of hepatitis B related ACLF[16]. This reflects the heterogeneity of the ACLF population even within the same continent. This could be a function of the different endemicity of various hepatotropic viruses in different regions along with differences in religious and social customs.
The predominance of active alcoholism could explain the high frequency of sepsis in our study as it has been demonstrated previously that alcohol predisposes to infections[17,18]. Infections were similarly common in the original CANONIC study which had a high prevalence of patients alcohol related cirrhosis and alcoholic hepatitis[3]. While it is possible that in some cases infections are a consequence of ACLF rather than a cause, the CANONIC study also demonstrated that non-ACLF patients who had infections at admission were more likely to develop ACLF in the same hospitalization. This suggests a role for infections in the development of ACLF.
Even in Asian-Indian patients with acute decompensation of cirrhosis, the CLIF-SOFA criteria better stratifies patients into ACLF according to short-term mortality as compared to the APASL criteria. This is the first study from Asia to validate the CLIF-SOFA definition of ACLF. Previous studies on prognosis of ACLF from Asia have focused on prognostic scores such as MELD, Child Pugh, APACHE II and SOFA[12-14], or have looked at individual parameters like presence of hepatic encephalopathy and renal dysfunction[15]. Along with no difference in mortality, there were also no significant clinical or biochemical differences between patients with and without ACLF on APASL criteria except for parameters used to define ACLF (bilirubin, INR and ascites) and the Child-Pugh score which is a composite of the previous 3 parameters. Hence, the APASL criteria were unable to identify patients with poor prognosis among the cohort of patients presenting with acute decompensation of cirrhosis. As the major need of accurately defining ACLF is to identify patients with poor prognosis who may benefit with newer treatment modalities such as artificial liver support systems[19], liver transplantation[20,21] or granulocyte-colony stimulating factor therapy[22,23]. any definition of ACLF should be able to predict mortality. The CLIF-SOFA definition seems to be more useful clinically for prognostication of these patients and evaluation for urgent liver transplantation. Also, CLIF-SOFA system seems to be better suited to be used as inclusion criteria for recruiting patients into therapeutic trials in ACLF. This may be because many patients who met CLIF-SOFA criteria for ACLF did not meet APASL criteria because APASL definition excludes patients with prior decompensation and does not include sepsis as cause of acute deterioration[1] both of which were seen in a majority of the patients in this study. Our previous experience from India has also suggested that non-hepatitic insults like systemic infections are a common cause of ACLF[12,13]. Similar to the European multicentre study[3], grading of severity of ACLF as per CLIF-SOFA criteria remains relevant for prognostication in Asian-Indian patients. Many studies, including one by us previously has found multi-organ failure scores like APACHE-II and SOFA to be better than liver specific scores like MELD and CTP for predicting mortality in patients with ACLF[10,24-26]. Similarly, the CLIF-SOFA score was better than liver specific scores in this study. These results suggest that ACLF is not a disease limited to the liver but is part of a systemic derangement resulting in multi-organ failure. This is probably related to development of systemic inflammatory response[27] and oxidative stress[28] leading to hemodynamic derangements[29] resulting in progressive tissue damage and organ failure. Systemic hemodynamic disturbances leading to multi-organ failure can theoretically result from non-hepatitic insults like infections and gastrointestinal bleeding. Infections as discussed above and variceal bleeding[3,12,13] have previously been demonstrated to be frequent precipitating events for ACLF. The CANONIC study had also found that only hepatic failure without any other organ failure was not associated with increased mortality[3]. The APASL definition does not consider non-hepatic organ involvement or non-hepatic insults in the definition of ACLF and hence fails to identify many patients at high risk of mortality.
The sample size in this was relatively small and being a single centre study the spectrum of ACLF reported by us may not be representative of all of Asia, thus further prospective multicenter and multinational studies are required.
In conclusion, the spectrum of ACLF seen in this study was similar to that in the West. Infections are frequently present in these patients and multi-organ failure is common and results in high mortality. Thus, we recommend the use of the CLIF-SOFA criteria which considers multiple organ failures rather than the liver-specific APASL criteria for definition of ACLF and prognostication even in Asian patients with ACLF.
Acute-on-chronic liver failure (ACLF) compared to chronic liver failure due to decompensation of cirrhosis is associated with high mortality. It is important to identify this sub-group of patients who need specialized management in intensive care units and may be candidates for newer therapeutic modalities. However, there are no universally accepted criteria to define ACLF and the definitions used in the Asia-Pacific region greatly differ from those used in Europe and North America. It is necessary to compare these definitions to ascertain the best criteria to identify patients with ACLF.
This is the first prospective study to directly compare the Asia-Pacific and Western criteria of defining ACLF and assessing their utility in identifying patients with high mortality. It also compares various liver-specific and general prognostic scores to identify the best prognostic scoring system in patients with ACLF.
This study demonstrated that among patients with acute decompensation of cirrhosis using the chronic liver failure (CLIF) Acute-on-Chronic Liver Failure in Cirrhosis (CANONIC) criteria to define ACLF, which was derived from a European study, was able to identifying patients with high mortality even in India patients. Conversely patients with and without ACLF as per the Asia-Pacific criteria had similar mortality, suggesting that the Asia-Pacific criteria were not useful in categorizing patients as having ACLF. Also, mortality increased with increasing number of organ failures as defined by the CANONIC criteria, suggesting that ACLF results in death by multi-organ failure rather than liver failure alone.
