Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14505
Revised: May 12, 2014
Accepted: June 13, 2014
Published online: October 21, 2014
Partial hepatectomy is still the treatment of choice aiming at a cure for patients with hepatocellular carcinoma (HCC), provided that the patient can tolerate the treatment. For patients with multiple recurrent HCC after partial hepatectomy which cannot be treated by re-hepatectomy or local ablative therapy, the prognosis is extremely poor. Sorafenib is a molecular-targeted agent which has been demonstrated in two global phase III randomized controlled trials to show survival benefit for advanced HCC. Here, we present a 56-year-old patient with HCC who showed complete clinical response after sorafenib was used for tumor recurrence which developed 3 mo after partial hepatectomy. There was no evidence of progression of disease for 60 mo till now after continuous treatment with sorafenib.
Core tip: For patients with multiple recurrent hepatocellular carcinoma (HCC) after partial hepatectomy which cannot be treated by re-hepatectomy or local ablative therapy, the prognosis is extremely poor. Our case showed that the patient with recurrent HCC treated with sorafenib can achieve a complete clinical response as it did in advanced HCC.
- Citation: Huan HB, Lau WY, Xia F, Ma KS, Bie P. Complete response to sorafenib in a patient with recurrent hepatocellular carcinoma. World J Gastroenterol 2014; 20(39): 14505-14509
- URL: https://www.wjgnet.com/1007-9327/full/v20/i39/14505.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i39.14505
Partial hepatectomy is still the treatment of choice aiming at a cure for patients with hepatocellular carcinoma (HCC), provided that the patient can tolerate the treatment. For patients with multiple recurrent HCC after partial hepatectomy which cannot be treated by re-hepatectomy or local ablative therapy, the prognosis is extremely poor[1,2].
Sorafenib is a molecular-targeted agent which has been demonstrated in two global phase III randomized controlled trials to show survival benefit for advanced HCC. It is an oral multi-kinase inhibitor that targets Raf kinases (Raf-1, wild-type B-Raf, and b-raf V600E), in addition to receptor tyrosine kinases associated with angiogenesis [vascular endothelial growth factor receptor (VEGFR)-2/-3, platelet-derived growth factor receptor (PDGFR)-β] or tumor progression (Flt-3, c-kit)[3]. However, in the SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) trial, only 7 sorafenib-treated patients had a partial response and no patient had a complete response[4]. In the Asia-Pacific trial, only 5 sorafenib-treated patients had a partial response and no patient had a complete response[5].
Here, we present a 56-year-old patient with HCC who showed a complete clinical response after sorafenib was used for tumor recurrence which developed 3 mo after partial hepatectomy. There was no evidence of progression of disease for 60 mo after continuous treatment with sorafenib.
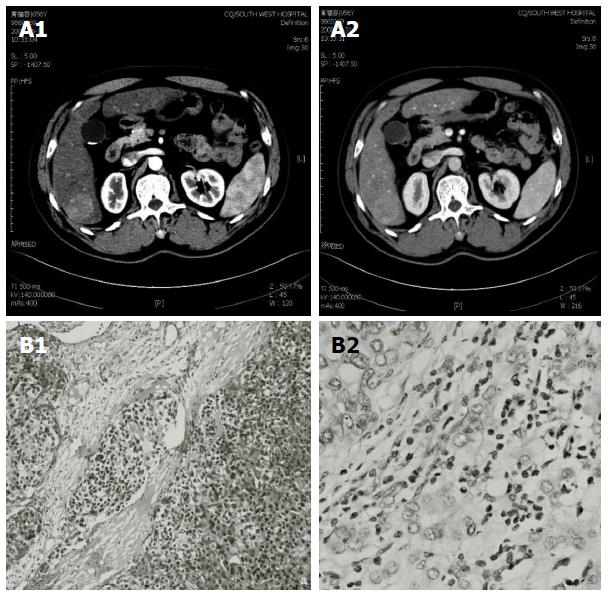
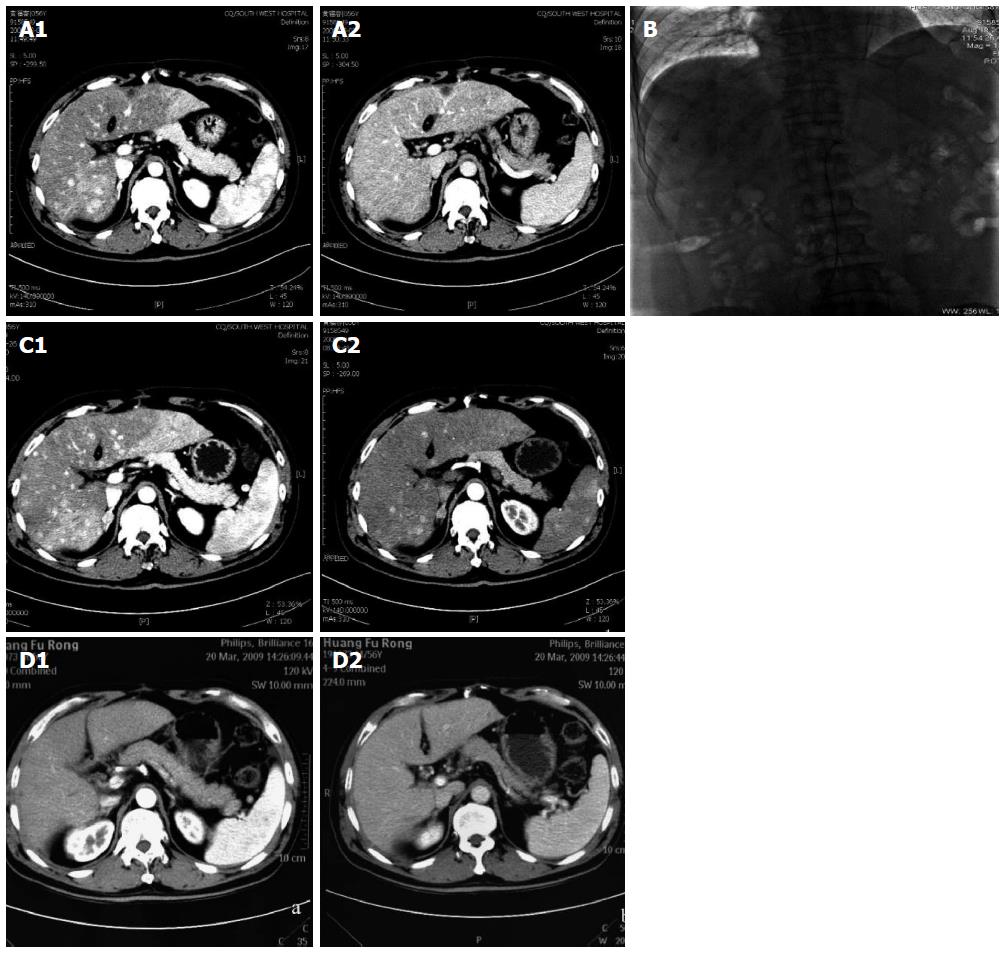
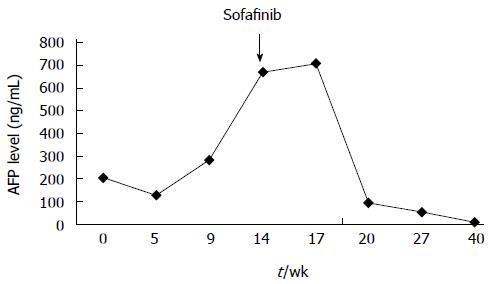
In June 2008, a 56-year-old male was diagnosed with HCC on a background of hepatitis B virus (HBV)-related cirrhosis. Computed tomography (CT) showed a 4 cm × 4 cm mass in liver segment 6 with an adjacent metastatic satellite tumor nodule (Figure 1A). The a-fetoprotein (AFP) level was raised to 205 ng/mL. Resection of liver segments 5 and 6 together with cholecystectomy was performed. Histopathological examination of the tumor specimen revealed HCC with intrahepatic metastasis and AFP(+) and CK8/18(+) (Figure 1B). Adjuvant transcatheter hepatic arterial chemoembolization (TACE) was performed 1 mo after hepatectomy. Hepatic angiography revealed no residual or recurrent lesion. The AFP level decreased to 127 ng/mL. On routine CT surveillance 2 mo later, multiple nodules were found in the right and left hemilivers (Figure 2A). Repeat hepatic angiography revealed multiple tumor nodules (maximum 41 mm × 24 mm) in the liver (Figure 2B). The AFP level rose to 283 ng/mL. The patient was diagnosed with multiple recurrent HCC and was treated by TACE once in August 2008. After 1 mo, the AFP continued to increase to 670 ng/mL. CT revealed an increase in the size of the recurrent lesions in the liver (Figure 2C). The patient’s Eastern Cooperative Oncology Group performance status was 1 (Child-Pugh class A). Treatment with oral sorafenib was initiated at 400 mg two times a day from September 26, 2008. After 2 wk, the sorafenib dosage was decreased to 400 mg/d (half the standard dose) for 1 wk because the patient developed thrombocytopenia together with laryngeal foreign body sensation and pain. Then, the drug dosage was returned to the standard dose of 400 mg twice a day. The AFP level increased to 708 ng/mL after 3 wk of treatment with sorafenib. At 6 wk of treatment, CT showed that the majority of the intrahepatic lesions shrank. The AFP level decreased to 93 ng/mL. At 3 mo, CT revealed only a small number of liver lesions. The AFP level further decreased to 54 ng/mL. At 6 mo, CT showed that the liver lesions completely disappeared (Figure 2D). The AFP level decreased to 9.3 ng/mL. A complete response was achieved. The patient continued to receive sorafenib till now. At a recent follow-up, the patient had a disease-free survival for more than 60 mo after complete clinical response. The AFP remained to be within normal limits and CT continued to show complete remission. The change in AFP level is shown in Figure 3.
Sorafenib acts on tumor cell proliferation and angiogenesis in patients with HCC[3]. It is therefore possible that HCC patients treated with sorafenib can achieve complete remission, although such a phenomenon is extremely rare. The effectiveness and safety of sorafenib have been confirmed in two multi-center clinical studies (the SHARP and the Asia-Pacific Studies)[4,5]. In the SHARP trial, 299 patients received sorafenib therapy. The survival rate at 1 year was 44% in the sorafenib group and 33% in the placebo group. However, only 7 patients (2%) developed a partial response and 211 (71%) showed stable disease (using the Response Evaluation Criteria in Solid Tumors). The disease-control rate was significantly higher in the sorafenib group than in the placebo group. There was no complete response in the trial[4]. In the Asia-Pacific trial, 150 patients received sorafenib, most of whom had associated chronic HBV infection. The disease-control rate was significantly greater in the sorafenib group (53%) than the placebo group (12%). Only 5 patients (3.3%) developed a partial response in the sorafenib group and there was no complete response[5]. So et al[6] reported on the first patient with HCC who developed a complete response following sorafenib for six months. Irtan et al[7] reported on two patients who achieved complete regression using sorafenib which was followed by radical resection surgery. In addition, there have been a small number of patients with HCC who developed a complete response with sorafenib treatment.
Our case provides additional evidence to show that sorafenib is effective for patients with HCC, and it can achieve complete remission, long disease-free survival and provide patients with good quality of life. This case report is special because, first, there has not been any report of complete tumor remission with sorafenib for recurrent HCC after partial hepatectomy. In our patient, the sorafenib treatment was initiated for disease progression 3 mo after surgery and the patient had a long disease-free survival of 60 mo. Second, the patient had a long history of HBV-related chronic hepatitis. The high HCC rate in China largely reflects the high prevalence of chronic HBV infection[8]. To the best of our knowledge, this is the first report on a Chinese patient who had a complete response with sorafenib. Third, disease progression was observed within 3 wk of treatment with sorafenib. However, when its use was continued, remission of the disease was observed after 6 wk, which indicates that the patient had a delay in responses. The patient continued sorafenib for 6 mo, and a complete response was achieved. This suggests that tumor progression during treatment is not a sign to withdraw sorafenib. Moreover, a recent study demonstrated that sorafenib administration beyond the first radiological evidence showing progressive disease could later show suppression of HCC growth and survival benefit[9]. Fourth, our patient received surgery and TACE before sorafenib therapy. The combination therapy may be beneficial to patients. There have been reports which showed that sorafenib, in combination with other drugs, radiation therapy, or immunotherapy, promotes better clinical outcomes in HCC patients. Fifth, our patient developed thrombocytopenia and laryngeal foreign body sensation and pain. Complications usually occur within 1-3 wk after initiation of sorafenib. Effective control of complications can make patients accept the treatment better. In our case, sorafenib was continued and 6 mo later the patient achieved complete remission.
In conclusion, our patient shows the effectiveness of sorafenib on disease progression in patients with recurrent HCC after partial hepatectomy.
A 56-year-old male patient with recurrent hepatocellular carcinoma (HCC) achieved a complete clinical response after administration of sorafenib.
Recurrent HCC.
Hepatic hemangioma, focal nodular hyperplasia, and hepatic cirrhosis nodule.
HbsAg(+), HbeAb(+), HbcAb(+), and AFP 670 ng/mL.
Computed tomography scan and hepatic angiography showed multiple tumor nodules in the liver.
Postoperative biopsy revealed hepatocellular carcinoma as well as a-fetoprotein, CK8/18 and CK19 positivity.
The patient was treated with sorafenib for recurrent HCC.
This case shows the effectiveness of sorafenib on disease progression in patients with recurrent HCC after partial hepatecotmy.
This case showed that the patient with recurrent HCC treated with sorafenib can achieve a complete clinical response as it did in advanced HCC.
P- Reviewer: Kakizaki S, Pellicelli AM, Piscaglia AC S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Hung H. Treatment modalities for hepatocellular carcinoma. Curr Cancer Drug Targets. 2005;5:131-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Del Pozo AC, López P. Management of hepatocellular carcinoma. Clin Liver Dis. 2007;11:305-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2966] [Cited by in F6Publishing: 3035] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9507] [Article Influence: 594.2] [Reference Citation Analysis (1)] |
| 5. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3854] [Cited by in F6Publishing: 4336] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 6. | So BJ, Bekaii-Saab T, Bloomston MA, Patel T. Complete clinical response of metastatic hepatocellular carcinoma to sorafenib in a patient with hemochromatosis: a case report. J Hematol Oncol. 2008;1:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Irtan S, Chopin-Laly X, Ronot M, Faivre S, Paradis V, Belghiti J. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int. 2011;31:740-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 9. | Miyahara K, Nouso K, Morimoto Y, Takeuchi Y, Hagihara H, Kuwaki K, Onishi H, Ikeda F, Miyake Y, Nakamura S. Efficacy of sorafenib beyond first progression in patients with metastatic hepatocellular carcinoma. Hepatol Res. 2014;44:296-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |