Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12132
Revised: April 2, 2014
Accepted: June 26, 2014
Published online: September 14, 2014
Human immunodeficiency virus (HIV) and hepatitis C virus (HCV) share the same transmission routes; therefore, coinfection is frequent. An estimated 5-10 million individuals alone in the western world are infected with both viruses. The majority of people acquire HCV by injection drug use and, to a lesser extent, through blood transfusion and blood products. Recently, there has been an increase in HCV infections among men who have sex with men. In the context of effective antiretroviral treatment, liver-related deaths are now more common than Acquired Immune Deficiency Syndrome-related deaths among HIV-HCV coinfected individuals. Morbidity and mortality rates from chronic HCV infection will increase because the infection incidence peaked in the mid-1980s and because liver disease progresses slowly and is clinically silent to cirrhosis and end-stage-liver disease over a 15-20 year time period for 15%-20% of chronically infected individuals. HCV treatment has rapidly changed with the development of new direct-acting antiviral agents; therefore, cure rates have greatly improved because the new treatment regimens target different parts of the HCV life cycle. In this review, we focus on the epidemiology, diagnosis and the natural course of HCV as well as current and future strategies for HCV therapy in the context of HIV-HCV coinfection in the western world.
Core tip: Hepatitis C virus (HCV) infection incidence has increased among men who have sex with men. Additionally, mortality and morbidity from chronic HCV infection has increased and liver-related deaths are now more common than Acquired Immune Deficiency Syndrome-related deaths. Several new direct-acting antiviral agents have been developed or are under development, and therapy strategies change faster than guidelines can be updated. This review focuses on the epidemiology, diagnosis, natural course and treatment of HCV infection in human immunodeficiency virus infected patients.
- Citation: Clausen LN, Lundbo LF, Benfield T. Hepatitis C virus infection in the human immunodeficiency virus infected patient. World J Gastroenterol 2014; 20(34): 12132-12143
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12132
A hallmark feature of hepatitis C virus (HCV) infection is its propensity for lifelong chronic infection. The majority (65%-80%) of all infected individuals remain chronically infected and at risk of severe liver disease (cirrhosis, end-stage liver disease and liver cancer); however, the remaining 15%-40% spontaneously resolve their infections[1]. Worldwide, this has resulted in more than 130 million chronically infected individuals. We conducted a narrative review to provide an overview of human immunodeficiency virus (HIV)-HCV coinfection. A comprehensive computerized literature search was carried out with PubMed and ClinicalTrials.gov to collect relevant articles. This review describes the epidemiology, diagnosis and the natural course of HCV as well as guidelines for HCV therapy in the context of HIV-HCV coinfection in the western world.
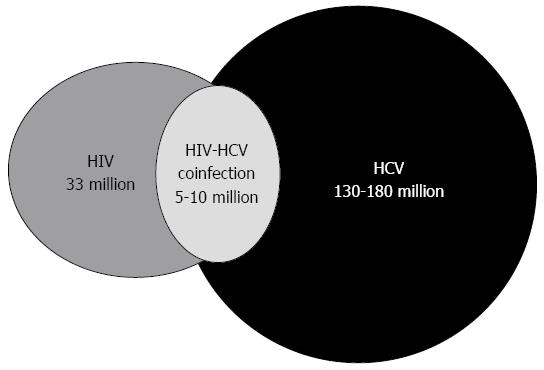
HCV and HIV share the same routes of transmission; therefore, coinfection with both viruses is frequent with an estimated 5-10 million coinfected individuals[2] (Figure 1). Contaminated blood product transmission used to be an important route of exposure, which explains why many hemophiliacs are coinfected with HIV-HCV. However, with the introduction of HIV and HCV screening, the blood product transmission rate has decreased in the western world[3]. Now, the primary routes of parenteral exposure are through injection drug use and, to a minor extent, through tattooing and piercing. The HIV and HCV transmission efficiency varies and transmission through percutaneous blood exposure is 10-fold higher for HCV compared with HIV[4]. As a consequence of this the incidence rate of HCV infection is higher than HIV among people who inject drugs (PWID)[5], and it is estimated that 50%-90% of all PWIDs are infected with HCV[6-10]. PWIDs with HIV-HCV coinfection are a population with behavioral and psychosocial problems, which together with clinical challenges, impacts not only the HIV and HCV infection course but also its treatment with antiviral therapy[11].
HCV also occurs through sexual transmission. Over the last decade, an increase in the HCV incidence rate among men who have sex with men (MSM) has emerged[12-15]. The background for the increased sexual transmission appears complex[16]. Sexual transmission has traditionally been considered inefficient, and truly, heterosexually HCV transmission is inefficient. Hence, the few prospective studies performed in heterosexual monogamous couples reported incident transmission rates of 0%-0.6% per year[17-19]. However, the increased transmission among MSM may be explained by changes in sexual behavior due to the expanded antiretroviral treatment (ART) availability and the lowered risk of HIV transmission, therefore, leading HIV infected individuals to engage in unprotected sexual activity with individuals with the same HIV status (termed serosorting)[8-10,16]. Further, traumatic sexual practices and substance abuse may increase bleeding during sexual activity and thereby increase the HCV transmission risk[16]. Finally, an increase in sexually transmitted diseases has been associated with serosorting and increased HCV transmission[14,20,21].
Transmission from mother-to-child is estimated at 4%-7% per pregnancy in women with HCV viremia (reviewed in[22]). Coinfection with HIV increases the transmission rates 4-5-fold; however, transmission is associated with levels of HCV and HIV viremia. Additionally, in a cohort of coinfected women who all received ART, no HCV transmission occurred[23]. The actual time and mode of transmission is unknown.
Acute HCV infection is defined as the first 6-mo period after HCV infection. The definition is arbitrary as the determination of when an acute infection becomes chronic is uncertain and no serological tests are available to distinguish between the two. Initial HCV infection is characterized by the detection of HCV RNA in the blood 2-14 d post exposure, increasing levels of liver-associated plasma enzymes and the gradual appearance of HCV antibodies[24]. In HIV infected individuals, the appearance of HCV antibodies may be delayed or absent, hypothetically, due to immunosuppression, with failure to mount or maintain HCV antibody titers for detection by standard serodiagnostic tests. The few studies conducted to assess the incidence of seronegative chronic HCV infection reported an incidence between 0%-13%[25-27].
Acute HCV clinical diagnosis has a low sensitivity as 75%-80% of cases are asymptomatic and are diagnosed based on risk history [e.g., needle stick injury or injection drug use (IDU)] or elevated liver-associated enzymes. Symptomatic acute HCV is often mild and involves nonspecific symptoms, such as lethargy and myalgia; however, jaundice might also be observed[28,29]. Little is known about the natural history of early HCV infection in HIV-positive individuals. Its clinical presentation has been described as being similar in HIV infected and HIV uninfected patients[14,30] as well as having less pronounced clinical symptoms with HIV infection[31].
The spontaneous HCV resolution rate is considered to be lower in HIV infected individuals than in HCV infected individuals without HIV, with estimates varying between 5% and 25%[4,32-34].
Several factors affect the HCV resolution rate. There is an overall consensus that female sex, younger age at infection, Caucasian race[33], coinfection with hepatitis B virus (HBV)[32,35-37] and symptomatic acute hepatitis[34,38-40] are associated with resolution. A strong genetic association with spontaneous HCV resolution has been mapped to a single nucleotide polymorphism (SNP) located approximately 3 kb upstream of interleukin 28B (IL28B; rs12979860)[41-46]. The genetic variations in SNPs marking IL28B explained approximately 10% of spontaneous HCV resolution cases[47]; however, the effect of IL28B on HCV may vary between HCV genotypes[48].
Both innate and adaptive immunity is believed to make important contributions to spontaneous resolution. HCV resolution has been associated with a rapid production of broadly neutralizing antibodies and a strong, broadly targeted T cell response. The innate immune response is induced upon recognition of viral pathogen-associated molecule patterns that are sensed by pathogen-recognition receptors. The sensing of HCV, which occurs through interaction with toll-like-receptors (TLR) and retinoic acid-inducible gene-I (RIG-I), leads to an intracellular signaling cascade that activates interferon (IFN) regulatory factors and ultimately induces production of IFNs. The production of IFNs leads to transcription of hundreds of IFN- stimulated genes (ISG) through the JAK-STAT signal pathway, which results in an antiviral state in the liver[49].
Individuals who resolve HCV infection have a broader and more sustained CD4 and CD8 T-cell response (reviewed in[50]) than individuals with chronic HCV. The detection of fully functional HCV-specific CD4 T lymphocytes during acute infection is associated with subsequent HCV resolution[51]. Further, a temporal association between the detection of HCV-specific CD8 T-cell responses indicates that CD8 T-cells are also important in HCV resolution. Hence, both CD4 and CD8 T-cell responses are required to achieve HCV resolution. The importance of CD4 T lymphocytes in resolution is further underscored by the lower resolution rate found with HIV coinfection, which may in part be due to CD4 T lymphocyte depletion.
Genetic associations with spontaneous HCV resolution have been investigated in many different parts of the human immune response. In the innate immune response, associations have been reported in killer immunoglobulin-like receptors (KIR)[52], inhibitor of NF-kB ε (IkBε)[53], TLR-7[54] and in the three main effector pathways of the IFN-mediated antiviral response, which include MxA, 2’5’-oligoadenylate-synthetase-directed ribonuclease L (OAS-1) and protein kinase R (PKR)[55]. In the adaptive immune response, the human-leukocyte-antigen (HLA) class II loci DQ and DRB1 are the loci with the most consistently association with spontaneous HCV resolution[40,56-63]. Further, regarding HLA class I, HLA-B57 and B27 have been associated with resolution[64-66]. The association with B57 is interesting because B57 has also been reported to be associated with slower HIV disease progression[67-70].
The risk factors for progression to chronic HCV infection include asymptomatic acute infection, male sex, older age at infection, black ethnicity, HIV co-infection and non-CC IL28B genotypes[9,33,71].
Chronic HCV infection in HIV infected individuals differs from that in individuals without an HIV infection in several ways. Coinfected individuals have a higher HCV viral load[72] and thereby have higher transmission rates. Coinfection is also associated with more rapid progression of fibrosis[73], cirrhosis[74], end-stage liver disease (ESLD) and hepatocellular carcinoma[75-79]. Recently, Kirk et al[77] reported that even after an adjustment for HCV viral load (VL) levels, HBV chronicity, sex, race and alcohol use, HIV-HCV coinfection was associated with liver fibrosis as advanced as those without HIV who were 10 years older.
The improvement of ART in the mid-1990’s led to greatly improved survival from HIV infection. With the decline in Acquired Immune Deficiency Syndrome (AIDS)-related deaths, non-AIDS causes of morbidity and mortality have become prevalent[80] and for HIV-HCV coinfected individuals, the burden of disease is largely related to their HCV disease[81-86]. A recent study of mortality from 2005-2009 in the Swiss HIV Cohort Study reported that 32% of deaths among individuals coinfected with HIV-HCV were caused by liver failure, including hepatocellular carcinoma[86]. HCV viral load[72,76,87-89], HCV genotype 3[87,89-93], ART[94], HCV treatment[95], CD4 T lymphocyte nadir[96], age, and drug use[97] have been shown to be associated with mortality in HIV-HCV coinfected individuals.
The differences in the causes of mortality among HCV infected individuals with and without HIV may reflect the differences in life-style-related risks for disease, drug use; the lasting chronic inflammation state, late presentation, treatment failure, prolonged immunodeficiency prior to treatment or no access to care or ART[80,98]. As a consequence of the high HCV prevalence in the HIV infected patient populations and accelerated disease progression, HCV-related morbidity and mortality is a substantial concern in HIV infected patients.
All HIV-infected patients should be tested for HCV infection. Initial screening for HCV should be performed by testing for anti-HCV antibodies; however, chronic HCV infection diagnosis is based on the presence of both anti-HCV antibodies (detected by enzyme-immunoassays) and HCV RNA (detected by molecular assays)[99].
False negative anti-HCV results may occur in HIV-infected persons with advanced immuno-suppression (CD4 < 100/mm3). Additionally, true negative anti-HCV results are common during acute HCV infection prior to seroconversion[26,100]. Different estimates of the duration from infection to seroconversion are specified and vary from 20-150 d. Most authors agree, however, that anti-HCV antibodies are expected to be detectable within the first 12 wk after exposure[24]. If anti-HCV is negative and HCV infection is still suspected due to elevated liver enzyme levels or risk factors, such as IDU or high-risk sexual behavior, HCV RNA testing should be performed.
HCV RNA is detectable in the plasma of most patients within 2 to 14 d following infection[24]. During the transition from an acute to a chronic infection, HCV RNA and serum alanine and aspartate aminotransferase (ALT and AST) levels may fluctuate, and some patients may have periods with undetectable HCV RNA and normal ALT levels[1]. While a single detectable HCV RNA result is sufficient to confirm the diagnosis of an active HCV infection, a single negative result cannot exclude active viremia because RNA levels might transiently decline below the detection limit; therefore, repeated testing is advised[1]. After the establishment of a chronic HCV infection, the HCV RNA levels stabilize and vary less than 1 log in the majority of cases[101]. The most recent HCV RNA assays are based on the use of the polymerase chain reaction and can detect HCV RNA between 10 IU/mL to 107 IU/mL[102,103]. Serum ALT and AST levels often fluctuate, and prolonged periods of normal serum liver enzyme levels may be observed. Although higher serum ALT and AST levels are clearly predictive of a more rapid disease progression and calls for an additional assessment of liver parenchymal changes, significant liver disease may be present even in the case of persistently normal ALT levels[104].
HCV has six clinically relevant genotypes that can be subdivided into multiple subtypes. HCV genotyping is essential when considering HCV treatments. It predicts the response to treatment and influences the decision to start a treatment and/or perform a liver biopsy. Several tests are available to determine HCV genotypes, and the most accurate method is to sequence an appropriate region that is divergent enough to discriminate subtypes and genotypes. Most assays target the highly conserved 5’untranslated region (5’UTR) as well as the E1 and NS5B regions of the HCV genome[105,106].
Hepatic parenchymal change severity assessments can be done by liver biopsy, liver transient elastography (TE) or by serum biomarker evaluation. Liver biopsy is considered the gold standard; however, its use is limited by the fact that its invasiveness carries the risk of rare but potentially lethal complications as well as the possibility of sampling error and intra- and inter-observer variability[107]. In contrast, liver biopsies provide histological fibrosis staging and the simultaneous evaluation of necro-inflammation, which enables for an assessment of the current fibrosis stage and activity level[108].
TE is non-invasive, painless, rapid and easy to perform at the bedside or in the outpatient clinic. The results are immediately available. TE reproducibility has been shown to be excellent in both inter- and intra-observer agreements[108,109]. One limitation of TE is that overestimation of liver stiffness may occur in patients with pronounced liver inflammation[110]. TE is obviously not capable of giving a direct histological liver description. The diagnostic accuracy of TE is high for cirrhosis, but poor for significant fibrosis[111].
Blood parameters combined with other biochemical factors have been validated to non-invasively predict the extent of liver fibrosis in a variety of liver diseases. Some tests are based on laboratory markers that are routinely available for most HIV-HCV coinfected individuals [e.g., the AST-to-Platelet-Index (APRI), FIB-4 ((Age × AST)/{Platelets × [sqr (ALT)]}), Forns {7.811 - 3.131 × ln [number of platelets (109/L)] × 0.781 ln [GGTP (U/L)] + 3.467 × ln [age (years)] - 0.014 [cholesterol (mg/dL)]}]. The tests have mostly been validated in chronic HCV infected individuals without HIV. In HIV-HCV coinfected individuals, APRI, Forns and FIB-4 were found to be accurate for cirrhosis diagnoses but relatively inaccurate for significant and advanced fibrosis diagnoses[112,113].
ART has been strongly associated with a slower fibrosis progression, decline in liver inflammation and decreased liver-associated mortality risk in HIV-HCV coinfected patients[73,114-118]. ART is, thus, indicated for every individual coinfected with HIV and HCV. However, the adverse impact of HIV coinfection on HCV disease is not entirely ameliorated by ART[76,119] and treatments aimed at chronic HCV infection eradication has been associated with a significantly lower risk of clinical events, as measured by ESLD, hepatocellular carcinoma or death rates. Interestingly, in a study by Limketkai et al[76], individuals who achieved viral suppression with either sustained (negative HCV RNA 24 wk after stopping therapy) or transient (undetectable HCV RNA during treatment but detectable in the follow-up period) HCV treatment experienced fewer clinical events post-HCV treatment in contrast to individuals who did not respond to HCV treatment or who did not undergo an HCV treatment. Thus, HCV treatment is required despite the many barriers, including decompensated liver disease, substance abuse, socioeconomic status and compliance issues.
Guidelines recommend HCV therapy initiation prior to ART if CD4 T lymphocytes are > 500/μL based on the evidence that HCV therapy is associated with lower responses in individuals with CD4 T lymphocytes < 500/μL[120,121]. In individuals with CD4 T lymphocytes < 500/μL, ART is initiated prior to HCV treatment.
HCV therapies change faster than the international guidelines are updated, and in the following section, we will attempt to provide an overview of the current and future possible treatment strategies for HIV-HCV coinfected individuals. Pegylated (peg) IFN will remain the backbone of some HCV therapy combinations in 2014 and 2015, but it is then expected to disappear from HCV therapy regimens. We provide an overview of interferon free clinical trials that include HIV infected individuals in Table 1. Ribavirin (RBV) can be used to increase sustained virological response (SVR) rates or to shorten the treatment durations with pegIFN or IFN-free regimens and will most likely remain in some HCV therapy strategies. Direct-acting antiviral (DAA) agents in HCV therapies target different steps of the viral lifecycle. DAAs are comprised of NS3/4A protease inhibitors, NS5B polymerase inhibitors, nucleoside/nucleotide analogues, non-nucleoside analogues inhibitors of the HCV RNA dependent RNA polymerase, cyclophilin inhibitors and miRNA-122 antagonists (as reviewed in[122]). Few DAAs, however, are approved for HCV therapy (Table 2) and the most of them are in phase II or III development. The antiviral effect of a DAA therapy can be optimized by combining several DAAs with or without RBV (Table 3) and this combination strategy has shown that it is possible to achieve SVR with interferon-free regimens.