Accurately identifying patients with ACLF at high risk of mortality with conventional management will allow better prognostication, selection of patients for intensive care and early liver transplantation as well as for inclusion into trials of newer therapeutic modalities like liver support devices and stem cell based therapies.
Acute-on-chronic liver failure is a condition in which a patient having underlying chronic liver disease has a rapid worsening of liver functions leading to liver failure, manifested by ascites, jaundice, altered sensorium or gastrointestinal bleeding. This is usually due to a superimposed injury to the liver due to alcohol, drugs or other toxins; sepsis; infections by viruses affecting the liver; or a rapid worsening of inflammation in the liver by the preexisting cause of liver disease.
This is an outstanding paper! Editorial suggestions are as follows: insert into the article under Materials and Methods the Asia-Pacific Association for the Study of Liver and Chronic Liver Failure-Sequential Organ Failure Assessment criteria for classification of ACLF for the benefit of the readers.
P- Reviewer: De Minicis S, Dang SS, Julie NL S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 610] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 2. | Olson JC, Wendon JA, Kramer DJ, Arroyo V, Jalan R, Garcia-Tsao G, Kamath PS. Intensive care of the patient with cirrhosis. Hepatology. 2011;54:1864-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 175] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 3. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-137, 1426-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1720] [Cited by in F6Publishing: 1860] [Article Influence: 169.1] [Reference Citation Analysis (3)] |
| 4. | Bajaj JS. Defining acute-on-chronic liver failure: will East and West ever meet? Gastroenterology. 2013;144:1337-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 602] [Cited by in F6Publishing: 553] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-1976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 409] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10902] [Cited by in F6Publishing: 10643] [Article Influence: 272.9] [Reference Citation Analysis (0)] |
| 8. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5490] [Cited by in F6Publishing: 5498] [Article Influence: 107.8] [Reference Citation Analysis (1)] |
| 9. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2125] [Cited by in F6Publishing: 2114] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 10. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1205] [Cited by in F6Publishing: 1132] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 11. | Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328-2335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 12. | Duseja A, Chawla YK, Dhiman RK, Kumar A, Choudhary N, Taneja S. Non-hepatic insults are common acute precipitants in patients with acute on chronic liver failure (ACLF). Dig Dis Sci. 2010;55:3188-3192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Duseja A, Choudhary NS, Gupta S, Dhiman RK, Chawla Y. APACHE II score is superior to SOFA, CTP and MELD in predicting the short-term mortality in patients with acute-on-chronic liver failure (ACLF). J Dig Dis. 2013;14:484-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Fan HL, Yang PS, Chen HW, Chen TW, Chan DC, Chu CH, Yu JC, Kuo SM, Hsieh CB. Predictors of the outcomes of acute-on-chronic hepatitis B liver failure. World J Gastroenterol. 2012;18:5078-5083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Huang K, Hu JH, Wang HF, He WP, Chen J, Duan XZ, Zhang AM, Liu XY. Survival and prognostic factors in hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol. 2011;17:3448-3452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Mahtab MA, Rahman S, Khan M, Karim MF. Hepatitis E virus is a leading cause of acute-on-chronic liver disease: experience from a tertiary centre in Bangladesh. Hepatobiliary Pancreat Dis Int. 2009;8:50-52. [PubMed] [Cited in This Article: ] |
| 17. | Rosa H, Silvério AO, Perini RF, Arruda CB. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am J Gastroenterol. 2000;95:1290-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, Verslype C, Fevery J, Nevens F. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10:R108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Duan BW, Lu SC, Wang ML, Liu JN, Chi P, Lai W, Wu JS, Guo QL, Lin DD, Liu Y. Liver transplantation in acute-on-chronic liver failure patients with high model for end-stage liver disease (MELD) scores: a single center experience of 100 consecutive cases. J Surg Res. 2013;183:936-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Bahirwani R, Shaked O, Bewtra M, Forde K, Reddy KR. Acute-on-chronic liver failure before liver transplantation: impact on posttransplant outcomes. Transplantation. 2011;92:952-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Duan XZ, Liu FF, Tong JJ, Yang HZ, Chen J, Liu XY, Mao YL, Xin SJ, Hu JH. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2013;19:1104-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 102] [Cited by in F6Publishing: 97] [Article Influence: 8.8] [Reference Citation Analysis (3)] |
| 23. | Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505-512.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 24. | Karvellas CJ, Pink F, McPhail M, Austin M, Auzinger G, Bernal W, Sizer E, Kutsogiannis DJ, Eltringham I, Wendon JA. Bacteremia, acute physiology and chronic health evaluation II and modified end stage liver disease are independent predictors of mortality in critically ill nontransplanted patients with acute on chronic liver failure. Crit Care Med. 2010;38:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Garg H, Kumar A, Garg V, Sharma P, Sharma BC, Sarin SK. Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis. 2012;44:166-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Katoonizadeh A, Laleman W, Verslype C, Wilmer A, Maleux G, Roskams T, Nevens F. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut. 2010;59:1561-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 370] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 28. | Mookerjee RP. Acute-on-chronic liver failure: the liver and portal haemodynamics. Curr Opin Crit Care. 2011;17:170-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Garg H, Kumar A, Garg V, Kumar M, Kumar R, Sharma BC, Sarin SK. Hepatic and systemic hemodynamic derangements predict early mortality and recovery in patients with acute-on-chronic liver failure. J Gastroenterol Hepatol. 2013;28:1361-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |