Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11033
Revised: April 14, 2014
Accepted: June 13, 2014
Published online: August 28, 2014
Chronic infection with hepatitis C virus (HCV) is a leading cause of liver-related morbidity and mortality worldwide and predisposes to liver fibrosis and end-stage liver complications. Liver fibrosis is the excessive accumulation of extracellular matrix proteins, including collagen, and is considered as a wound healing response to chronic liver injury. Its staging is critical for the management and prognosis of chronic hepatitis C (CHC) patients, whose number is expected to rise over the next decades, posing a major health care challenge. This review provides a brief update on HCV epidemiology, summarizes basic mechanistic concepts of HCV-dependent liver fibrogenesis, and discusses methods for assessment of liver fibrosis that are routinely used in clinical practice. Liver biopsy was until recently considered as the gold standard to diagnose and stage liver fibrosis. However, its invasiveness and drawbacks led to the development of non-invasive methods, which include serum biomarkers, transient elastography and combination algorithms. Clinical studies with CHC patients demonstrated that non-invasive methods are in most cases accurate for diagnosis and for monitoring liver disease complications. Moreover, they have a high prognostic value and are cost-effective. Non-invasive methods for assessment of liver fibrosis are gradually being incorporated into new guidelines and are becoming standard of care, which significantly reduces the need for liver biopsy.
Core tip: Chronic hepatitis C is a leading cause of liver-related morbidity and mortality and predisposes to liver fibrosis, the excessive accumulation of extracellular matrix proteins. The staging of liver fibrosis is critical for the management and prognosis of patients. This review provides an update on hepatitis C virus (HCV) epidemiology, summarizes basic mechanisms of HCV-dependent liver fibrogenesis, and discusses common methods for assessment of liver fibrosis. While liver biopsy was until recently considered as the gold standard, novel non-invasive methods, including serum biomarkers, transient elastography and combination algorithms, are gradually being incorporated into new guidelines and are becoming standard of care.
- Citation: Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol 2014; 20(32): 11033-11053
- URL: https://www.wjgnet.com/1007-9327/full/v20/i32/11033.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i32.11033
Chronic hepatitis C (CHC) is caused by infection with hepatitis C virus (HCV) and constitutes a major public health concern, affecting around 200 millions people worldwide[1]. It is the leading cause of hepatocellular carcinoma (HCC) and the main indication for liver transplantation in Western countries. Although some data indicated that HCV does not increase all-cause mortality[2], other studies postulated that CHC could reduce life expectancy by 8 to 12 years[3,4]. Thus, HCV was reported to cause more than 86000 deaths in Europe in 2002[5]. The mortality and morbidity attributable to CHC is expected to increase dramatically over the next 50 years, considering that the rate of new HCV infections dropped significantly only after 1989[6]. Markov model analysis suggested that by 2030, 30% of deaths due to HCV-related complications would be preventable by increasing 50% of the patients receiving treatment with interferon/ribavirin therapy[7]. With the development in new anti-HCV agents, including NS3/4A, NS5A and NS5B inhibitors, higher success rates for treatment are anticipated, even for patients with cirrhosis or post transplantation.
The acute infection with HCV frequently does not resolve spontaneously. Approximately 80% of the infected individuals become chronic carriers and may progress to severe liver disease. Based on the natural history of CHC it is estimated that 10%-20% of patients will develop liver cirrhosis and 1%-5% will develop HCC within 20-30 years[8]. Once liver cirrhosis is established, HCC develops at a yearly rate of 5%-7%[9]. Importantly, epidemiological studies have shown that most patients are unaware of their positive HCV antibody status[6]. A report commissioned by the Institute of Medicine of the National Academies highlighted shortcomings in care for viral hepatitis, and estimated that up to 75% of patients with CHC remain undiagnosed[10]. Along these lines, the Centers for Disease Control and Prevention (CDC) estimated that although persons born during 1945-1965 comprise approximately 27% of the United States population, they account for 75% of all HCV infections, 73% of HCV-related mortality, and are at greater risk of HCC and end-stage liver complications.
Given the fact that early diagnosis and treatment can prevent liver cirrhosis and HCC, it is reckoned that one-time testing of persons born during 1945-1965 (baby boomers) will prevent more than 120000 deaths in the United States. Based on these epidemiological data and on recent advances in treatment of CHC, the CDC is now recommending a general screening strategy with a one-time testing without prior ascertainment of HCV risk for baby boomers[6]. A recent study showed that broader screening for HCV would likely be cost-effective[11]. Nevertheless, significant reduction of HCV-related morbidity and mortality would also require improved rates of referral, treatment and follow-up[11]. Thus, once patients with CHC are recognized from a broader screening for HCV infection, they have to be offered appropriate clinical care and therapy. In this view, the assessment of liver fibrosis stage is the key event in clinical management of CHC, affecting both disease prognosis and treatment indication[12].
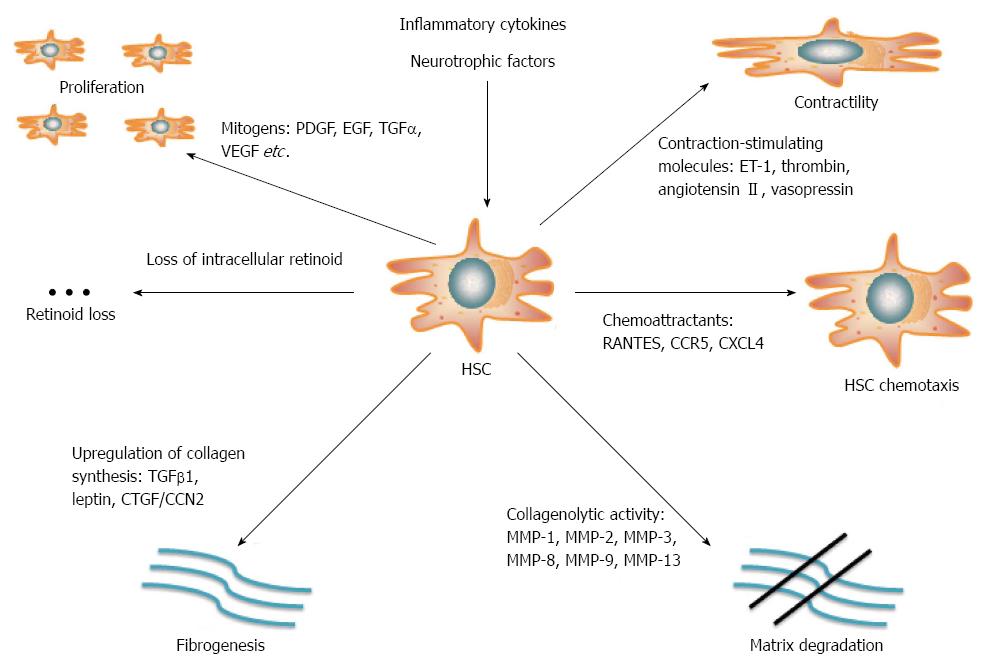
Elucidating the mechanisms underlying liver fibrogenesis is of paramount importance for management and prevention of end-stage liver disease. Liver fibrosis is defined by the excessive accumulation of extracellular matrix (ECM) proteins such as collagen, laminin, elastin, fibronectin, etc., and is currently considered as a wound healing response to chronic liver injury[13]. HCV infection directly modulates signaling and metabolic pathways by viral proteins. Moreover, it indirectly induces host antiviral immune responses leading to chronic inflammation. Together, these events promote liver fibrogenesis[14]. The hepatic stellate cell (HSC), a vitamin A (retinoid)-storing cell residing in the perisinusoidal space of Disse, is the key fibrogenetic element. Although quiescent in the absence of inflammatory stimuli, HSCs are activated in response to liver injury and undergo transformation to proliferative, contractile myofibroblasts. Activated HSCs constitute a prevalent source of ECM production[15] and thereby disrupt the equilibrium between deposition and dissolution of ECM proteins, which leads to fibrotic scarring and eventually to liver cirrhosis (Figure 1).
The development of cell culture and animal models that recapitulate main aspects of HCV infection and liver injury has been crucial for understanding the pathogenesis of CHC[16,17]. This involves pathways that are implicated in the initiation and the perpetuation of HSC activation. Initiation of HSC activation is mediated by paracrine stimuli from neighboring cells, reactive oxygen species (ROS), lipopolysaccharide (LPS), or apoptotic bodies. HSCs maintain their activity in response to fibrogenetic, proliferative, chemotactic and inflammatory signaling[14].
The HCV contains a positive sense single-stranded RNA that is translated to a large polyprotein precursor. The latter undergoes proteolytic cleavage by viral and host enzymes in order to generate mature structural proteins (core, E1, E2 and p7) and non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A and NS5B)[18]. These molecules may target multiple cell types, including hepatocytes, monocytes, lymphocytes and various secretory cells[19-21], and thereby modulate cell proliferation, apoptosis, oxidative stress and innate immunity[22].
Experimental evidence suggests that the HCV core protein, as well as non-structural HCV proteins may directly trigger HSC activation and, thus, the initiation of fibrogenesis. The core protein preferentially activates pro-mitogenic intracellular pathways within HSCs, whereas the NS3 and NS5 proteins specifically stimulate pro-inflammatory pathways via NF-κB and JNK[23]. The core and NS3 proteins promote increases in intracellular calcium [Ca2+]i and ROS levels; the effects of the core protein depend on its binding to the C1q receptor[23]. The induction of osteopontin by calcium and ROS signaling contributes to the epithelial to mesenchymal transition of hepatocytes[24]. The E2 glycoprotein of the HCV envelope is another potential fibrogenetic factor. It promotes the activation of matrix metalloproteinase 2 (MMP-2) upon binding to CD81 of HSCs, which results in degradation of normal ECM in areas with high HCV density, and may lead to infiltration of inflammatory cells[25].
It should also be noted that the core, NS3 and NS5A proteins induce oxidative stress in hepatocytes and monocytes via activation of the NADPH oxidase[26-28] and repression of heme oxygenase 1 (HO-1)[29]. In addition, the core and NS3 proteins activate inflammatory pathways via Toll-like receptor 2 (TLR2) in monocytes, which modulate innate immunity[30]. Furthermore, studies with HCV replicon models demonstrated the induction of oxidative stress and the activation of transforming growth factor β1 (TGFβ1) and other pro-fibrotic signals in response to HCV replication[31,32].
The immune response to HCV infection plays a key role in the enhancement of hepatic fibrogenesis. Multiple growth factors, inflammatory cytokines and chemokines may regulate the activation of HSCs and their transformation to myofibroblasts[33]. In particular, the immune-promoted induction of the platelet-derived growth factor (PDGF) and the subsequent mobilization of intracellular calcium elicit mitogenic effects to HSCs[34,35]. Kupffer cell-derived transforming growth factor α (TGFα)[36] and bile acid-induced activation of the epidermal growth factor (EGF) receptor[37] promote the proliferation of HSCs. Moreover, induction of the vascular endothelial growth factor (VEGF) contributes to activation and proliferation of HSCs, as well as to hepatic angiogenesis, rendering this molecule a key element of the fibrogenic process[38].
Next to the proliferative factors, fibrogenic cytokines that promote ECM production are positively regulated in the context of immune responses to HCV infection. TGFβ1 is the most potent pro-fibrotic cytokine, stimulating collagen production via Smad signaling[39,40]. Moreover, additional molecules such as the connective tissue growth factor (CTGF/CCN2)[41] and the adipokine leptin[42] promote liver fibrogenesis via TGFβ1 signaling. The fibrogenic activity of leptin is partly mediated by TGFβ1 and requires further Kupffer cell-derived stimuli[43]. Leptin also acts as a suppressor of the peroxisome proliferator-activated receptor γ (PPARγ), an anti-fibrotic nuclear receptor able to abrogate HSC activation and conserve its quiescence[44].
Chemokines enhance fibrogenesis through chemotaxis of fibrogenic cells and amplification of the inflammatory response. HSCs produce numerous receptors and secret several cytokines[45]; their role in the pathophysiology of fibrogenesis is currently a subject of investigation. Recent evidence suggests that the induction of C-C chemokine ligand 5 (CCL5, also known as RANTES) by the NF-κB signaling pathway promotes chemotactic and mitogenic effects to HSCs via its C-C chemokine receptor 5 (CCR5)[46]. Furthermore, platelet-derived chemokine (C-X-C motif) ligand 9 (CXCL9) exhibits anti-fibrotic properties that depend on its receptor CXCR3[47], whereas CXCL4 exerts a pro-fibrotic function[48].
Neurochemical and neurotrophic factors may also enhance the fibrogenetic function of the HSCs. Several cellular pathways of the neuroendocrine system are activated in response to chronic liver injury. Induction of opioid signaling by endogenous opioids stimulates proliferation of HSCs and enhances collagen deposition[49]. Along similar lines, the activation of the CB1 receptor by HSC-derived cannabinoids[50], the enhancement PDGF signaling in HSCs by serotonin[51] and the activation of HSCs by thyroid hormones[52] promote fibrogenetic pathways.
The direct interaction of HSCs with immune cells, through expression of adhesion molecules, results in bidirectional cellular stimulation and amplification of fibrosis. Tumor necrosis factor α and monocyte chemoattractant protein 1 (MCP-1), along with other pro-inflammatory cytokines are secreted by Kupffer cells in response to NF-κB activation[53]. This results once again in HSCs activation and in secretion of factors that amplify the inflammatory process and perpetuate the macrophage activity, such as the macrophage colony-stimulating factor[54], interleukin 6[55], MCP-1[56] and RANTES[46]. In addition, HSCs express cell adhesion molecules including vascular cell adhesion molecule 1[57] and intracellular adhesion molecule 1[58]. These are involved in further recruitment of inflammatory cells in the site of injury, which enhances the fibrogenetic process. Other cell types implicated in fibrosis progression include lymphocytes[59], macrophages[60] and endothelial cells[61]. Macrophages promote the survival of activated HSCs via NF-κB-dependent pathways[62]. By contrast, natural killer cells and T cells from HCV-infected patients promote apoptosis of HSCs and thereby exert anti-fibrotic function[63].
Last but not least, oxidative stress is a key component of hepatic fibrosis[64]. Apoptotic parenchymal cells are being phagocytosed by activated HSCs resulting in activation of the NADPH oxidase[65]. The latter mediates the generation of ROS, which are capable of both initiating and perpetuating fibrosis via activation of HSCs, hepatocytes, Kupffer cells and inflammatory cells[66]. This process is further enhanced in the presence of polyunsaturated fatty acids, ethanol and iron. Furthermore, the DNA of apoptotic hepatocytes may interact with HSCs’ TLR9 and thus enhance the collagen production and deposition[67].
Mild to moderate hepatic iron overload is a common manifestation of CHC patients. This is largely attributed to misregulation of the iron regulatory hormone hepcidin[68,69], which is transcriptionally inhibited by HCV-induced oxidative stress[70]. Even though iron antagonizes HCV replication by inactivating the viral polymerase NS5B[71,72], hepatic iron accumulation[73], elevated serum ferritin[74] or reduced serum hepcidin levels[75] are associated with progression of liver disease. The hemochromatosis protein HFE, an atypical major histocompatibility complex class I molecule, may also contribute to liver fibrogenesis as an upstream regulator of hepcidin and/or as possible immunological factor[76,77].
The accumulation of liver fibrosis is a significant incident with major consequences on the pathology development of CHC[78]. It indicates the onset of progressive disease, which may eventually lead to cirrhosis and end-stage liver complications[79]. Patients with absent or mild fibrosis at diagnosis have a relatively low risk (25%-30%) of developing cirrhosis over the next 20 years. Portal and septal fibrosis both cause cirrhosis, albeit with different progression rates (18-20 years for patients with portal fibrosis and 8-10 years for patients with septal fibrosis, respectively)[80]. Thus, the stage of liver fibrosis is critical for clinical management, especially in light of the new screening wave of HCV-infected patients[6].
The clinical management of CHC patients depends on two different stages of liver fibrosis[81]: (1) considerable fibrosis, histologically classified as septal fibrosis (stage F3 by METAVIR), represents a definitive indication to schedule, not defer, antiviral treatment; and (2) cirrhosis (stage F4 by METAVIR) necessitates specific and regular follow-up which should include screening for HCC and esophageal varices. Apart for indication to antiviral treatment, a more advanced liver fibrosis stage should require interventions to control known negative cofactors for disease progression (Table 1). These include life style modifications (diet, weight loss, regular physical exercise), alcohol and drug abstinence, referral to specialists (hepatologist, metabolic clinics, dietician, psychologist), specific medications (statins, insulin-sensitizing agents). Thus, the new screening strategies, which are opening to a large group of persons, the baby boomers, should be associated with diagnostic and therapeutic interventions to all newly identified patients.
| Non-modifiable | Modifiable |
| Duration of HCV infection | High alcohol consumption (≥ 20-50 g/d) |
| Older age at infection | Insulin resistance |
| Male sex | Obesity |
| Presence of baseline fibrosis | Metabolic syndrome |
| HIV or HBV co-infection | Daily cannabis use |
| Infection with HCV genotype 3 | |
| Gene polymorphisms involved in iron overload/inflammatory pathways | |
| Latin ethnicity |
For many years the assessment for liver fibrosis has been through liver biopsy, which has been considered the gold standard gauge for the direct histological evaluation of the severity of liver disease.
The representativeness of liver samples obtained through a liver biopsy and the pathologist’s experience remain the major determinants of diagnostic accuracy. Inadequate liver biopsy sample can lead to underestimation of liver fibrosis stage[82]. Samples taken from both lobes of the liver in a cohort of CHC patients highlighted in 33.1% of them a difference in the fibrosis stage by at least one grade, and in 14.5% of them underdiagnosis of fibrosis[83]. On single blind percutaneous liver biopsies, cirrhosis was missed in 10%-30% of samples[84-86]. Since liver biopsy involves only a very small part of the whole organ (approximately 1/50000), the diagnosis of fibrosis can be missed, especially in cases where the lesions are not uniformly distributed through the parenchyma.
Misclassification of the stage of liver fibrosis can be reduced by obtaining a specimen of adequate size and quality. It has been suggested by some authors that an adequate sample of the liver should be at least 15 mm in length and ought to contain more than 5 portals[87-89]. By critically evaluating published literature, Guido et al[90] concluded that unacceptable methodological limits often flaw liver biopsy results; moreover they proposed sample sizes of 20 mm or more containing at very least 11 complete portal tracts for reliable staging. Analyzing even larger size samples going up to 25 mm in length has been suggested by other authors[91,92]. According to the American Association for the Study of Liver Diseases (AASLD), a liver biopsy sample should contain at least 11 complete portal tracts and be no less than 20 mm in length, while liver fibrosis should be scored by a simple (METAVIR) rather than complex (Ishak) system[93].
There is also a significant degree of inter-/intra-observer variability in the pathologic assessment of liver biopsy samples. The practical knowledge and experience of pathologists demonstrated by a longer medical career, or affiliation within an academic realm, could have a greater influence on the interpretation of the diagnosis, more than the sample size[94]. A pathologist with specific expertise in liver disease should interpret the biopsy, preferably in coordination with the clinician who performed the procedure and is caring for the patient. In the absence of this interaction, diagnostic errors by non-specialist pathologists have been reported in more than 25% of patients[95,96]. If liberal use of second opinions from specialist liver pathologists has been recommended, this may result in increased costs and waiting time.
Recent studies have implied that liver biopsy should not be considered as the gold standard, but rather as the best point of reference for staging liver disease[97,98]. Surrogates in general are evaluated by utilizing the area under the curve (AUC), with liver biopsy as the reference. Mehta and coworkers argued that the ideal surrogate will at no time attain the maximal value (1)[97]. By taking into consideration a spectrum of accuracies of the biopsy plus a spectrum of prevalence of substantial fibrosis, they demonstrated that even under optimal conditions and with a perfect marker, it is not possible to achieve an AUC ≥ 0.90 when assessing substantial fibrosis[97,98].
There are definitely advantages in performing liver biopsy since it gives important and direct information relating to fibrosis, necroinflammatory activity, steatosis stage and also hepatic iron deposits, which are recurring histological appearances of CHC and potential comorbidities. However, there are also possible drawbacks for the clinician, such as the invasiveness of the procedure and the cost (Table 2). The most frequent complication (84%) for patients undergoing liver biopsy is pain. Bleeding occurs in 0.01%-0.04% of cases, whereas death is very rarely associated with the procedure (≤ 0.01%). Clinical studies have provided evidence that the rate of complications in percutaneous liver biopsy inversely correlates with the experience of the operator[99,100], but opposite data have also been reported[101].
| Liver biopsy | Serum biomarkers | Transient elastography | |
| Advantages | Direct assessment of liver fibrosis | Immediate result | Immediate result |
| Stage by stage fibrosis classification | Fast (one time blood sample) | Duration of examination 5 min | |
| Evaluation of coexisting disorders (inflammation, steatosis, iron overload) | Patient friendly | Operator and patient friendly | |
| Limitations | Complications (pain, bleeding) | Cost (unitary cost per patient for patented tests) | Cost (one time per machine) |
| Sampling error, intra-observer and inter-observer variability | High rates of unclassified patients (APRI, Fib-4, Forns’ index, Lok index) | Failure in 5% of cases (25% in obese patients) | |
| Hospitalization (day hospital) often required | Unreliable results in 15% of cases (obesity, ascites, limited operator experience) | ||
| Cost | Lower performance for diagnosis of significant fibrosis | Lower performance for diagnosis of significant fibrosis | |
| Delayed result (2-4 wk) | Unable to discriminate between intermediate stages of fibrosis | Unable to discriminate between intermediate stages of fibrosis | |
| Contraindications | Absolute: uncooperative patient, severe coagulopathy, extrahepatic biliary obstruction | None | Pacemaker, pregnancy |
| Relative: ascites, morbid obesity, possible vascular lesions, amyloidosis | |||
| Risk factors for error | Biopsy sample < 2 cm in length, containing < 10 complete portal tracts; inexperienced pathologist | Autoimmune thrombocytopenia (APRI); Gilbert’s sydrome, extrahepatic cholestasis, hemolytic anemia (Fibrotest) | Transaminases flares; acute viral hepatitis; non-fasting patient; vascular hepatic congestion; extrahepatic cholestasis; IQR ≥ 30% |
There is some ongoing debate amongst physicians about liver biopsy and its role in the assessment of fibrosis. A survey with 1177 general practitioners in France showed that up to 59% of patients with CHC refused the procedure due to its invasive nature, and some 22% of the physicians had similar considerations[102]. Liver biopsy was not performed by 29% from 112 American physicians due to following concerns: safety (72.7%), low reimbursement (66.7%), logistical issues (45.4%)[103].
A recent Canadian nationwide survey on patterns of diagnosing liver fibrosis showed that for almost half of the physicians, liver biopsy was the main diagnostic approach. Limitations in access/availability of non-invasive tools and lack of reimbursement represented a significant barrier[104]. A similar survey was earlier performed in France, the country where non-invasive diagnostic methods of liver fibrosis were first marketed, and appropriate reimbursement policies are being implemented since 2007. Interestingly, only 4% of physicians that responded, routinely requested liver biopsy[105]. A survey among Italian hepatologists uncovered discrepancies between them on how and when to perform liver biopsy in CHC patients[106].
Cost is a major issue for implementation of liver biopsy in clinical practice, especially in light of the recent broader screening strategies for hepatitis C. In the United States the cost is currently $1032 and can increase up to $2745 if complications occur during and after the procedure[107]. In Canada, the mean cost of a complicated liver biopsy requiring hospitalization is $4579[108].
Given the drawbacks of liver biopsy, non-invasive tools for assessment of liver fibrosis have attracted the attention of hepatologists. Table 3 compares guidelines in terms of recommendations for liver biopsy and/or non-invasive tools for the staging of liver fibrosis in HCV-infected patients. Overall, in spite of a previous consensus that a stage of liver fibrosis of at least F2 represents a definitive indication for antiviral therapy, recent guidelines recommend that there should be no threshold precluding patients from antiviral treatment. The Asian Pacific Association for the Study of the Liver (APASL), recommends treatment for patients with a histological score of F1 or above[109]. HCV patients with viral genotypes 1-3 can be treated regardless of the stage of the disease. It is not compulsory for patients infected with HCV genotypes 2 or 3 to have a liver biopsy in order to start therapy. However, obtaining a liver biopsy before starting therapy could offer prognostic information. At the time the APASL guidelines were issued, non-invasive methods were not recommended.
| Ref. | Threshold for definitive indication to antiviral therapy | Recommended methods for liver fibrosis staging | Can non-invasive methods replace liver biopsy? |
| APASL[109], 2007 | F1 | Liver biopsy | No |
| AASLD[190], 2009 | F2 | Liver biopsy, serum biomarkers, transient elastography | No |
| EASL[81], 2014 | F2 | Liver biopsy, serum biomarkers, transient elastography | Yes |
| CASL[111], 2012 | None | Liver biopsy, serum biomarkers, transient elastography | Yes |
AASLD guidelines state that in CHC, liver biopsy should be considered if the patient and the health care provider wish to know the fibrosis stage to enable an informed decision on treatment options and/or to predict possible outcomes. A liver biopsy may be unnecessary in persons infected with HCV genotypes 2 and 3, since more than 80% of them achieve a sustained virological response (SVR). There is, nevertheless, an ongoing argument on whether CHC patients with HCV genotype 1 warrant a biopsy because of their lower SVR rates. Likewise, the need for liver biopsy in CHC patients with less common HCV genotypes (4-6) is unclear. At present there are accessible non-invasive tools, which might be useful in determining the absence or presence of advanced fibrosis; however they should not take the place of liver biopsy in routine clinical care practices.
More up-to-date guidelines on management of specific chronic liver diseases, give a different perspective. Thus, according to the European Association for the Study of the Liver (EASL), although liver biopsy is still the gold standard of reference in CHC, non-invasive methods may also be used instead[110]. Similarly, the guidelines of the Canadian Association for the Study of the Liver (CASL) state that acceptable methods to stage liver fibrosis include liver biopsy, Fibroscan® and serum biomarkers[111]. Moreover, the CASL guidelines state clearly that if F2 is a threshold for definitive candidacy to antiviral therapy, no threshold of fibrosis should preclude a patient with CHC from treatment. Overall, the diagnostic value of liver biopsy and non-invasive methods for assessment of liver fibrosis has progressively evolved across the guidelines. In the most recent ones, a clear cut-off for indication to antiviral therapy is no longer recommended. Moreover, we witnessed an evolution in the strength of recommendation of liver biopsy vs non-invasive fibrosis assessment tools, with the recent guidelines being indifferent.
The CDC guidelines recommend a onetime screening test for HCV infection in baby boomers, meaning that a new wave of identified chronic carriers will soon present in the panorama of HCV epidemiology. Once these new patients are identified, appropriate management should be offered. Liver fibrosis staging is the single most important factor impacting on the natural history of CHC. It is critical for prognosis and expedited initiation of treatment. However, it is impractical and immensely expensive to stage fibrosis through liver biopsy in all affected persons. Nowadays, this procedure should be thought of as a diagnostic funnel for large-scale screening of liver fibrosis in HCV infection. Consequently, non-invasive tools are absolutely necessary in order to restrict biopsies. In general, non-invasive methods can be divided into two main classes: the serum biomarkers, based on a biological approach; and methods based on a physical approach, including transient elastography, acoustic radiation force impulse imaging, magnetic resonance elastography. Any non-invasive method should ideally fulfill certain characteristics: it should be simple, accessible, easy interpretable, highly accurate, liver-specific, and satisfactorily validated.
The concept of validation is critical and encompasses a number of features that the ideal serum biomarker should fulfill. First, a non-invasive method should demonstrate a good diagnostic accuracy. Specifically, an expensive and patented tool should demonstrate a clear advantage in terms of diagnostic accuracy when compared to simple and economic ones. Second, there should be a sufficient number of validation studies from independent researchers. Third, specific etiology-validation of the non-invasive methods should be provided considering that each etiology of chronic liver disease presents with specific pathogenesis, natural history and associated comorbidities. For example, when considering CHC and chronic hepatitis B (CHB), the former has specific associated comorbidities, such as steatosis and diabetes, the latter is characterized by a more vigorous necroinflammation[112]. Thus, a non-invasive tool developed in the setting of CHB should be specifically validated in CHC patients. Fourth, a careful evaluation of the risk factors for error and failure of a non-invasive tool should be carried out for adequate interpretation in clinical practice. Fifth, serum biomarkers should be specifically validated in special HCV-infected populations, such as patients co-infected with human immunodeficiency virus. Finally, when dealing with serum biomarkers, particularly the patented ones, analytic conditions, such as standardization of reagents and analyzers according to manufacturer’s recommendations, should be taken into account. An overview of the non-invasive diagnostic tools for liver fibrosis and their main validation features is shown in Table 4.
| Ref. | Parameters | Independent validation studies | Etiology-validation studies | Characterization of risk factors for error | Validation in special HCV populations |
| AAR[138] | AST, ALT | + | + | + | + |
| APRI[142] | AST, platelets | + | + | + | + |
| ELF[131] | Age, TIMP-1, hyaluronan, procollagen type III | +/- | + | + | - |
| Fib-4[145] | Age, ALT, AST, platelets | + | + | + | + |
| Fibrometer®[122] | Platelets, prothrombin index, AST, α2-macroglobulin, hyaluronan, urea, age | +/- | + | + | + |
| Fibroscan®[167] | Liver stiffness measurement | + | + | + | + |
| Fibrospect®[132] | Hyaluronan, TIMP-1, α2-macroglobulin | +/- | - | - | - |
| Fibrotest-Fibrosure®[132] | γGT, total bilirubin, haptoglobin, α2-macroglobulin, apolipo-protein A1, age, gender | + | + | + | + |
| Forns’ index[144] | Age, γGT, cholesterol, platelets | + | + | + | + |
| Hepascore[129] | Age, gender, bilirubin, γGT, hyaluronan, α2-macroglobulin | +/- | + | - | + |
| Hyaluronan | Hyaluronic acid | + | + | + | + |
| Lok index[191] | AST, ALT, platelets | - | - | + | - |
There are direct and indirect serum biomarkers for assessment of liver fibrosis. The former are fragments of compounds of the liver matrix; for instance, hyaluronan, collagen synthesis or degradation products, and regulators of fibrogenetic mechanisms. The latter are biochemical parameters that can be calculated from routine peripheral blood tests. Calculations use liver-derived molecules, such as clotting factors, bilirubin, cholesterol, albumin and transaminases. Direct biomarkers mirror the metabolism of liver ECM and can be potentially utilized to assess the dynamics of liver fibrogenesis. However, they may not be routinely provided in every hospital setting, limiting their clinical use. Indirect biomarkers correlate with liver fibrosis stage. Tables 4 and 5 provide an overview of the performance of the most proven biomarkers in CHC.
| Index | ≥F2/F4 | ||||||
| AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR- | |
| Hyaluronan[113-115,119,128] | 0.73-0.86/ 0.89-0.92 | 64.5-75/ 79.2-100 | 81.0-91.2/ 80.0-89.4 | 44.0-86.3/ 63.0-100 | 78.5-93/ 99.0-100 | 3.94-7.32/ 5.00-7.47 | 0.30-0.38/ 0.00-0.23 |
| Fibrometer[122,124] | 0.85-0.89/ 0.91 | 80.5-89/ 94.1 | 84.1-89.9/ 87.6 | 82.0-86.3/ 68 | 77.6-82.5/ 94.7 | 5.56-7.97/ 7.46 | 0.13-0.21/ 0.06 |
| FibroSpect[122,126-128] | 0.82-0.87/ NA | 71.8-93.0/ NA | 66.0-73.9/ NA | 60.9-82.6/ NA | 77.7-94/ NA | 2.73-2.75/ NA | 0.10-0.24/ NA |
| Hepascore[124,129,130] | 0.79-0.85/ 0.85-0.94 | 53.08-82/ 71.0-76.5 | 65.0-92.0/ 84.0-89.8 | 70-88/ 64.9 | 63.5-78/ 89.6-98 | 2.34-6.62/ 4.78-6.96 | 0.27-0.51/ 0.27-0.32 |
| ELF score[122,131] | 0.80/ NA | 90/ NA | 31/ NA | 27.5/ NA | 92/ NA | 1.30/ NA | 0.32/ NA |
| AAR[137,192] | NA/ 0.51-0.83 | NA/ 46.7-78.0 | NA/ 95.9-100 | NA/ 73.7-100 | NA/ 80.7-89 | NA/ 19.02 | NA/ 0.22-0.43 |
| APRI[122,124,133,137,142,192-194] | 0.69-0.88/ 0.61-0.94 | 41-91/ 57-89 | 47-95/ 75-93 | 61-88/ 38-57 | 64-86/ 93-98 | 1.71-8.20/ 3.56-8.14 | 0.19-0.62/ 0.10-0.46 |
| Lok Index[137,191] | NA/ 0.78-0.81 | NA/ 37-92 | NA/ 30-94 | NA/ 32-75 | NA/ 84-91 | NA/ 1.31-6.16 | NA/ 0.26-0.67 |
| Forns’ Index[122,124,133,144,192,193] | 0.60-0.86/ NA | 79.8-94/ NA | 61.2-95.0/ NA | 66-94.7/ NA | 63.8-96/ NA | 2.42-15.96/ NA | 0.09-0.21/ NA |
| Fib-4[145] | 0.82-0.89/ 0.79-0.91 | 37.6-74.3/ NA | 80.1-98.2/ NA | 82.1/ NA | 94.7/ NA | 3.73-20.77/ NA | 0.32-0.63/ NA |
| Fibrotest[122,124,132,133,135] | 0.74-0.87/ 0.71-0.87 | 65-77/ 50-87 | 72-91/ 70-92.9 | 76-80/ 57.9-93 | 66.7-81/ 44-90.5 | 2.75-7.22/ 2.9-7.04 | 0.31-0.38/ 0.17-0.53 |
The most common direct markers investigated for liver fibrosis in CHC include laminin, hyaluronan, procollagen III, collagen type IV, YKL-40, MMPs and their inhibitors (Tables 4 and 5). Hyaluronan is a glycosaminoglycan synthesized by HSCs and degraded in the liver sinusoidal cells[113]. In a study of 326 CHC patients, the AUC for significant fibrosis and cirrhosis were 0.86 and 0.92, respectively, and the cut off level was 110 μg/L[113]. Nevertheless, a different cohort study involving over 400 patients reported an AUC of only 0.73 for significant fibrosis[114]; cirrhosis was excluded with 100% negative predictive value (NPV), a cut-off of 50 μg/L and an AUC of 0.97. In yet another study with 486 patients, hyaluronan values of < 60 μg/L were used to exclude cirrhosis with a NPV of 99%[115]. Type IV collagen showed an AUC of 0.83 for the diagnosis of significant fibrosis[116]. Comparison of the diagnostic performance of hyaluronan and type IV collagen revealed superiority of the former as a marker in CHC[117].
Laminin is a non-collagenous glycoprotein synthesized by HSCs and deposited in the liver basement membrane. The diagnostic value of laminin is not as high as those of hyaluronan and type IV collagen[118]. Thus, a study involving 243 chronic liver disease patients reported a 77% accuracy for laminin for detecting significant liver fibrosis among a CHC subgroup[119]. MMP-2 and tissue inhibitors of MMP-1 and -2 (TIMP-1 and -2) have also demonstrated some diagnostic potential to detect liver fibrosis in CHC[120].
YKL-40 is a glycoprotein that is member of the chitinase family. It is strongly expressed in human cartilage and liver, and it is involved in the fibrogenetic process. In 109 CHC patients, it showed a discrete performance for significant liver fibrosis AUC 0.81, specificity of 81% and sensitivity of 78%. However, its accuracy for the prediction of liver cirrhosis was lower, with the AUC at 0.795 A possible diagnostic value of procollagen III assessment has also been evaluated; however, it was found to be inferior compared to type IV collagen and hyaluronan[113,121].
Direct markers have also been proposed as combination panels for increasing the diagnostic performance of the single parameter. Fibrometer® is a patented test combining age, platelets, hyaluronan, AST, prothrombin index, urea and α2-macroglobulin. In CHC patients, AUC values were reported to be between 0.85-0.89 for significant liver fibrosis and 0.91 for liver cirrhosis[122-124]. Fibrospect® is a combination of hyaluronan, TIMP-1 and α2-macroglobulin that showed an AUC of 0.82-0.87 for significant fibrosis[125-127]. A comparative study investigated the diagnostic performance of Fibrospect®, hyaluronan and YK-40 for significant fibrosis in CHC[128]. Interestingly, the recorded Fibrospect® AUC was 0.66, while that of hyaluronan was 0.76. Hepascore® is another patented test, combining age, gender, hyaluronan, bilirubin, γGT, and α2-marcoglobulin. In CHC patients, AUC values of Hepascore® were 0.79-0.85 for diagnosis of significant fibrosis, and 0.89-0.94 for diagnosis of cirrhosis, which indicates an excellent performance[124,129,130]. The panel of direct non-invasive markers proposed by the European liver fibrosis study group includes, hyaluronan, TIMP-1, type III collagen and age. In a cohort study involving more than one thousand patients with chronic liver disease, the panel detected significant liver fibrosis with an AUC of 0.77 in the CHC subgroup[131].
Among the patented panels combining parameters for diagnosis of liver fibrosis, Fibrotest-Fibrosure® is the most validated. The parameters included in its formula are γGT, total bilirubin, haptoglobin, α2-macroglobulin, apolipoprotein A1, age and gender[132]. Risk factors for error of this test include elevation of bilirubin levels unrelated to fibrosis (for example due to cholestatic or Gilbert syndromes), reduction of haptoglobin related to hemolysis, elevation of haptoglobin and α2-macroglobulin due to non-hepatic inflammation. The number of patients that have been included in independent studies is more than 5000. The AUC values range between 0.74-0.87 for significant fibrosis and 0.71-0.87 for cirrhosis[89,132-134]. A systematic review including 9 studies for a total number of 1679 CHC patients concluded that Fibrotest-Fibrosure® is excellent for its diagnostic accuracy in cirrhosis but not in early stages of fibrosis[135].
Non-invasive indirect biomarkers for liver fibrosis comprise serum parameters and their combination panels, such as platelets, transaminases, and albumin. Platelet count showed a discrete performance in ruling-out cirrhosis with a cut-off value of 150 × 109/L, with 84% to 95% NPV[119,136,137]. The prothrombin index, based on prothrombin time, showed a NPV ranging from 82% to 91% to rule-out cirrhosis[119,137]. However, these simple and inexpensive markers do not provide a classification of significant liver fibrosis.
One of the most adopted indirect biomarkers is the AST to ALT ratio (AAR), which is widely used for the staging of liver fibrosis in CHC patients. The normal value is < 0.8. An increase of AAR reflects a progressive liver functional impairment, while a ratio ≥ 1 is indicative of cirrhosis[138]. AAR distinguished cirrhotic patients from non-cirrhotic with 60%-83.6% accuracy, 31.5%-81.3% and 53%-100% specificity[138-141]. Its performance has been variable in difference studies, and the AUC ranged between 0.51-0.83. This test is easy to perform in the daily clinical setting and it comes with no cost; however, a major limitation is that it cannot diagnose significant fibrosis, while values may be affected in case of alcohol consumption[119].
The AST to platelet ratio index (APRI) is another simple score proposed for the classification of both significant fibrosis and cirrhosis. The APRI is calculated by using AST and platelet count, which makes it easily accessible to the clinician at virtually no cost[142]. It is a useful tool to manifest or exclude significant liver fibrosis (cut-off 0.5-1.5) and liver cirrhosis (cut-off 1-2). However, in a substantial number of patients (30%-50%) APRI values are within an intermediate area and thus classification is unreliable. Nonetheless, to date APRI remains one of the most validated non-invasive biomarkers for liver fibrosis, and among the most referenced by guidelines[89]. In the initial study, APRI demonstrated a high precision for the prediction of significant fibrosis (AUC 0.88) and cirrhosis (AUC 0.94)[142]. Subsequent studies nevertheless indicated an irregular performance with AUC for significant fibrosis ranging between 0.69-0.88 and for cirrhosis between 0.61-0.94[89,133]. This variability could be partially explained by different cut-off values chosen in each study and by population heterogeneity. A recent meta-analysis of 40 studies, which included 8739 patients with CHC, concluded that APRI can be used in clinical practice for the confirmation of severe fibrosis/cirrhosis when other clinical signs and examination are non-decisive[143]. Moreover, since it is cheap and simple, it should be considered a reference test against which other non-invasive methods should illustrate improved precision and cost-effectiveness. Moreover, APRI is still the first choice for CHC patients to identify fibrosis in regions with limited healthcare resources.
The Lok index is a modification of APRI that combines platelet count, INR and AAR102. Cut-off values of 0.2 or 0.5 are used to rule-out or rule-in cirrhosis, respectively. Nevertheless, the Lok index is unreliable in detecting significant fibrosis. To this end, Forns et al[144] developed a simple panel based on clinical variables routinely recorded: age, γGT, platelet count and cholesterol levels. The Forns’ index utilizes cut-off values of 4.2 or 6.9 to rule-out or rule-in significant fibrosis, respectively, while intermediate values cannot be classified. A study involving 476 CHC patients revealed a high diagnostic performance of the Forns’ index for the detection of significant fibrosis, with an AUC of 0.81-0.86[144]. Remarkably, the low cut-off value of 4.2 had a NPV of 96% in excluding significant liver fibrosis. Conversely, the high cut-off value of 6.9 had a positive predictive value of only 66% in manifesting significant fibrosis. Further studies uncovered a slightly decreased performance of Forns’ index, with AUC 0.76-0.79[124,133]. The major limitation of the Forns’ index is that it does not offer conclusive information regarding cirrhosis, while it leaves a high number of cases unclassified.
Fib-4 is another index combining simple biomarkers and is based on age, platelet count, AST, and ALT[145]. Fib-4 uses cut-off values of 1.45 or 3.25 to rule-out or rule-in significant fibrosis, respectively. In a study involving 529 CHC patients, Fib-4 enabled the correct identification of cases with severe fibrosis and cirrhosis, with AUC 0.85[145]. Similar conclusions were reached by other studies[146]. Nonetheless, overall Fib-4 does not offer sufficient clues about cirrhosis and consistently leaves several cases unclassified. On the other hand, it is simple and cheap, and has been validated in a number of studies.
The measurement of liver stiffness by transient elastography offers an accredited non-invasive method for the assessment of liver fibrosis[147]. It is performed by using Fibroscan® (Echosens, Paris), a device composed of an ultrasound transducer probe that is mounted on the axis of a vibrator. The transducer transmits vibrations of mild amplitude and low frequency. This generates an elastic shear wave, which disseminates through the underlying tissue. Dissemination of the shear wave is monitored by pulse-echo ultrasound acquisition. Its velocity directly correlates to tissue: the faster the shear wave disseminates the stiffer the tissue. Liver stiffness is measured by Fibroscan® in a volume that is approximately a cylinder 1 cm wide and 4 cm long, between 2.5 and 6.5 cm below the skin. This volume is substantially bigger (at least 100 times) than a typical biopsy sample.
The Fibroscan® examination is painless, fast (performed in less than 5 min), and easy to use. It is performed on a patient who is lying flat on his/her back, with the right arm tucked behind the head. The probe transducer is placed on the patient’s skin, in-between the rib bones at the same level as the right lobe of the liver that would be used to obtain a biopsy sample. The operator needs to acquire 10 valid measurements and then the Fibroscan® software calculates the median value. Success of each measurement is determined by the software itself. Liver stiffness ranges between 2.5-75 kPa. Fibroscan® cut-off values between 5.2-8.9 kPa are consistent with significant fibrosis, while values between 10.1-17.6 kPa indicate cirrhosis[79,148]. Main features on Fibroscan® studies in CHC patients are summarized in Table 6.
| Ref. | Cut-off for≥F2 (kPa) | Cut-off for F4 (kPa) | AUC for≥F2 | AUC for F4 | Number of patients included |
| Sandrin et al[147], 2003 | 7.6 | 14.4 | 0.88 | 0.99 | 106 |
| Castéra et al[167], 2005 | 7.1 | 12.5 | 0.83 | 0.95 | 183 |
| Ziol et al[195], 2005 | 8.7 | 14.5 | 0.79 | 0.97 | 327 |
| Kettaneh et al[196], 2007 | 6.8 | 17.6 | 0.79 | 0.91 | 935 |
| Arena et al[197], 2008 | 7.8 | 14.8 | 0.91 | 0.98 | 150 |
| Cross et al[198], 2010 | 8.9 | 10.1 | 0.89 | 0.97 | 187 |
| Degos et al[199], 2010 | 5.2 | 12.9 | 0.75 | 0.90 | 913 |
Overall, the accuracy of transient elastography is comparable to that of patented serum biomarkers that are used for assessment of significant liver fibrosis, with AUC < 0.80. However, transient elastography shows excellent performance for the diagnosis of cirrhosis since the AUC was ≥ 0.90 in all reported studies[79]. Meta-analysis data indicate that the Fibroscan® examination alone does not provide sufficient information to diagnose significant liver fibrosis. Instead, Fibroscan® may be used together with an algorithm combining non-invasive serum biomarkers[149]. On the other hand, the meta-analysis validated the excellent accuracy of transient elastography in the diagnosis of liver cirrhosis when other examinations and clinical signs are inconclusive. It should be noted that the French Haute Autorité de Santé recommends the utilization of either Fibroscan®, Fibrotest® or Fibrometer® for first line assessment of liver fibrosis in CHC patients.
Even though the Fibroscan® examination per se is straight forward, the interpretation of the result must be done by an expert clinician, knowledgeable on the clinical background of the individual patient and on the conditions that can influence liver stiffness measurement. Factors that influence the applicability of Fibroscan® in clinical practice can be divided into three categories: (1) risk factors of failure; (2) risk factors of low quality; and (3) risk factors of false positivity.
Risk factors of failure of liver stiffness measurement include obesity, narrow intercostal space and ascites[79]. Failure rates range between 2.4%-9.4%[150,151]. Obesity is a major factor for failure, given its frequency in the general population. A study of 2114 examinations showed that a body mass index (BMI) ≥ 28 kg/m2 was the only factor independently associated with failure[152]. On the same line, Wong and colleagues found a failure rate of 2.6% if BMI was < 30 kg/m2 and 25.5% if BMI was ≥ 30 kg/m2[153]. To overcome the high failure rates occurring in obese patients with the Fibroscan® standard probe (M), a new FibroScan® probe (the “XL” probe) has been developed. This utilizes a hypersensitive ultrasonic transducer with a lower frequency, larger vibration amplitude, deeper focal length and higher depth of measurement. Reliable results with the XL probe were obtained in 61% of obese patients in whom the M probe failed[154].
According to the manufacturer, the risk factors of poor quality of a Fibroscan® examination include an interquartile range (IQR) exceeding 30% of the median value, which reflects the variability of the validated measures, and a success rate less than 60%, that is the percentage of valid measurement. Interestingly, a study investigating 254 CHC patients showed that while IQR is indeed a factor of overestimation of liver fibrosis, success rate is not a factor significantly influencing the accuracy of Fibroscan®[151].
A number of conditions can lead to false positivity of Fibroscan® examination. Acute viral hepatitis increases liver stiffness[155,156]. Thus, the necroinflammatory status needs to be taken into consideration, particularly in patients with absent or low-stage liver fibrosis. In relevant studies[155,156], ALT levels correlated with Fibroscan® values. Conversely, another study showed that low AST is a variable associated with discordance between Fibroscan® measurement and liver biopsy for diagnosis of significant fibrosis[157]. The authors concluded that Fibroscan® is influenced by major variations in biochemical activity of liver disease in CHC and that liver stiffness, at low levels of AST, can underestimate fibrosis. For this reason, adjustments for age and AST of the Fibroscan® result may significantly improve accuracy.
In patients with extra-hepatic cholestasis, liver stiffness significantly correlates with bilirubin levels and leads to false positivity of Fibroscan® measurement[158]. Fibroscan® value was significantly reduced following successful bilirubin drainage. Likewise, vascular hepatic congestion can erroneously increase Fibroscan® values. This effect is entirely reversible upon correction of cardiovascular dysfunction[150]. Fasting is also important to avoid overestimation of Fibroscan® measurement. A study by Arena et al[159] showed the confounding effect of a meal on the accuracy of liver stiffness in CHC patients. The authors proposed a fasting period of 120 min before performing the examination. On the same line, Berzigotti et al[160] demonstrated that post-prandial hyperemia is accompanied by a marked increase in liver stiffness in patients with liver cirrhosis.
Transient elastography by using Fibroscan® is a highly reproducible technique[161]. Inter- and intra-observer fluctuations are affected by high grade hepatic steatosis, mild fibrosis (F1-F2 by METAVIR) and a BMI ≥ 25 kg/m2[161]. Nevertheless, the applicability of Fibroscan® may not be as good as that of biomarkers. Overall, in a study of 13369 examinations, liver stiffness data were not interpretable in nearly 20% of cases, mainly due to failure to obtain reliable measurements according to the manufacturer’s recommendations. The technical limitations were attributed to obesity of patients, and in particular to increased waist circumference, and to limited experience of the operator[162].
In order to increase the diagnostic performance of the single method, especially for the diagnosis of significant fibrosis, non-invasive methods have been combined in diagnostic algorithms. The rationale is to combine non-invasive methods, such as Fibroscan® and serum biomarkers, or different, unrelated serum biomarkers. Such a strategy led to a significant reduction in the number of liver biopsies and to an increase in diagnostic accuracy, and it has been recommended by guidelines, such as those from the EASL and CASL. In a recent review Pinzani et al[163] suggested to apply two unrelated non-invasive methods in CHC patients, and to obtain liver biopsy in only one subgroup of them. On the same line, Manning and Afdhal[164] have proposed to perform annually biomarkers analysis plus Fibroscan®. The utilization of combination algorithms does not completely eliminate the need for liver biopsies; however it can greatly reduce it and limit it to cases where serum biomarker data do not show a reliable accuracy. Combination algorithms used in clinical settings are able to provide the subsequent responses: (1) Presence or absence of significant liver fibrosis, which indicates whether to administer antiviral therapy or not; (2) Presence or absence of liver cirrhosis, which indicates whether to proceed with specific screening for esophageal varices and HCC or not; and (3) Liver biopsy needed to correctly stage hepatic fibrosis. Combination algorithms of non-invasive methods for assessment of liver fibrosis that have been proposed in the literature are summarized in Table 7.
| Algorithm’s name | Type | Non-invasive methods adopted | AUC for≥F2 | AUC for F4 | Saved liver biopsies for > F2 (%) | Saved liver biopsies for F4 (%) | Number of studies (patients) |
| SAFE biopsy[133,165] | Stepwise | APRI, Fibrotest® | 0.89-0.94 | 0.87-0.92 | 43.8-54.0 | 74.8-93.4 | 6 (4118) |
| Bordeaux algorithm[167,168] | Synchronous | Fibrotest, Fibroscan® | 0.88-0.91 | 0.93-0.95 | 71.9-77.0 | 78.8-79.0 | 3 (875) |
| Leroy algorithm[124] | Synchronous | APRI, Fibrotest® | 0.94 | NA | 19.0-29.2 | NA | 3 (1381) |
| Fibropaca algorithm[134] | Synchronous | APRI, Fibrotest, Forns’ index | 0.88 | 0.85 | 51.7 | 76.2-81.3 | 2 (1248) |
| Angers algorithms[171] | Synchronous | Fibrotest, Fibrometer® | 0.892 | 0.917 | 79.8 | 89.7 | 1 (390) |
| Bourliere’s algorithm[166] | Stepwise | APRI, Hepascore | 91%-96% (accuracy) | 33-45 | 1 (467) | ||
| Fibrometer® + Fibroscan[172] | Synchronous | Fibrometer, Fibroscan | 86.7% | 100 | 1 (1785) | ||
Sebastiani et al[133,165] proposed an approach that combines APRI and Fibrotest® sequentially. These methods were selected because they are highly validated and widely available. The Sequential Algorithm for Fibrosis Evaluation (SAFE) biopsy was aimed at reducing the amount of liver biopsies needed to accurately stage liver fibrosis, and at minimizing misclassifications. The stepwise modeling of the algorithms for significant liver cirrhosis and fibrosis was intended for achieving ≥ 90% accuracy. The model uses APRI as a first line test because of its simplicity and low cost, and Fibrotest® as a second line test because of its accuracy and higher cost. Importantly, it uses liver biopsy as a third line test only in cases where the combined non-invasive biomarkers fail to classify with adequate accuracy. The modeling of the stepwise algorithms was established on the single biomarkers predicted values. The SAFE biopsy has been validated by data obtained in a multi-centered study with more than 2035 CHC patients (Table 7). They show excellent diagnostic performance and substantial reduction of liver biopsies (50% for significant fibrosis and 80% for cirrhosis). Another proposed stepwise algorithm combines Hepascore®, a patented test, with APRI[166]. This approach yielded 91% diagnostic accuracy and reduced liver biopsies for significant fibrosis by 45%. To date, its main drawback is the lack of extensive validation data for Hepascore®, as compared to APRI, Fibrotest® and Forns’ index.
Castéra et al[167] proposed the Bordeaux algorithm, which combines Fibrotest® and Fibroscan®. This approach improves accuracy for the diagnosis of significant fibrosis. Performance of the Bordeaux algorithm and SAFE biopsy was subsequently compared in 302 patients with CHC[168]. Both algorithms saved a high number of liver biopsies to diagnose cirrhosis, while the Bordeaux algorithm was more effective in the prevention of liver biopsies for the diagnosis of significant fibrosis. The accuracy of the two algorithms was similar for significant fibrosis, whereas, the Bordeaux algorithm was more accurate for the diagnosis of cirrhosis. Nevertheless, the Bordeaux algorithm requires the use of Fibrotest® and Fibroscan® in all patients, which increases cost. The SAFE biopsy is much cheaper because it requires the use of Fibrotest® only in a subgroup of patients who cannot be categorized by APRI.
Another combination algorithm consisting of Forns’ index, Fibrotest® and APRI was proposed by Bourliere et al[169] and showed an exceedingly good performance for diagnosing both significant fibrosis and cirrhosis, saving around 50% and 80% of liver biopsies, respectively. Leroy et al[124], proposed a synchronous algorithm using Fibrotest® and APRI in concordance, which demonstrated exceptional performance in the diagnosing of significant fibrosis. However, the number of saved liver biopsies was relatively small as compared to the other combination algorithms.
The SAFE biopsy, Fibropaca algorithm and Leroy algorithm were applied to 1013 CHC patients[170]. The accuracy of the Fibropaca algorithm and the SAFE biopsy was similar; however, the SAFE biopsy reduced the number of biopsies and required the acquisition of fewer non-invasive biomarkers, thereby saving costs. Boursier et al[171] described the Angers’ algorithm, which combines Fibrotest® and Fibrometer®, and showed that this could save 44.8% of liver biopsies by exhibiting an overall accuracy of 95.3%. Moreover, they suggested that the synchronous combination algorithms could be more efficient than the sequential algorithms, including SAFE biopsy, which is at present debatable. On the same line, a study of 1785 CHC patients compared the performance of eight diagnostic algorithms[172]. The authors found an impressive 0% rate in liver biopsy need with a synchronous combination of Fibroscan® and Fibrometer®. However, even though it showed an excellent accuracy, Fibrometer® has been less evaluated independently compared to other established tests that are used for SAFE biopsy and Bordeaux algorithm (APRI, Fibroscan® and Fibrotest®), and is not licensed in as many countries as Fibrotest®.
In conclusion, combination algorithms can significantly improve the diagnostic accuracy of the single non-invasive method, particularly to diagnose significant liver fibrosis. Moreover, they can safely reduce the number of liver biopsies needed in clinical practice. The choice of the algorithm to be used in clinical practice may be based on some considerations: (1) what is locally available; (2) what is more validated; (3) what is not affected by patient co-morbidities; and (4) which methods the physicians feel more comfortable with.
Several studies suggest that complications of liver disease in compensated cirrhosis can be monitored by non-invasive techniques. As such, values of liver stiffness in cirrhotic patients increase with the progression of liver disease. In a retrospective study of 711 patients, values of liver stiffness significantly correlated with the severity of chronic liver disease in terms of Child-Pugh score, clinical parameters (ascites, varices, history of bleeding, HCC), biochemical parameters (albumin, bilirubin, platelets and INR) and other indications (large esophageal varices, splenomegaly on sonography, nodular surface, heterogeneous parenchyma)[152]. Fibroscan® cut-off values of 27.5, 49.1, 53.7 and 62.7 kPa had > 90% NPV for large esophageal varices, history of ascites, HCC and esophageal bleeding, respectively. On the same line, Vizzutti et al[173] reported a correlation between liver stiffness and portal hypertension, as assessed by the hepatic venous pressure gradient (HVPG). A cut-off of 17.6 kPa of Fibroscan® had 90% sensitivity to rule-in esophageal varices.
In a study of 99 cases Fibrotest® showed a high NPV (100%) to exclude large esophageal varices with a cut-off value of 0.75 in detecting large varices[174]. In another study of 70 patients, Fibrotest® showed 92% NPV for excluding large esophageal varices with a specific cut-off (0.78), with an overall AUC of 0.75; Fibroscan® showed an AUC of 0.87[137]. A low platelet count has been related to the presence of esophageal varices. The discriminating threshold ranged between 68000 and 160000/mm3[137,175]. However, other studies concluded that platelet count is not an adequate non-invasive marker for esophageal varices[176]. For the diagnosis of esophageal varices, Giannini et al[177] reported an overall accuracy of 86% and good sensitivity at 91.5%, and with the cut-off platelet count to spleen diameter ratio at 909.
The value of 7 non-invasive biomarkers of liver fibrosis in prediction of esophageal varices was investigated in one study with 510 patients with cirrhosis[175]. The presence of esophageal varices could be excluded with ≥ 96% NPV by Lok index with the cut-off of 1.5. Importantly, a combination of Forns’ index (8.5 cut-off) and Lok index (0.9 cut-off) could rule-out clinically significant esophageal varices, defined as varices requiring primary prophylaxis of bleeding (large esophageal varices or small varices with red signs or in Child-Pugh class C), with 91% NPV. Likewise, a good performance of Lok index for diagnosis of varices was also reported by Castéra et al[137], with a 0.87 AUC.
Complications of liver cirrhosis, including esophageal varices, ascites and hepatic encephalopathy, occur when portal hypertension develops. The gold standard of reference to diagnose portal hypertension, measurement of HVPG, is invasive and limited to highly specialized centers. Berzigotti et al[178] demonstrated that liver stiffness measurement by Fibroscan® predicts presence of portal hypertension with an AUC of 0.88 as compared to HVPG. Moreover, the performance increased significantly when Fibroscan® was combined with platelets or spleen size (up to 0.935 AUC). In a study of 100 consecutive patients with CHC, spleen stiffness was demonstrated to predict accurately HVPG. Moreover, a cut-off value of spleen stiffness of 41.3 was able to rule-out esophageal varices with 98% sensitivity and 66% specificity[179].
Even though at present non-invasive methods for liver fibrosis cannot replace endoscopy for screening of esophageal varices, they may help stratifying cirrhotic patients for risk classes and possibly reducing the number of endoscopies.
Evaluating the stage of liver fibrosis is a key point not only for management of the patient, but also for long-term prognosis. If CHC patients have mild fibrosis at diagnosis, only 25%-30% of them progress to become cirrhotic within 20 years. However, virtually all patients diagnosed with portal fibrosis will progress to liver cirrhosis within 18-20 years, whereas all patients diagnosed with septal fibrosis will progress to cirrhosis in only 8-10 years. Moreover, end-stage complications mainly occur in patients with advanced disease. Portal hypertension, ascites, or HCC are associated with a shorter survival. Given that the level of fibrosis predicts liver-related complications and survival, early assessment of the risk of bad prognosis helps the physician to manage patients with cirrhosis and to make decisions about liver transplantation.
Liver biopsy does not meet the criteria for serial monitoring and surrogate end-point marker tool because of its invasiveness, sampling error, intra- and inter-observer variability, cost, and patient reluctance to undergo serial monitoring. As such, the value of non-invasive methods for liver fibrosis in predicting clinical outcomes of CHC has been investigated. Ngo et al[180] showed that Fibrotest-Fibrosure® displays a significant correlation with survival, with a 5-year prognostic value similar to that of liver biopsy for the prediction of cirrhosis decompensation and survival. Along the same line, Nunes et al[181] showed that hyaluronic acid, APRI, and Fib-4 were significantly associated with mortality. An association between liver stiffness and risk of HCC development in CHC patients was also described[182].
A definitive demonstration of the long-term predictive role of non-invasive methods for liver fibrosis comes from a study by Vergniol et al[183]. In a consecutive cohort of 1457 CHC patients, the researchers investigated the role of Fibrotest-Fibrosure®, APRI, Fib-4 and liver stiffness in predicting death, liver-related death, and liver transplantation during a 5-year follow-up period[183]. All non-invasive fibrosis methods could predict shorter survival, with liver stiffness and Fibrotest® showing the higher predictive values. Moreover, patient outcomes worsened as liver stiffness and Fibrotest® values increased. On the same line, a recent study of 3927 patients with CHC showed that Fibrotest® and Fibroscan® predicted 10 years occurrence of severe liver-related complications, HCC, variceal bleeding and hepatic failure[184].
A study recently performed in our center investigated the value of Fibroscan® in diagnosing subclinical cirrhosis, as defined by liver stiffness ≥ 13 kPa and absence of thrombocytopenia, ultrasonographic signs of advanced liver disease/splenomegaly, esophageal varices, and ascites[185]. In 1492 consecutive patients with a mean follow-up of 18 mo, we found that patients with subclinical cirrhosis had a higher incidence of cirrhosis-related events as compared to non-cirrhotic patients, including HCC. We then concluded that screening with Fibroscan® may help early identification of subclinical cirrhosis, stratifying patients by risk and establishing a surveillance program for HCC and varices.
Antiviral therapies for CHC are medium term and expensive, and it may be clinically worthy to monitor histological data, in addition to virological and biochemical responses. Even in the rapidly changing panorama of antiviral therapy against HCV infection, the cost will remain a major issue. Initial data revealed significant alterations of Fibroscan® and Fibrotest® values in CHC patients during and after antiviral therapy. In 91 patients with CHC, Hezode et al[186] investigated the kinetics of liver stiffness during antiviral treatment with pegylated interferon alpha and ribavirin. A significant improvement in liver stiffness was observed during therapy, which continued after treatment only in patients who achieved SVR. Interestingly, similar dynamics of liver stiffness were observed in cirrhotic vs non-cirrhotic patients. In multivariate analysis, only the SVR was associated with long-term improvement of liver stiffness. The authors hypothesized that these changes reflect fibrosis regression. This is in keeping with reported improvement of histology in pair liver biopsies[187,188]. On the same line, patients were more likely to achieve SVR if the baseline value of Fibroscan® or Fibrotest® was lower, and mean value of patients at end of treatment was lower in responders[189]. Taken together, these data suggest that antiviral therapies promote regression of liver fibrosis. Larger prospective studies are required for further validation.
Staging of liver fibrosis is crucial for the management of CHC patients and for prognosis. Liver biopsy cannot be used as a screening tool due to its invasiveness and drawbacks, especially in light of recent recommendations for large scale screening against HCV infection. Non-invasive methods to stage liver fibrosis are accurate, cost-effective and patient-friendly. Combination algorithms can help optimize the implementation of non-invasive methods in clinical practice. A rational approach is to perform a first line screening of liver fibrosis with algorithms combining the most accredited non-invasive methods and to perform a biopsy only for patients where non-invasive tests yielded unreliable or inaccurate results. Non-invasive methods for assessment of liver fibrosis can also predict cirrhosis-related complications and long-term outcomes of CHC patients. Thus, they can be used to stratify patients by risk classes and to prioritize for antiviral treatment and liver transplantation. Finally, non-invasive methods can be used to monitor the regression of liver fibrosis in response to antiviral therapy.
We thank Lynda Lennox for editing the manuscript.
P- Reviewer: Adler MG, Fraquelli M, Yoshioka K S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 1020] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 2. | Harris HE, Ramsay ME, Andrews NJ. Survival of a national cohort of hepatitis C virus infected patients, 16 years after exposure. Epidemiol Infect. 2006;134:472-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Jacobson IM, Cacoub P, Dal Maso L, Harrison SA, Younossi ZM. Manifestations of chronic hepatitis C virus infection beyond the liver. Clin Gastroenterol Hepatol. 2010;8:1017-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Ryder SD. Outcome of hepatitis C infection: bleak or benign? J Hepatol. 2007;47:4-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Mühlberger N, Schwarzer R, Lettmeier B, Sroczynski G, Zeuzem S, Siebert U. HCV-related burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity, and mortality. BMC Public Health. 2009;9:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32. [PubMed] [Cited in This Article: ] |
| 7. | Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750-1755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Alberti A, Chemello L, Benvegnù L. Natural history of hepatitis C. J Hepatol. 1999;31 Suppl 1:17-24. [PubMed] [Cited in This Article: ] |
| 9. | Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-938. [PubMed] [Cited in This Article: ] |
| 10. | Ikeda K; Infections. CotPaCoVH. Hepatitis and liver cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington: Institute of Medicine of the Natioinal Academies 2010; . [Cited in This Article: ] |
| 11. | Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54:1259-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Sebastiani G, Gkouvatsos K, Plebani M. Non-invasive assessment of liver fibrosis: it is time for laboratory medicine. Clin Chem Lab Med. 2011;49:13-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3381] [Cited by in F6Publishing: 3806] [Article Influence: 200.3] [Reference Citation Analysis (3)] |
| 14. | Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 15. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in F6Publishing: 684] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 16. | Steinmann E, Pietschmann T. Cell culture systems for hepatitis C virus. Curr Top Microbiol Immunol. 2013;369:17-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Billerbeck E, de Jong Y, Dorner M, de la Fuente C, Ploss A. Animal models for hepatitis C. Curr Top Microbiol Immunol. 2013;369:49-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933-938. [PubMed] [Cited in This Article: ] |
| 19. | Shimizu YK, Feinstone SM, Kohara M, Purcell RH, Yoshikura H. Hepatitis C virus: detection of intracellular virus particles by electron microscopy. Hepatology. 1996;23:205-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Caussin-Schwemling C, Schmitt C, Stoll-Keller F. Study of the infection of human blood derived monocyte/macrophages with hepatitis C virus in vitro. J Med Virol. 2001;65:14-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Arrieta JJ, Rodríguez-Iñigo E, Ortiz-Movilla N, Bartolomé J, Pardo M, Manzarbeitia F, Oliva H, Macías DM, Carreño V. In situ detection of hepatitis C virus RNA in salivary glands. Am J Pathol. 2001;158:259-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Giannini C, Bréchot C. Hepatitis C virus biology. Cell Death Differ. 2003;10 Suppl 1:S27-S38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529-540. [PubMed] [Cited in This Article: ] |
| 24. | Iqbal J, McRae S, Banaudha K, Mai T, Waris G. Mechanism of hepatitis C virus (HCV)-induced osteopontin and its role in epithelial to mesenchymal transition of hepatocytes. J Biol Chem. 2013;288:36994-37009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Mazzocca A, Sciammetta SC, Carloni V, Cosmi L, Annunziato F, Harada T, Abrignani S, Pinzani M. Binding of hepatitis C virus envelope protein E2 to CD81 up-regulates matrix metalloproteinase-2 in human hepatic stellate cells. J Biol Chem. 2005;280:11329-11339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599-9604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 486] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 27. | Bureau C, Bernad J, Chaouche N, Orfila C, Béraud M, Gonindard C, Alric L, Vinel JP, Pipy B. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J Biol Chem. 2001;276:23077-23083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Li K, Prow T, Lemon SM, Beard MR. Cellular response to conditional expression of hepatitis C virus core protein in Huh7 cultured human hepatoma cells. Hepatology. 2002;35:1237-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Abdalla MY, Mathahs MM, Ahmad IM. Reduced heme oxygenase-1 expression in steatotic livers infected with hepatitis C virus. Eur J Intern Med. 2012;23:649-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513-1524. [PubMed] [Cited in This Article: ] |
| 31. | Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, Schuppan D. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129:246-258. [PubMed] [Cited in This Article: ] |
| 32. | Lin W, Tsai WL, Shao RX, Wu G, Peng LF, Barlow LL, Chung WJ, Zhang L, Zhao H, Jang JY. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology. 2010;138:2509-218, 2518.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223-229. [PubMed] [Cited in This Article: ] |
| 34. | Failli P, Ruocco C, De Franco R, Caligiuri A, Gentilini A, Giotti A, Gentilini P, Pinzani M. The mitogenic effect of platelet-derived growth factor in human hepatic stellate cells requires calcium influx. Am J Physiol. 1995;269:C1133-C1139. [PubMed] [Cited in This Article: ] |
| 35. | Wong L, Yamasaki G, Johnson RJ, Friedman SL. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94:1563-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 241] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Meyer DH, Bachem MG, Gressner AM. Modulation of hepatic lipocyte proteoglycan synthesis and proliferation by Kupffer cell-derived transforming growth factors type beta 1 and type alpha. Biochem Biophys Res Commun. 1990;171:1122-1129. [PubMed] [Cited in This Article: ] |
| 37. | Svegliati-Baroni G, Ridolfi F, Hannivoort R, Saccomanno S, Homan M, De Minicis S, Jansen PL, Candelaresi C, Benedetti A, Moshage H. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology. 2005;128:1042-1055. [PubMed] [Cited in This Article: ] |
| 38. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Yamazaki M. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52:1347-1354. [PubMed] [Cited in This Article: ] |
| 39. | Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 40. | Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1739] [Cited by in F6Publishing: 1786] [Article Influence: 89.3] [Reference Citation Analysis (1)] |
| 41. | Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1-9. [PubMed] [Cited in This Article: ] |
| 42. | Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 43. | Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, George J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Zhou Y, Jia X, Wang G, Wang X, Liu J. PI-3 K/AKT and ERK signaling pathways mediate leptin-induced inhibition of PPARgamma gene expression in primary rat hepatic stellate cells. Mol Cell Biochem. 2009;325:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Sahin H, Trautwein C, Wasmuth HE. Functional role of chemokines in liver disease models. Nat Rev Gastroenterol Hepatol. 2010;7:682-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949-G958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 47. | Sahin H, Borkham-Kamphorst E, Kuppe C, Zaldivar MM, Grouls C, Al-samman M, Nellen A, Schmitz P, Heinrichs D, Berres ML. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology. 2012;55:1610-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Zaldivar MM, Pauels K, von Hundelshausen P, Berres ML, Schmitz P, Bornemann J, Kowalska MA, Gassler N, Streetz KL, Weiskirchen R. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2010;51:1345-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 49. | De Minicis S, Candelaresi C, Marzioni M, Saccomano S, Roskams T, Casini A, Risaliti A, Salzano R, Cautero N, di Francesco F. Role of endogenous opioids in modulating HSC activity in vitro and liver fibrosis in vivo. Gut. 2008;57:352-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Jeong WI, Osei-Hyiaman D, Park O, Liu J, Bátkai S, Mukhopadhyay P, Horiguchi N, Harvey-White J, Marsicano G, Lutz B. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 251] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 51. | Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, Mann DA. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006;169:861-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Zvibel I, Atias D, Phillips A, Halpern Z, Oren R. Thyroid hormones induce activation of rat hepatic stellate cells through increased expression of p75 neurotrophin receptor and direct activation of Rho. Lab Invest. 2010;90:674-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Liu C, Tao Q, Sun M, Wu JZ, Yang W, Jian P, Peng J, Hu Y, Liu C, Liu P. Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab Invest. 2010;90:1805-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Pinzani M, Abboud HE, Gesualdo L, Abboud SL. Regulation of macrophage colony-stimulating factor in liver fat-storing cells by peptide growth factors. Am J Physiol. 1992;262:C876-C881. [PubMed] [Cited in This Article: ] |
| 55. | Tiggelman AM, Boers W, Linthorst C, Brand HS, Sala M, Chamuleau RA. Interleukin-6 production by human liver (myo)fibroblasts in culture. Evidence for a regulatory role of LPS, IL-1 beta and TNF alpha. J Hepatol. 1995;23:295-306. [PubMed] [Cited in This Article: ] |
| 56. | Czaja MJ, Geerts A, Xu J, Schmiedeberg P, Ju Y. Monocyte chemoattractant protein 1 (MCP-1) expression occurs in toxic rat liver injury and human liver disease. J Leukoc Biol. 1994;55:120-126. [PubMed] [Cited in This Article: ] |
| 57. | Knittel T, Dinter C, Kobold D, Neubauer K, Mehde M, Eichhorst S, Ramadori G. Expression and regulation of cell adhesion molecules by hepatic stellate cells (HSC) of rat liver: involvement of HSC in recruitment of inflammatory cells during hepatic tissue repair. Am J Pathol. 1999;154:153-167. [PubMed] [Cited in This Article: ] |
| 58. | Hellerbrand SC, Tsukamoto H, Brenner DA, Rippe RA. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology. 1996;24:670-676. [PubMed] [Cited in This Article: ] |
| 59. | Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 896] [Cited by in F6Publishing: 966] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 61. | Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21-34. [PubMed] [Cited in This Article: ] |
| 62. | Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, Jang MK, Guenther ND, Mederacke I, Friedman R. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461-1473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 403] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 63. | Muhanna N, Abu Tair L, Doron S, Amer J, Azzeh M, Mahamid M, Friedman S, Safadi R. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011;60:90-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | Choi J, Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol. 2006;290:G847-G851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 65. | Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 66. | MacDonald GA, Bridle KR, Ward PJ, Walker NI, Houglum K, George DK, Smith JL, Powell LW, Crawford DH, Ramm GA. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol. 2001;16:599-606. [PubMed] [Cited in This Article: ] |
| 67. | Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509-1518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 68. | Fujita N, Sugimoto R, Motonishi S, Tomosugi N, Tanaka H, Takeo M, Iwasa M, Kobayashi Y, Hayashi H, Kaito M. Patients with chronic hepatitis C achieving a sustained virological response to peginterferon and ribavirin therapy recover from impaired hepcidin secretion. J Hepatol. 2008;49:702-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Girelli D, Pasino M, Goodnough JB, Nemeth E, Guido M, Castagna A, Busti F, Campostrini N, Martinelli N, Vantini I. Reduced serum hepcidin levels in patients with chronic hepatitis C. J Hepatol. 2009;51:845-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 71. | Fillebeen C, Rivas-Estilla AM, Bisaillon M, Ponka P, Muckenthaler M, Hentze MW, Koromilas AE, Pantopoulos K. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C Virus. J Biol Chem. 2005;280:9049-9057. [PubMed] [Cited in This Article: ] |
| 72. | Fillebeen C, Pantopoulos K. Iron inhibits replication of infectious hepatitis C virus in permissive Huh7.5.1 cells. J Hepatol. 2010;53:995-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Lambrecht RW, Sterling RK, Naishadham D, Stoddard AM, Rogers T, Morishima C, Morgan TR, Bonkovsky HL. Iron levels in hepatocytes and portal tract cells predict progression and outcomes of patients with advanced chronic hepatitis C. Gastroenterology. 2011;140:1490-500.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Lange CM, Kutalik Z, Morikawa K, Bibert S, Cerny A, Dollenmaier G, Dufour JF, Gerlach TJ, Heim MH, Malinverni R. Serum ferritin levels are associated with a distinct phenotype of chronic hepatitis C poorly responding to pegylated interferon-alpha and ribavirin therapy. Hepatology. 2012;55:1038-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Tsochatzis E, Papatheodoridis GV, Koliaraki V, Hadziyannis E, Kafiri G, Manesis EK, Mamalaki A, Archimandritis AJ. Serum hepcidin levels are related to the severity of liver histological lesions in chronic hepatitis C. J Viral Hepat. 2010;17:800-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Pantopoulos K. Function of the hemochromatosis protein HFE: Lessons from animal models. World J Gastroenterol. 2008;14:6893-6901. [PubMed] [Cited in This Article: ] |
| 77. | Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. 2003;124:1509-1523. [PubMed] [Cited in This Article: ] |
| 78. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1928] [Cited by in F6Publishing: 2053] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 79. | Sebastiani G, Alberti A. How far is noninvasive assessment of liver fibrosis from replacing liver biopsy in hepatitis C? J Viral Hepat. 2012;19 Suppl 1:18-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, Hashimoto E, Lefkowitch JH, Ludwig J, Okuda K. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 375] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 81. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 646] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 82. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [PubMed] [Cited in This Article: ] |
| 83. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1504] [Cited by in F6Publishing: 1504] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 84. | Pagliaro L, Rinaldi F, Craxì A, Di Piazza S, Filippazzo G, Gatto G, Genova G, Magrin S, Maringhini A, Orsini S. Percutaneous blind biopsy versus laparoscopy with guided biopsy in diagnosis of cirrhosis. A prospective, randomized trial. Dig Dis Sci. 1983;28:39-43. [PubMed] [Cited in This Article: ] |
| 85. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [PubMed] [Cited in This Article: ] |
| 86. | Poniachik J, Bernstein DE, Reddy KR, Jeffers LJ, Coelho-Little ME, Civantos F, Schiff ER. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568-571. [PubMed] [Cited in This Article: ] |
| 87. | Hübscher SG. Histological grading and staging in chronic hepatitis: clinical applications and problems. J Hepatol. 1998;29:1015-1022. [PubMed] [Cited in This Article: ] |
| 88. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 410] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 89. | Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12:3682-3694. [PubMed] [Cited in This Article: ] |
| 90. | Guido M, Rugge M. Liver biopsy sampling in chronic viral hepatitis. Semin Liver Dis. 2004;24:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1193] [Cited by in F6Publishing: 1334] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 92. | Scheuer PJ. Liver biopsy size matters in chronic hepatitis: bigger is better. Hepatology. 2003;38:1356-1358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1449] [Cited by in F6Publishing: 1419] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 94. | Rousselet MC, Michalak S, Dupré F, Croué A, Bedossa P, Saint-André JP, Calès P. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 426] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 95. | Bejarano PA, Koehler A, Sherman KE. Second opinion pathology in liver biopsy interpretation. Am J Gastroenterol. 2001;96:3158-3164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Hahm GK, Niemann TH, Lucas JG, Frankel WL. The value of second opinion in gastrointestinal and liver pathology. Arch Pathol Lab Med. 2001;125:736-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 97. | Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol. 2009;50:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 98. | Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009;50:1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 222] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 99. | Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36:437-441. [PubMed] [Cited in This Article: ] |
| 100. | Szymczak A, Simon K, Inglot M, Gladysz A. Safety and effectiveness of blind percutaneous liver biopsy: analysis of 1412 procedures. Hepat Mon. 2012;12:32-37. [PubMed] [Cited in This Article: ] |
| 101. | Chevallier P, Ruitort F, Denys A, Staccini P, Saint-Paul MC, Ouzan D, Motamedi JP, Tran A, Schnyder P, Bruneton JN. Influence of operator experience on performance of ultrasound-guided percutaneous liver biopsy. Eur Radiol. 2004;14:2086-2091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Bonny C, Rayssiguier R, Ughetto S, Aublet-Cuvelier B, Baranger J, Blanchet G, Delteil J, Hautefeuille P, Lapalus F, Montanier P. [Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region]. Gastroenterol Clin Biol. 2003;27:1021-1025. [PubMed] [Cited in This Article: ] |
| 103. | Muir AJ, Trotter JF. A survey of current liver biopsy practice patterns. J Clin Gastroenterol. 2002;35:86-88. [PubMed] [Cited in This Article: ] |
| 104. | Sebastiani G, Ghali P, Wong P, Klein MB, Deschenes M, Myers RP. Physicians’ practices for diagnosing liver fibrosis in chronic liver diseases: a nationwide, Canadian survey. Can J Gastroenterol Hepatol. 2014;28:23-30. [PubMed] [Cited in This Article: ] |
| 105. | Castera L, Denis J, Babany G, Roudot-Thoraval F. Evolving practices of non-invasive markers of liver fibrosis in patients with chronic hepatitis C in France: time for new guidelines? J Hepatol. 2007;46:528-59; author reply 529-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Almasio PL, Niero M, Angioli D, Ascione A, Gullini S, Minoli G, Oprandi NC, Pinzello GB, Verme G, Andriulli A. Experts’ opinions on the role of liver biopsy in HCV infection: a Delphi survey by the Italian Association of Hospital Gastroenterologists (A.I.G.O.). J Hepatol. 2005;43:381-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Wong JB, Koff RS. Watchful waiting with periodic liver biopsy versus immediate empirical therapy for histologically mild chronic hepatitis C. A cost-effectiveness analysis. Ann Intern Med. 2000;133:665-675. [PubMed] [Cited in This Article: ] |
| 108. | Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28:705-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 109. | Asian Pacific Association for the Study of the Liver (APASL) Hepatitis C Working Party; McCaughan GW, Omata M, Amarapurkar D, Bowden S, Chow WC, Chutaputti A, Dore G, Gane E, Guan R, Hamid SS, Hardikar W, Hui CK, Jafri W, Jia JD, Lai MY, Wei L, Leung N, Piratvisuth T, Sarin S, Sollano J, Tateishi R. Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:615-633. [PubMed] [Cited in This Article: ] |
| 110. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 889] [Cited by in F6Publishing: 968] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 111. | Myers RP, Ramji A, Bilodeau M, Wong S, Feld JJ. An update on the management of hepatitis C: consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol. 2012;26:359-375. [PubMed] [Cited in This Article: ] |
| 112. | Sebastiani G, Vario A, Guido M, Alberti A. Sequential algorithms combining non-invasive markers and biopsy for the assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2007;13:525-531. [PubMed] [Cited in This Article: ] |
| 113. | Guéchot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem. 1996;42:558-563. [PubMed] [Cited in This Article: ] |
| 114. | Halfon P, Bourlière M, Pénaranda G, Deydier R, Renou C, Botta-Fridlund D, Tran A, Portal I, Allemand I, Rosenthal-Allieri A. Accuracy of hyaluronic acid level for predicting liver fibrosis stages in patients with hepatitis C virus. Comp Hepatol. 2005;4:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 115. | McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945-951. [PubMed] [Cited in This Article: ] |
| 116. | Walsh KM, Fletcher A, MacSween RN, Morris AJ. Basement membrane peptides as markers of liver disease in chronic hepatitis C. J Hepatol. 2000;32:325-330. [PubMed] [Cited in This Article: ] |
| 117. | Murawaki Y, Ikuta Y, Okamoto K, Koda M, Kawasaki H. Diagnostic value of serum markers of connective tissue turnover for predicting histological staging and grading in patients with chronic hepatitis C. J Gastroenterol. 2001;36:399-406. [PubMed] [Cited in This Article: ] |
| 118. | Misaki M, Shima T, Yano Y, Sumita Y, Kano U, Murata T, Watanabe S, Suzuki S. Basement membrane-related and type III procollagen-related antigens in serum of patients with chronic viral liver disease. Clin Chem. 1990;36:522-524. [PubMed] [Cited in This Article: ] |
| 119. | Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, Gallois Y, Rifflet H, Maïga MY, Penneau-Fontbonne D. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609-1616. [PubMed] [Cited in This Article: ] |
| 120. | Walsh KM, Timms P, Campbell S, MacSween RN, Morris AJ. Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases -1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Dig Dis Sci. 1999;44:624-630. [PubMed] [Cited in This Article: ] |
| 121. | Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, Sugimoto K, Murata K, Nakano T. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005;11:476-481. [PubMed] [Cited in This Article: ] |
| 122. | Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, Gallois Y, Ternisien C, Chevailler A, Lunel F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 123. | Calès P, Lainé F, Boursier J, Deugnier Y, Moal V, Oberti F, Hunault G, Rousselet MC, Hubert I, Laafi J. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol. 2009;50:165-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 124. | Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, Morel F, Zarski JP. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46:775-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 125. | Christensen C, Bruden D, Livingston S, Deubner H, Homan C, Smith K, Oh E, Gretch D, Williams J, McMahon B. Diagnostic accuracy of a fibrosis serum panel (FIBROSpect II) compared with Knodell and Ishak liver biopsy scores in chronic hepatitis C patients. J Viral Hepat. 2006;13:652-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 126. | Zaman A, Rosen HR, Ingram K, Corless CL, Oh E, Smith K. Assessment of FIBROSpect II to detect hepatic fibrosis in chronic hepatitis C patients. Am J Med. 2007;120:280.e9-280.14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 127. | Patel K, Nelson DR, Rockey DC, Afdhal NH, Smith KM, Oh E, Hettinger K, Vallée M, Dev A, Smith-Riggs M. Correlation of FIBROSpect II with histologic and morphometric evaluation of liver fibrosis in chronic hepatitis C. Clin Gastroenterol Hepatol. 2008;6:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 128. | Mehta P, Ploutz-Snyder R, Nandi J, Rawlins SR, Sanderson SO, Levine RA. Diagnostic accuracy of serum hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2008;103:928-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 129. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 370] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 130. | Becker L, Salameh W, Sferruzza A, Zhang K, ng Chen R, Malik R, Reitz R, Nasser I, Afdhal NH. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701. [PubMed] [Cited in This Article: ] |
| 131. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ; European Liver Fibrosis Group. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [PubMed] [Cited in This Article: ] |
| 132. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 133. | Sebastiani G, Vario A, Guido M, Noventa F, Plebani M, Pistis R, Ferrari A, Alberti A. Stepwise combination algorithms of non-invasive markers to diagnose significant fibrosis in chronic hepatitis C. J Hepatol. 2006;44:686-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 134. | Halfon P, Bourliere M, Deydier R, Botta-Fridlund D, Renou C, Tran A, Portal I, Allemand I, Bertrand JJ, Rosenthal-Allieri A. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol. 2006;101:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 135. | Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589-2600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 136. | Lackner C, Struber G, Bankuti C, Bauer B, Stauber RE. Noninvasive diagnosis of cirrhosis in chronic hepatitis C based on standard laboratory tests. Hepatology. 2006;43:378-39; author reply 379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 137. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 138. | Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Ceppa P, Testa R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218-224. [PubMed] [Cited in This Article: ] |
| 139. | Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, Bauer B, Stauber RE. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41:1376-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 140. | Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 341] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 141. | Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J Gastroenterol Hepatol. 2000;15:386-390. [PubMed] [Cited in This Article: ] |
| 142. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 2988] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 143. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 698] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 144. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 751] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 145. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1288] [Cited by in F6Publishing: 1425] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 146. | Adler M, Gulbis B, Moreno C, Evrard S, Verset G, Golstein P, Frotscher B, Nagy N, Thiry P. The predictive value of FIB-4 versus FibroTest, APRI, FibroIndex and Forns index to noninvasively estimate fibrosis in hepatitis C and nonhepatitis C liver diseases. Hepatology. 2008;47:762-763; author reply 763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 147. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [Cited in This Article: ] |
| 148. | Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293-1302.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 461] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 149. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1011] [Article Influence: 63.2] [Reference Citation Analysis (1)] |
| 150. | Lebray P, Varnous S, Charlotte F, Varaut A, Poynard T, Ratziu V. Liver stiffness is an unreliable marker of liver fibrosis in patients with cardiac insufficiency. Hepatology. 2008;48:2089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 151. | Lucidarme D, Foucher J, Le Bail B, Vergniol J, Castera L, Duburque C, Forzy G, Filoche B, Couzigou P, de Lédinghen V. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1083-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 152. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 897] [Cited by in F6Publishing: 903] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 153. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 876] [Article Influence: 62.6] [Reference Citation Analysis (1)] |
| 154. | Myers RP, Pomier-Layrargues G, Kirsch R, Pollett A, Duarte-Rojo A, Wong D, Beaton M, Levstik M, Crotty P, Elkashab M. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 343] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 155. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 156. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 405] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 157. | Calvaruso V, Cammà C, Di Marco V, Maimone S, Bronte F, Enea M, Dardanoni V, Manousou P, Pleguezuelo M, Xirouchakis E. Fibrosis staging in chronic hepatitis C: analysis of discordance between transient elastography and liver biopsy. J Viral Hepat. 2010;17:469-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 158. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 425] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 159. | Arena U, Lupsor Platon M, Stasi C, Moscarella S, Assarat A, Bedogni G, Piazzolla V, Badea R, Laffi G, Marra F. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology. 2013;58:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 160. | Berzigotti A, Abraldes JG, Bosch J. Regarding “Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution”. Hepatology. 2014;59:350-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 161. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 618] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 162. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 163. | Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 164. | Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670-1681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 165. | Sebastiani G, Halfon P, Castera L, Pol S, Thomas DL, Mangia A, Di Marco V, Pirisi M, Voiculescu M, Guido M. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1821-1827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 166. | Bourliere M, Penaranda G, Ouzan D, Renou C, Botta-Fridlund D, Tran A, Rosenthal E, Wartelle-Bladou C, Delasalle P, Oules V. Optimized stepwise combination algorithms of non-invasive liver fibrosis scores including Hepascore in hepatitis C virus patients. Aliment Pharmacol Ther. 2008;28:458-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 167. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] [Cited in This Article: ] |
| 168. | Castéra L, Sebastiani G, Le Bail B, de Lédinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 169. | Bourliere M, Penaranda G, Renou C, Botta-Fridlund D, Tran A, Portal I, Lecomte L, Castellani P, Rosenthal-Allieri MA, Gerolami R. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J Viral Hepat. 2006;13:659-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 170. | Sebastiani G, Halfon P, Castera L, Mangia A, Di Marco V, Pirisi M, Voiculescu M, Bourliere M, Alberti A. Comparison of three algorithms of non-invasive markers of fibrosis in chronic hepatitis C. Aliment Pharmacol Ther. 2012;35:92-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 171. | Boursier J, Vergniol J, Sawadogo A, Dakka T, Michalak S, Gallois Y, Le Tallec V, Oberti F, Fouchard-Hubert I, Dib N. The combination of a blood test and Fibroscan improves the non-invasive diagnosis of liver fibrosis. Liver Int. 2009;29:1507-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 172. | Boursier J, de Ledinghen V, Zarski JP, Fouchard-Hubert I, Gallois Y, Oberti F, Calès P; multicentric groups from SNIFF 32, VINDIAG 7, and ANRS/HC/EP23 FIBROSTAR studies. Comparison of eight diagnostic algorithms for liver fibrosis in hepatitis C: new algorithms are more precise and entirely noninvasive. Hepatology. 2012;55:58-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 173. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 493] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 174. | Thabut D, Trabut JB, Massard J, Rudler M, Muntenau M, Messous D, Poynard T. Non-invasive diagnosis of large oesophageal varices with FibroTest in patients with cirrhosis: a preliminary retrospective study. Liver Int. 2006;26:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 175. | Sebastiani G, Tempesta D, Fattovich G, Castera L, Halfon P, Bourliere M, Noventa F, Angeli P, Saggioro A, Alberti A. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large-scale study. J Hepatol. 2010;53:630-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 176. | Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Maurer R, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Makuch R; Portal Hypertension Collaborative Group. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology. 2008;47:153-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 177. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [PubMed] [Cited in This Article: ] |
| 178. | Berzigotti A, Bosch J, Boyer TD. Use of noninvasive markers of portal hypertension and timing of screening endoscopy for gastroesophageal varices in patients with chronic liver disease. Hepatology. 2014;59:729-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 179. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 180. | Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, Thibault V, Benhamou Y, Moussalli J. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 181. | Nunes D, Fleming C, Offner G, Craven D, Fix O, Heeren T, Koziel MJ, Graham C, Tumilty S, Skolnik P. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 182. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 183. | Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, Couzigou P, de Ledinghen V. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970-199, 1970-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 184. | Poynard T, Vergniol J, Ngo Y, Foucher J, Munteanu M, Merrouche W, Colombo M, Thibault V, Schiff E, Brass CA. Staging chronic hepatitis C in seven categories using fibrosis biomarker (FibroTest™) and transient elastography (FibroScan®). J Hepatol. 2014;60:706-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 185. | Chen T, Wong RE, Alshaalan R, Wong P, Deschenes M, Ghali P, Sebastiani G. Subclinical cirrhosis: defining a new clinical entity with transient elastography. Hepatology. 2013;58:123A. [Cited in This Article: ] |
| 186. | Hézode C, Castéra L, Roudot-Thoraval F, Bouvier-Alias M, Rosa I, Roulot D, Leroy V, Mallat A, Pawlotsky JM. Liver stiffness diminishes with antiviral response in chronic hepatitis C. Aliment Pharmacol Ther. 2011;34:656-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 187. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [PubMed] [Cited in This Article: ] |
| 188. | Cammà C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, Marcellin P, Balart L, Alberti A, Craxì A. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 189. | Patel K, Friedrich-Rust M, Lurie Y, Grigorescu M, Stanciu C, Lee CM, Schiff ER, Häussinger D, Manns MP, Gerken G. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 190. | Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2252] [Cited by in F6Publishing: 2192] [Article Influence: 146.1] [Reference Citation Analysis (1)] |
| 191. | Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di Bisceglie AM. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 192. | Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat. 2008;15:212-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 193. | Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 194. | Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 195. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1090] [Cited by in F6Publishing: 1062] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 196. | Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Lédinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 197. | Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 198. | Cross TJ, Calvaruso V, Maimone S, Carey I, Chang TP, Pleguezuelo M, Manousou P, Quaglia A, Grillo F, Dhillon AP. Prospective comparison of Fibroscan, King’s score and liver biopsy for the assessment of cirrhosis in chronic hepatitis C infection. J Viral Hepat. 2010;17:546-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 199. | Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 332] [Article Influence: 23.7] [Reference Citation Analysis (0)] |