| Direct acting antiviral | Ribavirin | Study name/identifier1 | Patient population | Phase | Primary endpoint | Presented results | ||
| Ns3/4a protease inhibitor | Ns5b polymerase inhibitors | Ns5a inhibitors | ||||||
| No | Sofosbuvir | No | Yes | Photon-I (Ncto1667731) | GT 1: | 3 | SVR 12 | GT 1: 76%2 |
| TN and IFN ineligible | GT 2: 88% | |||||||
| GT 2 + 3: TE | GT 3: 67% | |||||||
| No | Sofosbuvir | No | Yes | Ncto1783678 | GT 1 + 2 + 3 + 4: | 3 | SVR 12 | No |
| TN and IFN ineligible | ||||||||
| GT 2 + 3: TE | ||||||||
| ABT-450(r) | ABT-333 | ABT-267 | Yes | Turquoise I (Ncto1939197) | GT 1 | 3 | SVR 12 | No |
| TN + TE | ||||||||
| MK-5172 | No | MK-8742 | Yes | c-WORHTy (Ncto01717326) | GT 1 | 2 | SVR 12 | No |
| Direct acting antiviral | Ribavirin/Peg Inf | Study name/ identifier1 | Treatment duration | Phase | Primary endpoint | Presented results | ||
| Ns3/4a protease inhibitor | Ns5b polymerase inhibitors | Ns5a inhibitors | ||||||
| Boceprevir | No | No | Yes | NCT00959699 | 48 wk | APPROVED | SVR24 | GT 1: 63%[123] |
| Telaprevir | No | No | Yes | NCT00983853 | 48 wk | APPROVED | SVR12 | GT 1: 74%[124] |
| Simeprevir | No | No | Yes | C212 | 24/48 wk2 | III3 | SVR12 | GT 1: 79%-87% |
| NCT01479868 | ||||||||
| Faldaprevir | No | No | Yes | Startverso4 | 24/48 wk2 | III | SVR12 | GT 1: 71%-72% |
| NCT01399619 | ||||||||
| Treatment strategy | Compounds | Genotype 1 | Genotype 2 | Genotype 3 | Genotype 4 | Genotype 5 | Genotype 6 |
| Interferon Based | Sofosbuvir | × | × | × | × | × | × |
| Simeprevir | × | × | |||||
| Daclatasavir | × | × | × | × | × | ||
| Interferon free | Sofosbuvir + ribavirin | × | × | ||||
| Sofosbuvir + simeprevir | × | × | |||||
| Sofosbuvir + daclatasavir | × | × | × | × | × |
The first DAAs to be approved for HCV treatment were the NS3/4A protease inhibitor, telaprevir or boceprevir in combination with peg IFN and ribavirin. These compounds are on their way out of HCV therapy and are being replaced with the nucleotide analogue, sofosbuvir, in combination with RBV with or without peg IFN as the best treatment option in HCV genotype 1 and 4 infections. The addition of sofosbuvir to pegIFN/RBV (triple therapy) for 24 wk increases the 12-wk post-treatment termination SVR rate (SVR12) to 76%. For genotype 2 infections, the current best treatment option is an IFN-free regimen with sofosbuvir and RBV for 12 wk. This results in an 88% SVR12 rate. In genotype 3 infections, the same regimen is approved; however, the treatment duration is extended to 24 wk, which results in a 67% SVR12 rate. Data regarding HCV genotype 5 and 6 is limited, however, sofosbuvir + pegIFN + RBV appears to be the best treatment option for these HCV types.
The next DAAs to be approved will most likely be simeprevir, faldaprevir and daclatasvir (the available data from these DAA studies regarding HIV-HCV coinfected patients are summarized in Table 2). However, treatment with DAAs is costly and the high costs will most likely influence the treatment strategy choice and result in first-, second- and third-line HCV treatment strategies.
With the potent therapeutic strategies combining different DAAs with or without RBV, the SVR rates are not fundamentally different between HCV mono-infected and HCV-HIV coinfected patients. However, drug-drug interactions (DDI) with ART are of concern as most HIV infected individuals are also treated for their HIV infection. Briefly, no clinically significant DDIs have been reported to occur between sofosbuvir and antiretrovirals. With respect to simeprevir, co-administration with antiretroviral CYP34A inducers is not recommended; however, with respect to daclatasvir dose reduction, it is recommended when administered with specific antiretroviral drugs. Still, data are limited and more studies are warranted to assess DDIs with ART. When initiating HCV therapy, the antiretroviral agents should be carefully assessed and if bidirectional interactions are present, then the HIV regimen should be switched to an acceptable combination. After an ART switch, the HCV therapy is suspended until the patient is virally stable and tolerating the new ART regimen.
Treatment indications for HCV in HIV-HCV coinfected individuals are based on liver disease progression. The decision to initiate or defer treatments to wait for more potent treatment regimens - which are expected to be available within the next 2-3 years - depends on the liver fibrosis stage, rapidity of fibrosis development and the former treatment responses. In individuals without fibrosis or with mild fibrosis (Metavir F0/F1), treatment deferral that is independent of any former treatment response may be reasonable[76]. In individuals with significant fibrosis (Metavir F2/F3), treatment can also be deferred; however, annual liver fibrosis progression assessments are required because the progression may be rapid[125]. Others suggest that individuals with significant fibrosis who are treatment naïve or relapsed with former anti-HCV treatments should be treated according to current guidelines (see below)[125,126]. The reason for not deferring therapy is the risk of liver decompensation among the coinfected individuals with bridging fibrosis within a short time period[125]. In individuals with cirrhosis (Metavir F4), HCV treatment is recommended independent of former treatment responses. Initiation of HCV therapy should be taken with a cautious approach because new DAAs with higher efficacy, lower pill burden, less pronounced interaction profiles and, hopefully, fewer side effects as well as new drug classes (also for non-genotype 1 patients) are under way. Further, the potential risk of resistance development, especially in this group of co-infected individuals who have a higher viral load than mono-infected individuals, should be taken into consideration.
The standard of care treatment for acute HCV infection is pegIFN/RBV, and the therapy duration is based on rapid virological response regardless of genotypes. Treatment in the acute HCV phase leads to SVR rates of 50%-91%[127]. In persons with acute HCV, HCV RNA should be measured at initial presentation and 4 wk later. Treatment should be offered to patients without a 2 log10 HCV RNA decrease at 4 wk compared with the initial HCV RNA level and to persons with persistent detectable serum HCV RNA levels 12 wk after acute HCV diagnoses. Persons who do not achieve more than a 2 log10 decrease in HCV RNA level at week 12 should discontinue therapy[128]. Currently, DAAs are not recommended as a first-line treatment for acute HCV infections.
The effect of ART is modified by HCV resulting in an earlier time to virological failure, lower CD4 T lymphocytes and a smaller and slower CD4 T lymphocyte increase than observed in HIV-treated infected individuals without HCV[129,130]. This is clinically relevant as HCV/HIV coinfection is associated with a higher risk of developing a new AIDS-related diagnosis or death. Thus, early ART initiation in HIV-HCV coinfected individuals is justified not only to delay liver fibrosis development but also to gain the protective effects of HIV therapy on HIV disease progression.
The total number of circulating CD4 T lymphocytes has well-validated predictive value for assessing progression to clinical AIDS and AIDS-related death, determining ART eligibility and monitoring responses to therapy. The number of CD4 T lymphocytes expressed as a percentage of lymphocytes (CD4%) has been suggested as a more appropriate HIV disease marker and predictor of HIV progression in individuals with HCV and CD4 T lymphocytes > 350 cells/mm3[131]. CD4 discordance describes the phenomenon where the absolute and relative CD4 cell relationship differs from what would be expected. CD4 discordance has been reported with high frequency in individuals with fibrosis, cirrhosis and ESLD[132,133] and thus, the consideration of CD4% measurements in individuals with HCV may be appropriate.
Due to shared routes of transmission, HIV and HCV coinfection is frequent and, in the western world, the majority of infected individuals are PWID. A minor subset of infected individuals has the ability to spontaneously resolve HCV infections, but the majority develop chronic HCV infection with the risk of cirrhosis, end-stage-liver-disease and hepatocellular carcinoma. With the decline in AIDS-related deaths, non-AIDS causes of morbidity and mortality have become prevalent and for HIV-HCV coinfected individuals, the burden of disease is largely related to their HCV disease. A consensus of the influence of HIV on the natural course of HCV exists; however, how HCV influences the natural course of HIV is still debated and no final conclusions have been drawn.
Treatment options for HCV are rapidly evolving towards an interferon-free all-oral treatment regimen. Currently, treatment responses to HCV therapy are lower than what is seen in individuals without HIV. With interferon- and even ribavirin-free combination treatment regimens, the response rates do not differ significantly from the ones seen in chronic HCV infected patients without HIV, and any coinfected individual should be evaluated for HCV therapy on an individual basis.
P- Reviewer: Robaeys GK, Sung FC, Verlato G, Zehender G S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21-S29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 2. | Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep. 2011;8:12-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Coste J, Reesink HW, Engelfriet CP, Laperche S, Brown S, Busch MP, Cuijpers HT, Elgin R, Ekermo B, Epstein JS. Implementation of donor screening for infectious agents transmitted by blood by nucleic acid technology: update to 2003. Vox Sang. 2005;88:289-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Sulkowski MS, Thomas DL. Hepatitis C in the HIV-Infected Person. Ann Intern Med. 2003;138:197-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 309] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 484] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Garten RJ, Lai S, Zhang J, Liu W, Chen J, Vlahov D, Yu XF. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int J Epidemiol. 2004;33:182-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Quan VM, Go VF, Nam le V, Bergenstrom A, Thuoc NP, Zenilman J, Latkin C, Celentano DD. Risks for HIV, HBV, and HCV infections among male injection drug users in northern Vietnam: a case-control study. AIDS Care. 2009;21:7-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6-S9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 627] [Cited by in F6Publishing: 620] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 9. | Thomas DL, Vlahov D, Solomon L, Cohn S, Taylor E, Garfein R, Nelson KE. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore). 1995;74:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 284] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35:3274-3277. [PubMed] [Cited in This Article: ] |
| 11. | Larsen MV, Omland LH, Gerstoft J, Larsen CS, Jensen J, Obel N, Kronborg G. Impact of injecting drug use on mortality in Danish HIV-infected patients: a nation-wide population-based cohort study. Addiction. 2010;105:529-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Danta M, Brown D, Bhagani S, Pybus OG, Sabin CA, Nelson M, Fisher M, Johnson AM, Dusheiko GM. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 13. | Götz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men--results from contact tracing and public health implications. AIDS. 2005;19:969-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Luetkemeyer A, Hare CB, Stansell J, Tien PC, Charlesbois E, Lum P, Havlir D, Peters M. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Serpaggi J, Chaix ML, Batisse D, Dupont C, Vallet-Pichard A, Fontaine H, Viard JP, Piketty C, Rouveix E, Rouzioux C. Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. AIDS. 2006;20:233-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Taylor LE, Swan T, Mayer KH. HIV coinfection with hepatitis C virus: evolving epidemiology and treatment paradigms. Clin Infect Dis. 2012;55 Suppl 1:S33-S42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Piazza M, Sagliocca L, Tosone G, Guadagnino V, Stazi MA, Orlando R, Borgia G, Rosa D, Abrignani S, Palumbo F. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537-1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Tahan V, Karaca C, Yildirim B, Bozbas A, Ozaras R, Demir K, Avsar E, Mert A, Besisik F, Kaymakoglu S. Sexual transmission of HCV between spouses. Am J Gastroenterol. 2005;100:821-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Vandelli C, Renzo F, Romanò L, Tisminetzky S, De Palma M, Stroffolini T, Ventura E, Zanetti A. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99:855-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Browne R, Asboe D, Gilleece Y, Atkins M, Mandalia S, Gazzard B, Nelson M. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80:326-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Matthews GV, Pham ST, Hellard M, Grebely J, Zhang L, Oon A, Marks P, van Beek I, Rawlinson W, Kaldor JM. Patterns and characteristics of hepatitis C transmission clusters among HIV-positive and HIV-negative individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2011;52:803-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Roberts EA, Yeung L. Maternal-infant transmission of hepatitis C virus infection. Hepatology. 2002;36:S106-S113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 707] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 25. | Chamie G, Bonacini M, Bangsberg DR, Stapleton JT, Hall C, Overton ET, Scherzer R, Tien PC. Factors associated with seronegative chronic hepatitis C virus infection in HIV infection. Clin Infect Dis. 2007;44:577-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Thio CL, Nolt KR, Astemborski J, Vlahov D, Nelson KE, Thomas DL. Screening for hepatitis C virus in human immunodeficiency virus-infected individuals. J Clin Microbiol. 2000;38:575-577. [PubMed] [Cited in This Article: ] |
| 27. | Thomson EC, Nastouli E, Main J, Karayiannis P, Eliahoo J, Muir D, McClure MO. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS. 2009;23:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Boesecke C, Rockstroh JK. Acute hepatitis C in patients with HIV. Semin Liver Dis. 2012;32:130-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Orland JR, Wright TL, Cooper S. Acute hepatitis C. Hepatology. 2001;33:321-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Matthews GV, Hellard M, Haber P, Yeung B, Marks P, Baker D, McCaughan G, Sasadeusz J, White P, Rawlinson W. Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis. 2009;48:650-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Deterding K, Wiegand J, Grüner N, Hahn A, Jäckel E, Jung MC, Buggisch P, Galle P, Berg T, Hinrichsen H. The German Hep-Net acute hepatitis C cohort: impact of viral and host factors on the initial presentation of acute hepatitis C virus infection. Z Gastroenterol. 2009;47:531-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Clausen LN, Weis N, Schønning K, Fenger M, Krarup H, Bukh J, Benfield T. Correlates of spontaneous clearance of hepatitis C virus in a Danish human immunodeficiency virus type 1 cohort. Scand J Infect Dis. 2011;43:798-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, Nelson KE, Strathdee SA, Johnson L. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 820] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 34. | Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 349] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Zhang M, Rosenberg PS, Brown DL, Preiss L, Konkle BA, Eyster ME, Goedert JJ. Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood. 2006;107:892-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Grebely J, Page K, Sacks-Davis R, van der Loeff MS, Rice TM, Bruneau J, Morris MD, Hajarizadeh B, Amin J, Cox AL. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59:109-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 37. | Soriano V, Mocroft A, Rockstroh J, Ledergerber B, Knysz B, Chaplinskas S, Peters L, Karlsson A, Katlama C, Toro C. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis. 2008;198:1337-1344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Thomson EC, Fleming VM, Main J, Klenerman P, Weber J, Eliahoo J, Smith J, McClure MO, Karayiannis P. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2011;60:837-845. [PubMed] [Cited in This Article: ] |
| 39. | Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 589] [Cited by in F6Publishing: 598] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 40. | Alric L, Fort M, Izopet J, Vinel JP, Bureau C, Sandre K, Charlet JP, Beraud M, Abbal M, Duffaut M. Study of host- and virus-related factors associated with spontaneous hepatitis C virus clearance. Tissue Antigens. 2000;56:154-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Clausen LN, Weis N, Astvad K, Schønning K, Fenger M, Krarup H, Bukh J, Benfield T. Interleukin-28B polymorphisms are associated with hepatitis C virus clearance and viral load in a HIV-1-infected cohort. J Viral Hepat. 2011;18:e66-e74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Jiménez-Sousa MA, Fernández-Rodríguez A, Guzmán-Fulgencio M, García-Álvarez M, Resino S. Meta-analysis: implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013;11:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 43. | Montes-Cano MA, García-Lozano JR, Abad-Molina C, Romero-Gómez M, Barroso N, Aguilar-Reina J, Núñez-Roldán A, González-Escribano MF. Interleukin-28B genetic variants and hepatitis virus infection by different viral genotypes. Hepatology. 2010;52:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 44. | Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338-45, 1345 e1-7. [PubMed] [Cited in This Article: ] |
| 45. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1667] [Cited by in F6Publishing: 1635] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 46. | Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, Lokhnygina Y, Kullig U, Göbel U, Capka E. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586-192, 1592.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 47. | Duggal P, Thio CL, Wojcik GL, Goedert JJ, Mangia A, Latanich R, Kim AY, Lauer GM, Chung RT, Peters MG. Genome-wide association study of spontaneous resolution of hepatitis C virus infection: data from multiple cohorts. Ann Intern Med. 2013;158:235-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 48. | Lindh M, Lagging M, Färkkilä M, Langeland N, Mørch K, Nilsson S, Norkrans G, Pedersen C, Buhl MR, Westin J. Interleukin 28B gene variation at rs12979860 determines early viral kinetics during treatment in patients carrying genotypes 2 or 3 of hepatitis C virus. J Infect Dis. 2011;203:1748-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Terilli RR, Cox AL. Immunity and hepatitis C: a review. Curr HIV/AIDS Rep. 2013;10:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Burke KP, Cox AL. Hepatitis C virus evasion of adaptive immune responses: a model for viral persistence. Immunol Res. 2010;47:216-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1202] [Cited by in F6Publishing: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 52. | Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 916] [Cited by in F6Publishing: 895] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 53. | Clausen LN, Ladelund S, Weis N, Bukh J, Benfield T. Genetic variation in toll-like receptors and retinoic acid-inducible gene I and outcome of hepatitis C virus infection: a candidate gene association study. J Viral Hepat. 2014;21:578-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Schott E, Witt H, Neumann K, Bergk A, Halangk J, Weich V, Müller T, Puhl G, Wiedenmann B, Berg T. Association of TLR7 single nucleotide polymorphisms with chronic HCV-infection and response to interferon-a-based therapy. J Viral Hepat. 2008;15:71-78. [PubMed] [Cited in This Article: ] |
| 55. | Knapp S, Yee LJ, Frodsham AJ, Hennig BJ, Hellier S, Zhang L, Wright M, Chiaramonte M, Graves M, Thomas HC. Polymorphisms in interferon-induced genes and the outcome of hepatitis C virus infection: roles of MxA, OAS-1 and PKR. Genes Immun. 2003;4:411-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Cramp ME, Carucci P, Underhill J, Naoumov NV, Williams R, Donaldson PT. Association between HLA class II genotype and spontaneous clearance of hepatitis C viraemia. J Hepatol. 1998;29:207-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Harris RA, Sugimoto K, Kaplan DE, Ikeda F, Kamoun M, Chang KM. Human leukocyte antigen class II associations with hepatitis C virus clearance and virus-specific CD4 T cell response among Caucasians and African Americans. Hepatology. 2008;48:70-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Minton EJ, Smillie D, Neal KR, Irving WL, Underwood JC, James V. Association between MHC class II alleles and clearance of circulating hepatitis C virus. Members of the Trent Hepatitis C Virus Study Group. J Infect Dis. 1998;178:39-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Thio CL, Thomas DL, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O’Brien SJ, Karacki P, Marti D, Astemborski J. Racial differences in HLA class II associations with hepatitis C virus outcomes. J Infect Dis. 2001;184:16-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Thursz M, Yallop R, Goldin R, Trepo C, Thomas HC. Influence of MHC class II genotype on outcome of infection with hepatitis C virus. The HENCORE group. Hepatitis C European Network for Cooperative Research. Lancet. 1999;354:2119-2124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 219] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 61. | Yee LJ. Host genetic determinants in hepatitis C virus infection. Genes Immun. 2004;5:237-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Yenigün A, Durupinar B. Decreased frequency of the HLA-DRB1*11 allele in patients with chronic hepatitis C virus infection. J Virol. 2002;76:1787-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Zavaglia C, Martinetti M, Silini E, Bottelli R, Daielli C, Asti M, Airoldi A, Salvaneschi L, Mondelli MU, Ideo G. Association between HLA class II alleles and protection from or susceptibility to chronic hepatitis C. J Hepatol. 1998;28:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Chuang WC, Sarkodie F, Brown CJ, Owusu-Ofori S, Brown J, Li C, Navarrete C, Klenerman P, Allain JP. Protective effect of HLA-B57 on HCV genotype 2 infection in a West African population. J Med Virol. 2007;79:724-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Hraber P, Kuiken C, Yusim K. Evidence for human leukocyte antigen heterozygote advantage against hepatitis C virus infection. Hepatology. 2007;46:1713-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Thio CL, Gao X, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O’Brien SJ, Karacki P, Astemborski J, Carrington M. HLA-Cw*04 and hepatitis C virus persistence. J Virol. 2002;76:4792-4797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Costello C, Tang J, Rivers C, Karita E, Meizen-Derr J, Allen S, Kaslow RA. HLA-B*5703 independently associated with slower HIV-1 disease progression in Rwandan women. AIDS. 1999;13:1990-1991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Klein MR, Keet IP, D’Amaro J, Bende RJ, Hekman A, Mesman B, Koot M, de Waal LP, Coutinho RA, Miedema F. Associations between HLA frequencies and pathogenic features of human immunodeficiency virus type 1 infection in seroconverters from the Amsterdam cohort of homosexual men. J Infect Dis. 1994;169:1244-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 69. | Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, Kaslow R, Buchbinder S, Hoots K, O’Brien SJ. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 920] [Cited by in F6Publishing: 853] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 70. | Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551-1557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 940] [Cited by in F6Publishing: 908] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 71. | Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383-98, vi. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 72. | Daar ES, Lynn H, Donfield S, Gomperts E, O’Brien SJ, Hilgartner MW, Hoots WK, Chernoff D, Arkin S, Wong WY. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001;183:589-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 895] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 74. | Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, Koziel MJ. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 705] [Cited by in F6Publishing: 688] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 75. | Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183:1112-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 76. | Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, Moore RD, Thomas DL, Sulkowski MS. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308:370-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 77. | Kirk GD, Mehta SH, Astemborski J, Galai N, Washington J, Higgins Y, Balagopal A, Thomas DL. HIV, age, and the severity of hepatitis C virus-related liver disease: a cohort study. Ann Intern Med. 2013;158:658-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 78. | Martín-Carbonero L, Benhamou Y, Puoti M, Berenguer J, Mallolas J, Quereda C, Arizcorreta A, Gonzalez A, Rockstroh J, Asensi V. Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C: a European collaborative study. Clin Infect Dis. 2004;38:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 79. | Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, Moore RD, Afdhal NH, Thomas DL. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21:2209-2216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 80. | Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S. Liver-related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med. 2006;166:1632-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 859] [Cited by in F6Publishing: 900] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 81. | Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 726] [Cited by in F6Publishing: 702] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 82. | Mocroft A, Katlama C, Johnson AM, Pradier C, Antunes F, Mulcahy F, Chiesi A, Phillips AN, Kirk O, Lundgren JD. AIDS across Europe, 1994-98: the EuroSIDA study. Lancet. 2000;356:291-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 343] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 83. | Mocroft A, Soriano V, Rockstroh J, Reiss P, Kirk O, de Wit S, Gatell J, Clotet B, Phillips AN, Lundgren JD. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS. 2005;19:2117-2125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 84. | Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6891] [Cited by in F6Publishing: 6645] [Article Influence: 255.6] [Reference Citation Analysis (0)] |
| 85. | Pineda JA, García-García JA, Aguilar-Guisado M, Ríos-Villegas MJ, Ruiz-Morales J, Rivero A, del Valle J, Luque R, Rodríguez-Baño J, González-Serrano M. Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007;46:622-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 86. | Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, Cavassini M, Calmy A, Bernasconi E, Schmid P. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14:195-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 87. | Clausen LN, Astvad K, Ladelund S, Larsen MV, Schønning K, Benfield T. Hepatitis C viral load, genotype 3 and interleukin-28B CC genotype predict mortality in HIV and hepatitis C-coinfected individuals. AIDS. 2012;26:1509-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Hisada M, Chatterjee N, Kalaylioglu Z, Battjes RJ, Goedert JJ. Hepatitis C virus load and survival among injection drug users in the United States. Hepatology. 2005;42:1446-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | McMahon BJ, Bruden D, Bruce MG, Livingston S, Christensen C, Homan C, Hennessy TW, Williams J, Sullivan D, Rosen HR. Adverse outcomes in Alaska natives who recovered from or have chronic hepatitis C infection. Gastroenterology. 2010;138:922-31.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 90. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 813] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 91. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2953] [Cited by in F6Publishing: 2955] [Article Influence: 113.7] [Reference Citation Analysis (1)] |
| 92. | de Mendoza C, Soriano V. The role of hepatitis C virus (HCV) in mitochondrial DNA damage in HIV/HCV-coinfected individuals. Antivir Ther. 2005;10 Suppl 2:M109-M115. [PubMed] [Cited in This Article: ] |
| 93. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 94. | Qurishi N, Kreuzberg C, Lüchters G, Effenberger W, Kupfer B, Sauerbruch T, Rockstroh JK, Spengler U. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708-1713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 95. | Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 96. | Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, Hogg RS, Deeks SG, Eron JJ, Brooks JT. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815-1826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 869] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 97. | Grebely J, Dore GJ. What is killing people with hepatitis C virus infection? Semin Liver Dis. 2011;31:331-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 98. | Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, Bertisch B, Bernasconi E, Weber R. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 449] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 99. | European Association of the Study of the Liver. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 2012;32 Suppl 1:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 100. | George SL, Gebhardt J, Klinzman D, Foster MB, Patrick KD, Schmidt WN, Alden B, Pfaller MA, Stapleton JT. Hepatitis C virus viremia in HIV-infected individuals with negative HCV antibody tests. J Acquir Immune Defic Syndr. 2002;31:154-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 101. | Yeo AE, Ghany M, Conry-Cantilena C, Melpolder JC, Kleiner DE, Shih JW, Hoofnagle JH, Alter HJ. Stability of HCV-RNA level and its lack of correlation with disease severity in asymptomatic chronic hepatitis C virus carriers. J Viral Hepat. 