INTRODUCTION
Pancreatic cancer is a common cancer of the digestive system and is a leading cause of morbidity and mortality in both developed and developing countries. Pancreatic cancer is characterized by a covert anatomical structure, aggressive biology, resistance to conventional therapeutic agents, and no early detectable biomarkers[1], resulting in a 5-year survival rate of only 5% among patients[2]. Therefore, searching for new strategies for the prevention and treatment of pancreatic cancer is essential.
The etiology of pancreatic cancer is not clearly understood. A prospective study showed that patients with hereditary pancreatitis had a significantly higher standardized incidence rate of pancreatic cancer than the general population[3]. Furthermore, primary pancreatic cancer without pancreatitis is accompanied by a large number of inflammatory cells and the expression of high levels of inflammatory factors[4]. Therefore, evidence suggests that a close relationship exists between inflammation and pancreatic cancer.
Oridonin, which has been extracted from Rabdosia rubescens, is a tetracyclic diterpenoid compound. Structural and pharmacological studies indicate that oridonin is one of the most important therapeutic compounds found in Rabdosia rubescens, due to its heat-clearing, detoxifying, anti-inflammatory, anti-nociceptive, and anti-tumorigenic properties[5]. Hanahan and Weinberg[6] revisited the original hallmarks of cancer and considered tumor-promoted inflammation to be included in this list. Few papers have focused on the anti-tumor mechanism of oridonin and, in particular, on its anti-inflammatory effect in pancreatic cancer. In a previous study, we found that oridonin inhibited the growth of human pancreatic cancer (BxPC-3) cells and down-regulated the mRNA expression of interleukin-1β (IL-1β), IL-6, IL-33, and bone morphogenetic protein-2 (BMP-2)[7]. Therefore, we proposed that oridonin could regulate the association of inflammatory factors with the tumor microenvironment (by altering related pathways) and modify nuclear transcription factors, thereby modulating the expression of downstream target genes and, in turn, changing cancer hallmarks.
MATERIALS AND METHODS
Cell line and culture conditions
The human pancreatic cancer cell line BxPC-3 was supplied by the Shanghai Institute of Biological Sciences of the Chinese Academy of Sciences and was maintained as a monolayer culture in RPMI1640 (GIBCO, NY, United States) medium supplemented with 10% fetal bovine serum at 37 °C in a humidified atmosphere containing 5% CO2.
Cell viability assay
Cell viability was quantified using a short-term microculture tetrazolium (MTT, Sigma, United States) assay. In a 96-well microplate (the outer wells were left empty for the later addition of water), 5 × 103 cells per well were treated with 2, 4, 8, 16, 32, 64, or 128 μg/mL of oridonin (Xiya Chemtech Co., Chengdu) for 24 h. As a negative control, cells were treated with 0.1% dimethyl sulfoxide (DMSO), the same DMSO concentration used for drug delivery. After treatment, the media was replaced with 180 μL of serum-free medium and 20 μL of MTT solution (5 mg/mL in sterile phosphate-buffered saline). After a 4-h incubation at 37 °C, the MTT solution in the wells was replaced with 150 μL DMSO. The absorption at 570 nm (A570) was measured using a microplate reader (Bio-Rad, Model 680, United States), and the results are expressed as the percentage of cell viability. The IC50 value was calculated as the concentration of oridonin that inhibited cell growth by 50%. The percentage of cell viability was calculated based on the following equation: Viability ratio % = A570(oridonin) /A570(control)× 100%. Each assay was performed at least three times.
Detection of IL-1β, IL-6, and IL-33 production
Secretion of IL-1β, IL-6, or IL-33 in response to treatment of cells with oridonin (0, 8, 16, or 32 μg/mL) was determined using a bioassay. Briefly, culture supernatants of BxPC-3 cells were collected at 24 h post-treatment and cleared of cell debris by brief centrifugation. IL-1β, IL-6, and IL-33 levels were measured using human enzyme-linked immunosorbent assay (ELISA) kits (Human interleukin 1β ELISA Kit, Cusabio, CSB-E08053h; Human interleukin 6 ELISA Kit, Cusabio, CSB-E04638h; Human interleukin 33 ELISA Kit, Cusabio, CSB-E13000h) according to the manufacturer’s instructions. The absorbance was measured at 490 nm using a microplate reader.
Immunofluorescence staining
For immunofluorescence experiments, cells were plated onto glass coverslips one day before treatment with oridonin (0, 8, 16, or 32 μg/mL) for 24 h. Cells were fixed in acetone for 30 min and incubated with an antibody raised against IL-33 (Santa Cruz Biotechnology, sc98660, United States) in PBS containing 2% FBS for 2 h. After three washes with PBS, the samples were incubated with the appropriate secondary antibody for 1 h in PBS containing 2% FBS and washed again as described above. Finally, the coverslips containing the cells were incubated with DAPI (0.5 μg/mL) for 1 min, and images were obtained using a fluorescence microscope.
Western blot analysis
To detect various proteins, BxPC-3 cells treated with oridonin (0, 8, 16, or 32 μg/mL) were washed twice with PBS and then lysed in RIPA lysis buffer (1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate). The lysates were then centrifuged, and the supernatants were collected. Protein concentrations were quantitated using a bicinchoninic acid assay kit (MultiSciences Biotech, PQ0011, China). Protein extracts (40 μg) were resolved using 10%-15% sodium dodecylsulfate polyacrylamide gel electrophoresis. After transferring the proteins to membranes, the membranes were blocked using 5% nonfat dry milk for 2 h at room temperature and incubated overnight with primary antibodies raised against C-Jun (Bioworld, BS1737, United States), nuclear factor κB (NF-κB)1 (Abcam, ab32360, United States), RelA (Abcam, ab31481, United States), phosphorylated RelA (P-RelA, Abcam, ab106129, United States), signal transducer and activator of transcription 3 (STAT3) (Abcam, ab50761, United States), phosphorylated STAT3 (P-STAT3, Abcam, ab76315, United States), transforming growth factor β1 (TGFβ1) (Abcam, ab64715, United States), BMP-2 (Bioworld, BS3473, United States), Smad2 (Abcam, ab33875, United States), phosphorylated Smad2 (P-Smad2, Abcam, ab53100, United States), Smad3 (Abcam, ab40854, United States), phosphorylated Smad3 (P-Smad3, Cell Signaling Technology, C25A9, United States), Smad4 (Abcam, ab40759, United States), phosphorylated Smad1/5/8 (P-Smad1/5/8, Cell Signaling Technology, 9511S, United States), Smad6 (Abcam, ab63713, United States), Smad7 (Abcam, ab124890, United States), vascular endothelial growth factor (VEGF) (Abcam, ab46154, United States), survivin (Abcam, ab76424, United States), MMP-2 (Abcam, ab86607, United States), and β-actin (MultiSciences, Mab1445, China) at 4 °C. The membranes were then incubated with secondary antibodies conjugated with horseradish peroxidase (goat anti-mouse IgG, MultiSciences Biotech, GAM0072, China or goat anti-rabbit IgG, MultiSciences Biotech, GAR0072, China) for 2 h at room temperature. The membranes were then developed using an ECL substrate kit (MultiSciences Biotech, P1425, China) and visualized using an Omega Lum G imaging system. The amount of protein present was normalized against β-actin. Image J software was used to quantify the bands.
Statistical analysis
For comparisons between the treatment groups, one-way analysis of variance was performed. The data are presented as mean ± SD in each figure. Statistical analyses were performed using the SPSS (Statistical Package for the Social Sciences) statistical package, version 17.0. The Mann-Whitney rank test was used to compare different groups. A P value less than 0.05 was considered statistically significant.
RESULTS
Oridonin-mediated hallmark changes in BxPC-3 cells
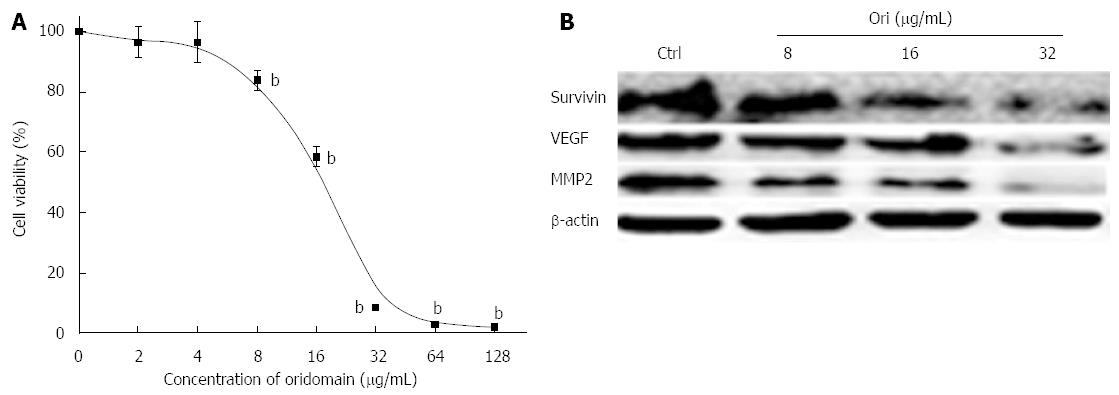
To investigate the possible effect of oridonin on changes in hallmarks in BxPC-3 cells, cells were treated with various concentrations of oridonin for 24 h. As shown in Figure 1A, oridonin induced BxPC-3 cell death in a dose-dependent manner. At low doses, oridonin did not show any inhibitory effect on BxPC-3 cells. No significant differences were observed between the negative control and the low-dose treatment (2 and 4 μg/mL) groups. Growth was inhibited in BxPC-3 cells following treatment with 8 μg/mL (13.05% ± 3.21%, P < 0.01) oridonin compared to without treatment. When treated with 16 μg/mL oridonin, the cell death rate was nearly 50%, and the IC50 of oridonin was determined to be 19.32 μg/mL in the BxPC-3 cell line. The highest inhibitory ratio was 90.64% ± 0.70% (obtained using 32 μg/mL oridonin). We next examined the effect of oridonin on the expression of hallmark-related proteins in BxPC-3 cells. BxPC-3 cells were treated with oridonin at 0, 8, 16, or 32 μg/mL for 24 h, and we observed that 8, 16, and 32 μg/mL oridonin caused obvious decreases in the expression of survivin, VEGF, and MMP-2 (Figure 1B).
Figure 1 Oridonin-mediated hallmark changes in BxPC-3 cells.
A: BxPC-3 cells were treated with 0.1% dimethyl sulfoxide (DMSO) or with 0.1% DMSO containing various concentrations of oridonin. The percentage of inhibition in each sample was determined using an MTT assay. Cytotoxicity is expressed as the percentage of cell inhibition compared with the control (DMSO only). Data are expressed as mean ± SD. bP < 0.01 vs the control treatment; B: Western blot analysis of the effect of oridonin treatment on the target genes of survivin, vascular endothelial growth factor (VEGF), and matrix metallopeptidase 2 (MMP-2) in BxPC-3 cells. The BxPC-3 cells were treated with 0, 8, 16, or 32 μg/mL oridonin for 24 h. Three independent experiments were performed, and a representative result is shown.
Oridonin down-regulates expression of proteins in the NF-κB/AP-1 pathway
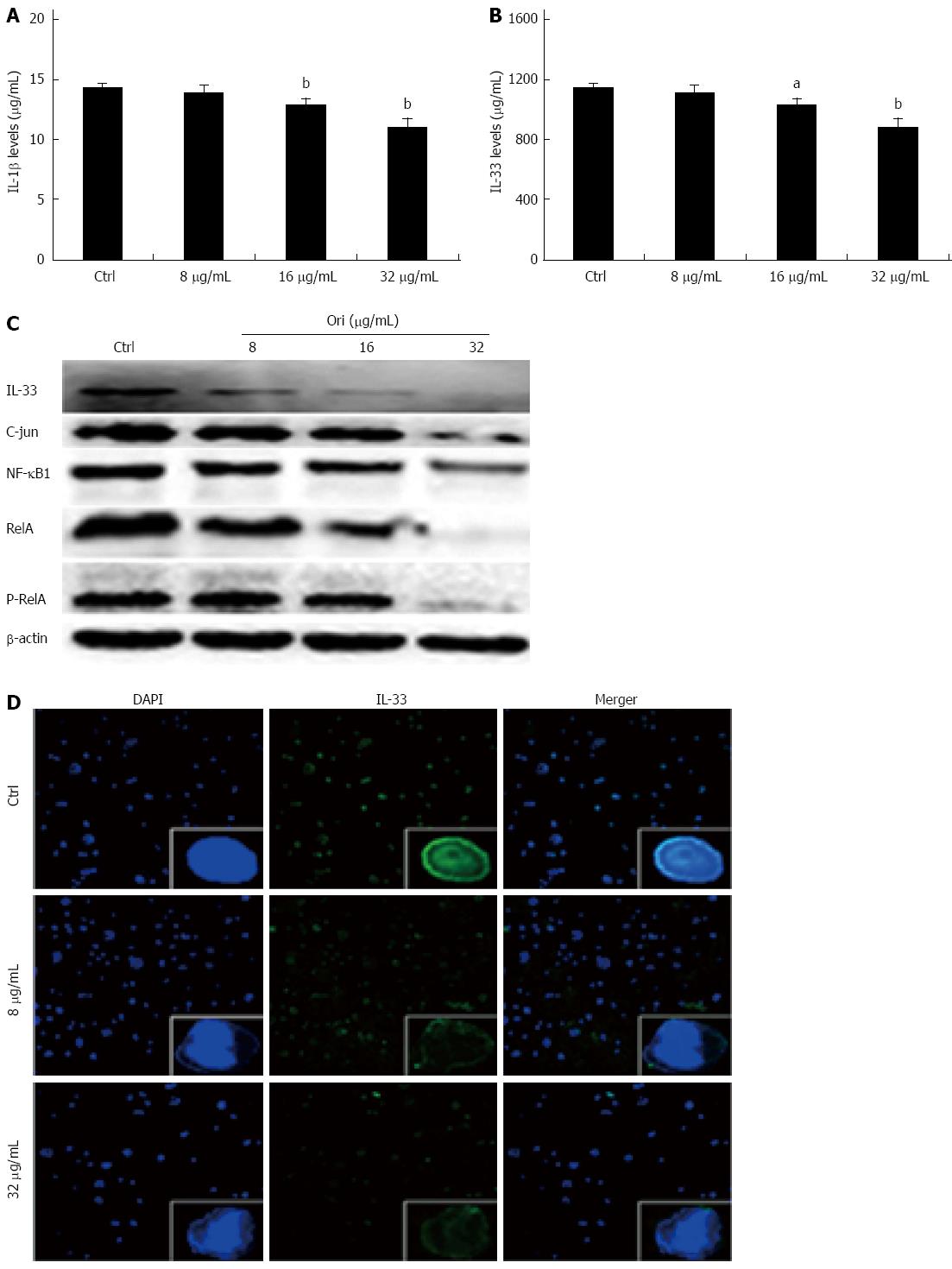
We next investigated the effect of oridonin on NF-κB/AP-1-dependent protein expression, including the expression of IL-1β, IL-33, NF-κB1, RelA, P-RelA, and AP-1. BxPC-3 cells were treated with 0, 8, 16, or 32 μg/mL oridonin for 24 h. The ELISA results showed that treatment of BxPC-3 cells with 8, 16, or 32 μg/mL oridonin altered the expression of IL-1β and IL-33 in a dose-dependent manner (Figure 2A and B). IL-1β expression was significantly reduced in the 16 and 32 μg/mL treatment groups compared to the negative control group (12.97 ± 0.45 pg/mL, 11.17 ± 0.63 pg/mL vs 14.40 ± 0.38 pg/mL, P < 0.01). Similar trends were observed for IL-33 expression, which was significantly reduced in the 8, 16, and 32 μg/mL treatment groups compared to the negative control group (911.05 ± 14.18 pg/mL vs 945.25 ± 12.09 pg/mL, P < 0.05; 802.70 ± 11.88 pg/mL, 768.54 ± 10.98 pg/mL vs 945.25 ± 12.09 pg/mL, P < 0.01). These trends were also observed for NF-κB1, RelA, P-RelA, and AP-1 expression. Western blot analysis showed that the protein expression levels of NF-κB1, RelA, P-RelA, and AP-1 decreased as the concentration of oridonin increased (Figure 2C).
Figure 2 Oridonin decreases both the expression of the nuclear factors nuclear factor κB/activating protein-1/interleukin-33 in BxPC-3 cells and the release of interleukin-1β and interleukin-33 into the supernatant.
A and B: Enzyme-linked immunosorbent assay analysis of the effect of oridonin on interleukin (IL)-1β and IL-33 in BxPC-3 cells. Data are expressed as mean ± SD. aP < 0.05, bP < 0.01 vs the control treatment; C: Western blot analysis of the effect of oridonin on the levels of signal transducer and activator of transcription 3 (STAT3) and phospho-STAT3 in BxPC-3 cells treated with 0, 8, 16, or 32 μg/mL oridonin for 24 h. Three independent experiments were performed, and a representative result is shown; D: Immunofluorescent staining analysis of the effect of oridonin on IL-33 in BxPC-3 cells (× 100). The BxPC-3 cells were treated with 0, 8, or 32 μg/mL oridonin for 24 h. NF-κB: Nuclear factor κB.
Oridonin reduces the nuclear expression of IL-33
To determine the effect of oridonin on nuclear IL-33 expression, BxPC-3 cells were treated with 0, 8, or 32 μg/mL of oridonin for 24 h. Immunofluorescence staining analysis (Figure 2D) showed that the control group retained intact cell nuclei and exhibited obvious green fluorescence, indicating a high level of IL-33 expression in both the nucleus and the cytoplasm. Interestingly, the green fluorescence was significantly and dose-dependently decreased in the nuclei of BxPC-3 cells following treatment with 8 and 32 μg/mL oridonin. Additionally, the nuclei of oridonin-treated BxPC-3 cells were broken or disintegrated and exhibited only minor levels of fluorescence.
Oridonin exerts a negative effect on the STAT3 pathway
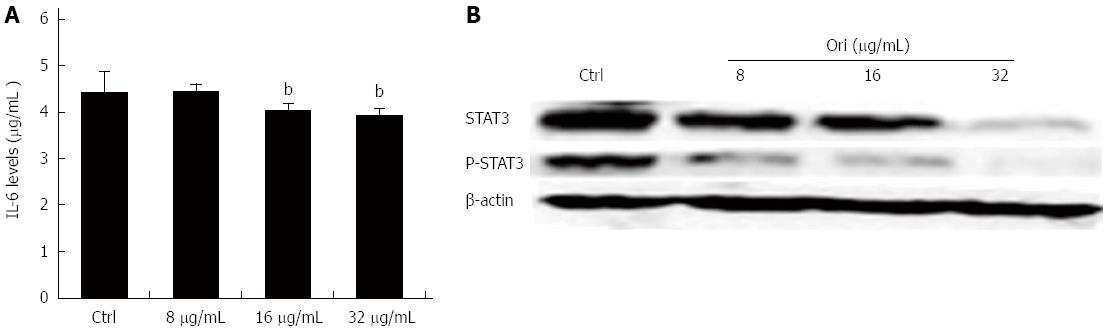
To investigate the effect of oridonin on STAT3-driven protein expression, including that of IL-6, STAT3, and P-STAT3, BxPC-3 cells were treated with 0, 8, 16, or 32 μg/mL oridonin for 24 h. The ELISA results showed that treatment of BxPC-3 cells with 8, 16, or 32 μg/mL oridonin significantly and dose-dependently altered the expression of IL-6 (Figure 3A). IL-6 expression was significantly reduced in the 16 and 32 μg/mL treatment groups compared to the negative control group (4.05 ± 0.14 pg/mL vs 4.45 ± 0.43 pg/mL, P < 0.05; 3.95 ± 0.13 pg/mL vs 4.45 ± 0.43 pg/mL, P < 0.01). Similar trends were observed for STAT3 and P-STAT3 (P-STAT3) expression. Western blot analysis showed that the protein expression levels of STAT3 and P-STAT3 decreased as the concentration of oridonin increased (Figure 3B). These results indicate that oridonin-mediated reductions in IL-6 levels might be regulated through the STAT3 signaling pathway.
Figure 3 Oridonin reduces both the expression of signal transducer and activator of transcription 3 in BxPC-3 cells and the release of interleukin-6 into the supernatant.
A: Enzyme-linked immunosorbent assay analysis of the effect of oridonin treatment on interleukin (IL)-6 in BxPC-3 cells. Data are expressed as mean ± SD. bP < 0.01 vs the control treatment; B: Western blot analysis of the effect of oridonin treatment on the levels of signal transducer and activator of transcription 3 (STAT3) and phospho-STAT3 in BxPC-3 cells treated with 0, 8, 16, or 32 μg/mL oridonin for 24 h. Three independent experiments were performed, and a representative result is shown.
The effect of oridonin on Smads is related to the TGFβ1 and BMP2 pathways
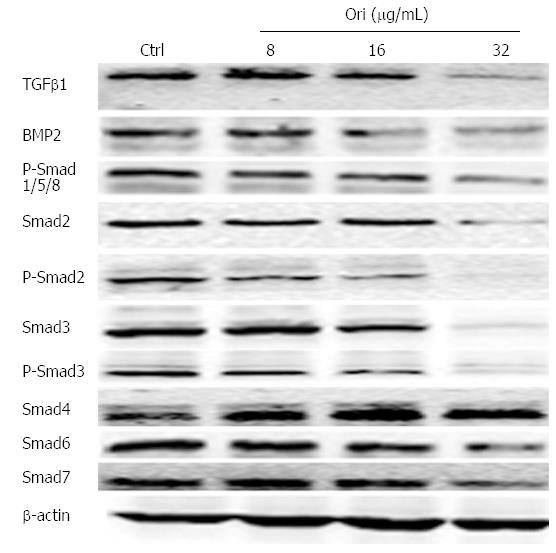
To characterize whether the effect of oridonin on protein expression is mediated by the TGFβ and Smad signaling pathways, BxPC-3 cells were treated with 0, 8, 16, or 32 μg/mL oridonin for 24 h. Western blot results showed that the protein expression levels of TGFβ1, BMP-2, Smad2/3/6/7, and P-Smad1/2/3/5/8 decreased as the concentration of oridonin increased. Oridonin down-regulated TGFβ1, BMP-2, Smad2/3/6/7, and P-Smad1/2/ 3/5/8 but increased the expression of Smad4 (Figure 4).
Figure 4 Oridonin regulates the expression of proteins in the transforming growth factor β/Smad pathway.
Western blot analysis of the effect of oridonin on proteins in the Smad signaling pathway in BxPC-3 cells. The BxPC-3 cells were treated with 0, 8, 16, or 32 μg/mL oridonin for 24 h. Three independent experiments were performed, and a representative result is shown. TGFβ1: Transforming growth factor β1; BMP-2: Bone morphogenetic proteins 2; Smads: Sma and mad homologues.
DISCUSSION
Oridonin is one of the most effective antitumor agents obtained from Rabdosia rubescens and exhibits antitumor activity against a number of cancer cell types[8]. The results obtained in this study demonstrate that oridonin inhibits the growth of human BxPC-3 pancreatic cancer cells, consistent with previous data. It is known that survivin, VEGF, and MMP-2 play important biological roles in pancreatic cancer growth, angiogenesis, and metastasis, respectively. Our results also indicate that oridonin specifically inhibits the expression of survivin, VEGF, and MMP-2.
In addition, tumor-promotion inflammation is also a hallmark of cancer. Cytokines are important inflammatory mediators that regulate a wide range of processes involved in cancer pathogenesis[9]. We could find that IL-1β, IL-33, IL-6, TGF-β1 and BMP2 were decreased by oridonin treatment. Pancreatic cancer can express receptors on the cell surface, such as IL1R[10], ST2[11], IL6R[12], and TGFR[13], that are activated by specific cytokines. These signaling pathways can regulate cellular growth, differentiation, proliferation and apoptosis. Jones et al[14] found that pancreatic cancer contains an average of 63 genetic alterations within a core set of 12 cellular signaling pathways, including the C-jun, invasion, and TGF-β pathways. In the present study, we found that nuclear transcription factor-related inflammatory cytokines were indeed regulated by oridonin treatment in pancreatic cancer cells.
NF-κB and AP-1 are pleiotropic transcription factors that are expressed in response to extracellular inflammation signals, such as IL-1β, and IL-33. NF-κB/Rel binding activity involves both NF-κB1 (p50) and RelA (p65). Previous studies support the idea that NF-κB plays important roles in cellular resistance to apoptosis, the promotion of tumor metastasis, and angiogenesis. The transcription factor NF-κB/Rel has been found to be constitutively activated in human pancreatic cancer and potentially activates epidermal growth factor receptor. In our previous study, inhibition of IKK activity induced cell apoptosis[15]. Moreover, after L3.3 cells transfected with mutated I kappa B alpha were implanted in the pancreas of nude mice, they produced rapidly growing tumors that metastasized to the liver[16]. C-Jun is a central component of all AP-1 complexes and is a positive regulator of cell proliferation[17]. Hypoxic environments up-regulate the IL-8 gene via cooperation between NF-κB and AP-1, which can be induced and can accumulate in the nucleus in hypoxic environments[18], thereby contributing to the progression and metastasis of human pancreatic cancer[19]. In this study, we found that NF-κB1, RelA, phospho-RelA, and AP-1 were down-regulated, suggesting that the NF-κB/AP-1 signaling pathway might be involved in the mechanism of oridonin-induced hallmark changes in BxPC-3 cell.
IL-33, a recently described member of the IL-1 family, is a dual-function protein that acts as both a proinflammatory cytokine and as an intracellular nuclear factor with transcriptional regulatory properties[20]. Higher serum levels of IL-33 in patients with gastric cancer correlate with depth of invasion, distant metastasis and advanced stage (stage III/IV)[21]. Moreover, in patients with head and neck squamous cell carcinoma (HNSCC), most cases with a low invasion pattern grading score (IPGS) exhibit little to no IL-33 expression, whereas most HNSCC cases with a high IPGS exhibit IL-33 over-expression in cancer cells[22]. In addition, recent studies indicate that IL-33 is a chromatin-associated nuclear factor with transcriptional regulatory properties[20], such as binding to the p65 promoter region to participate in inflammatory reactions[23]. Moreover, IL-33 peptide can regulate chromatin compaction by promoting nucleosome-nucleosome interactions[24] and might regulate PDGF-induced proliferation in pancreatic stellate cells[25]. However, the function of IL-33 as a nuclear factor is poorly defined in pancreatic cancer. In this study, we determined that the expression of extracellular or nuclear IL-33 is decreased by treatment with oridonin, suggesting that this compound can reduce IL-33-induced carcinogenesis in BxPC-3 cells. In our further study, the role of IL-33 in the regulation of BxPC-3 functions will be examined using recombinant IL-33 and small interfering RNA.
STAT3, a member of the Janus kinase (JAK)/STAT signaling pathway, is activated by the phosphorylation of a conserved tyrosine residue Tyr705 (P-STAT3) in response to extracellular signals, such as IL-6[12]. The abnormal activation of STAT3 participates in pancreatic cancer[26] and plays a critical role in oncogenesis, metastasis, and tumor growth. Inhibition of activated STAT3 decreases the level of cell cycle control factors such as p21, cyclin A, cyclin B1[27], and cyclin D1[28]. Additionally, this inhibition also decreases the expression of VEGF and MMP-2, factors that are related to cell metastasis. Also, STAT3 transcriptionally regulates the expression of anti-apoptotic proteins, such as Bcl-xL and survivin[29]. In this study, we found that the expression levels of IL-6, STAT3, and phospho-STAT3 are decreased by treatment with oridonin, suggesting that this compound can down-regulate the STAT3 signaling pathway, which is responsible for the hallmarks of growth, invasion, and metastasis of human pancreatic cancer. Type I interferons (IFNs) may have a possible role in the treatment of pancreatic adenocarcinoma, but the limitation of their clinical use is due to the activation of tumor resistance mechanisms, including the JAK2/STAT3 pathway[30,31]. In this light, oridonin provides a promising way of interfering with STAT-3 signaling, therefore, we cannot exclude the possibility of future use of oridonin in combination with some chemotherapeutic compounds in pancreatic cancer.
The TGFβ superfamily comprises TGFβs, BMPs, activins, and related proteins. TGFβs are pleiotropic factors that regulate cell proliferation, angiogenesis, metastasis, and immune suppression. Dysregulation of the TGFβ pathway in pancreatic cancer cells often leads to tumor invasiveness and growth. SD-208, a 2,4-disubstituted pteridine and an ATP-competitive inhibitor of TGFβ receptor I kinase (TGFβRI), has been used in PANC-1 cell line. cDNA microarray analysis and functional gene clustering identified groups of TGFβ-regulated genes that are involved in metastasis, angiogenesis, cell proliferation, survival, and apoptosis. The expression of these genes is inhibited by treatment with SD-208[32]. Additionally, Smads have different functions in pancreatic cancer cells. Smad1 induces and up-regulates MMP-2, stimulating pancreatic cancer cell invasiveness[33]. siRNA-mediated silencing of Smad3 in PANC-1 cells, a TGFβ-responsive pancreatic ductal adenocarcinoma cell line, decreased TGFβ1-induced growth inhibition but increased the migratory response; in contrast, silencing of Smad2 enhanced growth inhibition but decreased chemokinesis[34]. The tumor suppressor gene, Smad4/deleted in pancreatic carcinoma, locus 4, is lost at a high frequency in pancreatic cancer. Loss of Smad4, which leads to aberrant activation of STAT3, contributes to the switch of TGF-β from a tumor-suppressive to a tumor-promoting pathway in pancreatic cancer[35]. In one study, restoration of Smad4 expression in human pancreatic carcinoma cells suppressed tumor formation in vivo but was unable to restore sensitivity to TGF-β. Instead, Smad4 restoration affected angiogenesis by decreasing VEGF expression[36]. Smad7 is over-expressed in pancreatic cancer cell lines, enhancing tumorigenicity[37], promoting growth[38], and suppressing cisplatin-induced apoptosis[39]. Few reports are available regarding Smad6 expression in pancreatic cancer. Smad6 and Smad7 expression is elevated in pancreatic cancer and has been demonstrated to suppress TGF-β-induced growth inhibition in COLO-357 pancreatic cancer cells[40]. The present study also showed that oridonin down-regulates TGFβ1, BMP-2, Smad1/2/3/5/6/7, and phospho-Smad1/2/3/5/8 but increases Smad4 expression, suggesting that oridonin can beneficially revert the abnormal expression of Smads.
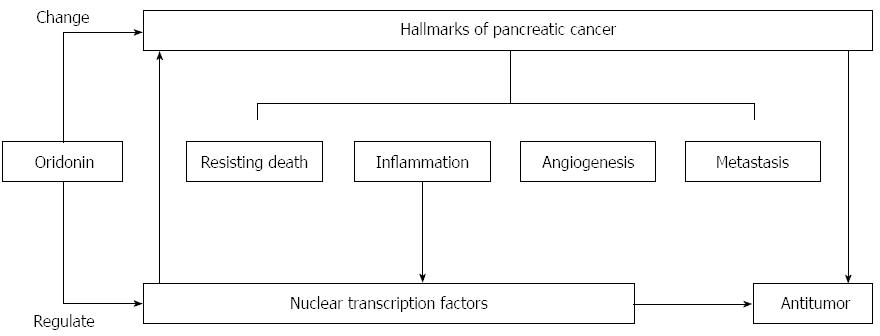
A better understanding of the relationship between neoplastic hallmarks and nuclear transcription factors will help us to interpret the novel mechanisms of oridonin-mediated decreases in cancer growth, angiogenesis, and metastasis. In conclusion, we have demonstrated that oridonin reduces growth, angiogenesis, and metastasis perhaps through the regulation of inflammatory factors and downstream transcription factors (Figure 5). Oridonin therefore exhibits potential for use in the treatment of pancreatic cancer. Afterwards, we will focus on exploring its therapeutic effects, toxicity and the underlying mechanism in animal models of pancreatic cancer.
Figure 5 Proposed mechanism of oridonin-induced hallmark changes in BxPC-3 cells.
Oridonin induces anticancer actions, which reflected by hallmarks changes, through regulation of transcription factors.
COMMENTS
Background
Pancreatic cancer is a common cancer of the digestive system and is a leading cause of morbidity and mortality in both developed and developing countries. The etiology of pancreatic cancer is currently unclear. A prospective study demonstrated that patients with hereditary pancreatitis had a significantly higher standardized incidence rate of pancreatic cancer than the general population. Cytokines are important inflammatory mediators that regulate many processes involved in cancer pathogenesis. Moreover, pancreatic cancer cells can express cell-surface receptors that bind specific cytokines and induce downstream signaling pathways that can regulate cellular growth, differentiation, proliferation, and apoptosis.
Research frontiers
Hanahan and Weinberg revisited the original hallmarks of cancer and considered tumor-promoted inflammation to be included in this list. Few papers have studied the anti-tumor mechanism of oridonin by focusing on its anti-inflammatory effects in pancreatic cancer.
Innovations and breakthroughs
A better understanding of the relationships among inflammatory factors, nuclear transcription factors and tumor behavior will help us to understand the novel mechanisms of anti-inflammatory agent-induced alterations in cancer biology, such as growth, angiogenesis, and metastasis. Analogous shifts caused by treatment with other drugs may also improve the efficacy of similar inflammation-targeted therapies.
Applications
Oridonin is one of the most effective antitumor compounds extracted from Rabdosia rubescens and holds promise for the treatment of pancreatic cancer.
Terminology
The nuclear transcription factor, nuclear factor κB (NF-κB), which consists of NF-κB1 (p50) and RelA (p65), regulates the expression of many genes that are critical for the control of apoptosis, viral replication, tumorigenesis, inflammation, and various autoimmune diseases. The hallmarks of cancer include self-sufficiency in growth signals; insensitivity to antigrowth signals; resisting cell death; limitless replicative potential; sustained angiogenesis; tissue invasion and metastasis; avoiding immune destruction; tumor promotion inflammation; deregulating cellular energetics; genome instability and mutation. The transforming growth factor β (TGFβ) superfamily comprises TGFβs, bone morphogenetic proteins, activins, and related proteins. Smads exhibit various functions in cancer and are classified into three categories: (1) regulatory Smads (R-Smads, which include Smads 1, 2, 3, 5 and 8); (2) common Smads (co-Smads, i.e., Smad 4); and (3) inhibitory Smads (I-Smads, including Smad 6 and Smad 7).
Peer review
This is a well-conducted study that carries forward previous work by the authors. This study shows that oridonin, a compound extracted from Rabdosia rubescens, exhibits anti-inflammatory properties that may be useful in controlling pancreatic cancer by regulating the expression of several nuclear transcription and inflammatory factors.