2001;8:256-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Chevaliez S, Bouvier-Alias M, Pawlotsky JM. Performance of the Abbott real-time PCR assay using m2000sp and m2000rt for hepatitis C virus RNA quantification. J Clin Microbiol. 2009;47:1726-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 103. | Vermehren J, Colucci G, Gohl P, Hamdi N, Abdelaziz AI, Karey U, Thamke D, Zitzer H, Zeuzem S, Sarrazin C. Development of a second version of the Cobas AmpliPrep/Cobas TaqMan hepatitis C virus quantitative test with improved genotype inclusivity. J Clin Microbiol. 2011;49:3309-3315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Puoti C, Guarisco R, Spilabotti L, Bellis L, Mitidieri Costanza O, Dell’ Unto O, Elmo MG. Should we treat HCV carriers with normal ALT levels? The ‘5Ws’ dilemma. J Viral Hepat. 2012;19:229-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Murphy DG, Willems B, Deschênes M, Hilzenrat N, Mousseau R, Sabbah S. Use of sequence analysis of the NS5B region for routine genotyping of hepatitis C virus with reference to C/E1 and 5’ untranslated region sequences. J Clin Microbiol. 2007;45:1102-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 106. | Corbet S, Bukh J, Heinsen A, Fomsgaard A. Hepatitis C virus subtyping by a core-envelope 1-based reverse transcriptase PCR assay with sequencing and its use in determining subtype distribution among Danish patients. J Clin Microbiol. 2003;41:1091-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 461] [Cited by in F6Publishing: 475] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 108. | Castera L. Invasive and non-invasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best Pract Res Clin Gastroenterol. 2011;25:291-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 109. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 618] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 110. | Vispo E, Barreiro P, Del Valle J, Maida I, de Ledinghen V, Quereda C, Moreno A, Macías J, Castera L, Pineda JA. Overestimation of liver fibrosis staging using transient elastography in patients with chronic hepatitis C and significant liver inflammation. Antivir Ther. 2009;14:187-193. [PubMed] [Cited in This Article: ] |
| 111. | Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 332] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 112. | Resino S, Sánchez-Conde M, Berenguer J. Coinfection by human immunodeficiency virus and hepatitis C virus: noninvasive assessment and staging of fibrosis. Curr Opin Infect Dis. 2012;25:564-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Resino S, Asensio C, Bellón JM, Carmona R, Miralles P, López JC, Cosín J, Álvarez E, Berenguer J. Diagnostic accuracy of the APRI, FIB-4, and the Forns index for predicting liver fibrosis in HIV/HCV-coinfected patients: a validation study. J Infect. 2011;63:402-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 114. | Bräu N, Salvatore M, Ríos-Bedoya CF, Fernández-Carbia A, Paronetto F, Rodríguez-Orengo JF, Rodríguez-Torres M. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 300] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 115. | Loko MA, Bani-Sadr F, Valantin MA, Lascoux-Combe C, Fontaine H, Bonnard P, Gervais A, Bouchaud O, Garipuy D, Quertainmont Y. Antiretroviral therapy and sustained virological response to HCV therapy are associated with slower liver fibrosis progression in HIV-HCV-coinfected patients: study from the ANRS CO 13 HEPAVIH cohort. Antivir Ther. 2012;17:1335-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Rockstroh JK. How important is HIV therapy for preventing liver fibrosis progression in HIV-HCV-coinfected individuals? Antivir Ther. 2012;17:1223-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 117. | Thorpe J, Saeed S, Moodie EE, Klein MB. Antiretroviral treatment interruption leads to progression of liver fibrosis in HIV-hepatitis C virus co-infection. AIDS. 2011;25:967-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 118. | Woreta TA, Sutcliffe CG, Mehta SH, Brown TT, Higgins Y, Thomas DL, Torbenson MS, Moore RD, Sulkowski MS. Incidence and risk factors for steatosis progression in adults coinfected with HIV and hepatitis C virus. Gastroenterology. 2011;140:809-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979-1991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 120. | Rockstroh JK, Bhagani S, Benhamou Y, Bruno R, Mauss S, Peters L, Puoti M, Soriano V, Tural C; EACS Executive Committee. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 121. | Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Günthard HF, Hammer SM, Reiss P. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 616] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 122. | Shah N, Pierce T, Kowdley KV. Review of direct-acting antiviral agents for the treatment of chronic hepatitis C. Expert Opin Investig Drugs. 2013;22:1107-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 123. | Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, Rivero A, Mak C, Thompson S, Howe AY. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13:597-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 124. | Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, Gharakhanian S, McCallister S, Henshaw J, Girard PM. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159:86-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 125. | Macías J, Márquez M, Téllez F, Merino D, Jiménez-Aguilar P, López-Cortés LF, Ortega E, von Wichmann MA, Rivero A, Mancebo M. Risk of liver decompensation among HIV/hepatitis C virus-coinfected individuals with advanced fibrosis: implications for the timing of therapy. Clin Infect Dis. 2013;57:1401-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Ingiliz P, Rockstroh JK. HIV-HCV co-infection facing HCV protease inhibitor licensing: implications for clinicians. Liver Int. 2012;32:1194-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Danta M, Dusheiko GM. Acute HCV in HIV-positive individuals - a review. Curr Pharm Des. 2008;14:1690-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 128. | European AIDS Treatment Network (NEAT) Acute Hepatitis C Infection Consensus Panel. Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS. 2011;25:399-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 129. | Hua L, Andersen JW, Daar ES, Glesby MJ, Hollabaugh K, Tierney C. Hepatitis C virus/HIV coinfection and responses to initial antiretroviral treatment. AIDS. 2013;27:2725-2734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 130. | Antonucci G, Girardi E, Cozzi-Lepri A, Capobianchi MR, De Luca A, Puoti M, Petrelli E, Carnevale G, Rizzardini G, Grossi PA. Role of hepatitis C virus (HCV) viremia and HCV genotype in the immune recovery from highly active antiretroviral therapy in a cohort of antiretroviral-naive HIV-infected individuals. Clin Infect Dis. 2005;40:e101-e109. [PubMed] [Cited in This Article: ] |
| 131. | Hulgan T, Raffanti S, Kheshti A, Blackwell RB, Rebeiro PF, Barkanic G, Ritz B, Sterling TR. CD4 lymphocyte percentage predicts disease progression in HIV-infected patients initiating highly active antiretroviral therapy with CD4 lymphocyte counts & gt; 350 lymphocytes/mm3. J Infect Dis. 2005;192:950-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 132. | Hull MW, Rollet K, Odueyungbo A, Saeed S, Potter M, Cox J, Cooper C, Gill J, Klein MB. Factors associated with discordance between absolute CD4 cell count and CD4 cell percentage in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2012;54:1798-1805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 133. | Claassen CW, Diener-West M, Mehta SH, Thomas DL, Kirk GD. Discordance between CD4+ T-lymphocyte counts and percentages in HIV-infected persons with liver fibrosis. Clin Infect Dis. 2012;54:1806-1813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |