Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.10944
Revised: April 1, 2014
Accepted: May 19, 2014
Published online: August 21, 2014
AIM: To determine the prognostic value of alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT) for hepatocellular carcinoma (HCC) .
METHODS: We analyzed the outcome of 172 HCC patients who underwent liver resection. Receiver operating characteristic (ROC) curve analysis was performed to determine the cut-off value of ALP and GGT. Then, preoperative risk factors for survival were evaluated by multivariate analysis. Based on the significant factors, a prognostic score model was established.
RESULTS: By ROC curve analysis, ALP > 120 U/L and GGT > 115 U/L were considered elevated. Overall survival (OS) and tumor-free survival (TFS) for patients with elevated ALP and GGT were significantly worse than for patients with ALP and GGT within the normal range. Multivariate analysis showed that the elevated levels of ALP, GGT and tumor size were independent prognostic factors. Giving each positive factor as a score of 1, we established a preoperative prognostic score model. The 5-year OS for patients with a score of 0, 1, 2 and 3 were 84.0%, 45.9%, 44.1% and 0%, respectively, while the TFS was 80.6%, 40.0%, 38.8% and 0%, respectively. When combining patients with scores of 1 and 2 into the middle risk group, and patients with scores of 0 and 3 into the low-risk and high-risk groups, respectively, different outcomes would be significantly distinguished by the risk groups.
CONCLUSION: Elevated ALP and GGT levels were risk predictors in HCC patients. Our prognostic model might vary the outcomes of patients from different risk groups.
Core tip: To determine the optimal cut-off value of alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT) to predict hepatocellular carcinoma (HCC) prognosis after liver resection, and to establish a scoring model, we analyzed the outcome of 172 HCC patients who underwent liver resection. Receiver operating characteristic curve analysis was performed to determine the cut-off value of ALP and GGT. Preoperative risk factors for survival were evaluated by multivariate analysis. Based on the significant factors, a prognostic scoring model was established. Our model might affect the outcome of patients in different risk groups, and was superior to the traditional risk markers.
- Citation: Xu XS, Wan Y, Song SD, Chen W, Miao RC, Zhou YY, Zhang LQ, Qu K, Liu SN, Zhang YL, Dong YF, Liu C. Model based on γ-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World J Gastroenterol 2014; 20(31): 10944-10952
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/10944.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.10944
Hepatocellular carcinoma (HCC), the fifth most common cancer worldwide, is rarely detected early and is usually fatal within months of diagnosis[1]. Liver resection remains the gold standard for patients with resectable HCC that develops in the setting of normal liver substance. However, most patients with HCC have diseased liver parenchyma, especially HBV-related cirrhosis in China, and resection in this population is more fraught, with the potential for more complications[2].
Thus, a balance for choice of therapy is urgently needed prior to treatment, such as liver transplantation, transcatheter hepatic arterial chemoembolization, and radiofrequency ablation. Yet, these choices are mostly based on traditional guidelines such as the Milan Criteria (single nodule ≤ 5 cm or two or three nodules ≤ 3 cm)[3] and the University of California, San Francisco (UCSF) Criteria (single nodule ≤ 6.5 cm, or two or three nodules with the largest nodule ≤ 4.5 cm and the total tumor burden ≤ 8 cm)[4]. Great suspicions were raised about these criteria, because they solely rely on inaccurate preoperative imaging findings such as tumor number and tumor size, but neglect the essential features of tumors such as carcinoembryonic antigen and α-fetoprotein (AFP). However, although AFP is frequently used to predict post-hepatectomy outcomes in patients with HCC, contradictory results have been reported from different studies, and the predictive accuracy was far from satisfactory[5,6].
Building a scoring model combining the imaging findings and serum parameters to predict the prognosis of HCC patients undergoing liver resection is useful in guiding us to choose the best treatment. Many trials have focused on exploring the prognostic markers of HCC in patients undergoing liver resection, such as serum miRNAs and other potential gene markers, however, they have had either unsatisfactory results, or were confined to laboratory experiments, which were not implementable clinically[7-9].
Serum liver enzymes such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and γ-glutamyltransferase (GGT), are routinely tested in patients. These enzymes are commonly elevated in patients with liver diseases and thus may reflect the status of liver injury[10]. Of the liver enzymes, ALP and GGT have long been recognized to play potential roles in the diagnosis of cancer. For instance, Xu et al[11] systemically analyzed the isoenzymes of GGT, and reported that GGT could be applied as an additional marker for HCC, valuable not only for the diagnosis of clinical HCC, but also for the detection of small or subclinical HCC. Hann et al[12] studied the associations of liver enzymes with the risk of HCC in HBV-infected patients, and found that compared to patients with normal baseline GGT, those with elevated GGT exhibited a significantly increased HCC risk with a hazard ratio of 2.60. Lopez et al[13] reported that raised ALP level in the presence of normal bilirubin was more often a feature of HCC than benign liver diseases, although the specific mechanism was not clear.
However, these studies were mainly focused on the diagnostic roles of ALP and GGT. Few studies have systematically explored the prognostic roles of these liver enzymes. In the current study, we sought to evaluate the effects of ALP and GGT on the long-term prognosis of patients with HCC undergoing liver resection. In addition, we tried to combine these serum markers such as ALP and GGT, and tumor characteristics such as tumor number and tumor size, to establish a scoring model consisting of comprehensive features of tumors to predict better the prognosis of HCC.
Prospectively collected data in our unit (First Affiliated Hospital, Xi’an Jiaotong University, Xi’an, China) were reviewed retrospectively. We enrolled 172 HCC patients underwent liver resection with complete follow-up during the 10-year period from December 2002 to July 2012. For this study, we included those patients who met all the following criteria: (1) patients were diagnosed with only HCC, but with no concomitant intrahepatic cholangiocarcinoma, or any other malignancies, to eliminate the confounding effects from disease etiology; (2) patients had serum liver enzymes (ALP and GGT) and AFP measured simultaneously at study entry, making the baseline analyses comparable; (3) liver resection was performed on the resectable HCC; and (4) patients had a minimum follow-up time of 1 year from the study entry point.
Patient baseline and clinical data, including age, sex, liver enzymes such as ALP and GGT, serum AFP, HBV infection, HBV-DNA level, HCV infection, preoperative imaging data (tumor size, number, and invasion), surgery procedure records, and tumor pathology were recorded. Other synthetic liver function was also assessed, such as total bilirubin, albumin and international normalized ratio (INR), to evaluate the Child classification of every patient. Liver resection specimens were assessed by two independent liver pathologists, blinded to all patient demographics and clinical outcomes. All patients gave written informed consent to this study, and approval for the study was obtained from the Institutional Review Board, which conformed to the standards of the Declaration of Helsinki.
A systematic examination was performed to exclude peritoneal metastases. The resection techniques principally involved were either anatomical or nonanatomical according to the patients’ preoperative liver function and tumor anatomical status. Hepatic pedicle and celiac lymph nodes were removed for frozen section histological examination. Systematic, intraoperative hepatic ultrasonography was performed to detect additional nodules or portal thrombosis. After resection, the macroscopic features of the tumor, including size, number of tumors, portal vein invasion, and hepatic vein invasion, were recorded. Histological examinations of microscopic vascular invasion as well as satellite lesions were examined. Postoperative management included symptomatic therapy if any surgical complications occurred, such as bleeding, infection, or hypoalbuminemia.
After liver resection, patients were followed every 3 mo in the first year, every 4 mo in the second year, and every 6 mo thereafter. Imaging with computed tomography or magnetic resonance imaging was obtained for each patient on every follow-up visit, along with liver function analysis and serum AFP level. Tumor recurrence was diagnosed based on the combined findings of these clinical examinations. Patients who developed recurrence were treated with repeat hepatic resection whenever possible, or otherwise with transcatheter arterial embolization.
All data were analyzed by the SPSS version 19.0 software (SPSS, Chicago, IL, United States). Comparisons between the two groups were done using a t-test for continuous data and the χ2 test for categorical data, with P < 0.05 considered significant. The survival curves were constructed by the Kaplan-Meier method and compared by the log-rank test, stratified by GGT and ALP, with the cutoff points determined by the receiver operating characteristic (ROC) curve analysis. Multivariate Cox regression analysis was performed to evaluate the prognostic significance of the variables in predicting overall survival (OS). Results are given as mean ± SD.
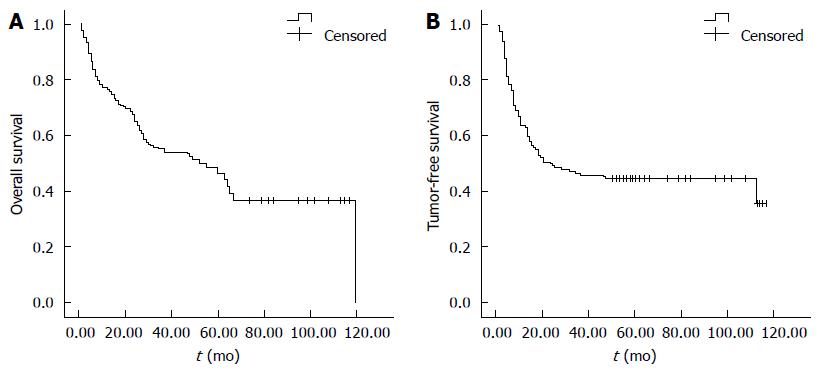
One hundred and thirty-nine patients (80.8%) were men and 33 (19.2%) women. The mean age was 53.5 years (range: 24-80 years). We were able to determine Child-Pugh classification from the available clinical records in all the enrolled patients, based on which, 160 cases were classified as Class A and 12 as Class B. No Class C patients were enrolled in this study, because Class C disease is a contraindication for hepatic resection in our department. Altogether, 87 patients died during follow-up. Of the 76 patients who developed tumor recurrence, 46 (60.5%) developed recurrence within 1 year and 69 (90.7%) within 2 years after surgery. Mean follow-up time was 2.91 years (range: 0.1-10 years). The 1-, 3- and 5-year OS for all patients included in this study were 74.1%, 54.4% and 46.6%, respectively (Figure 1).
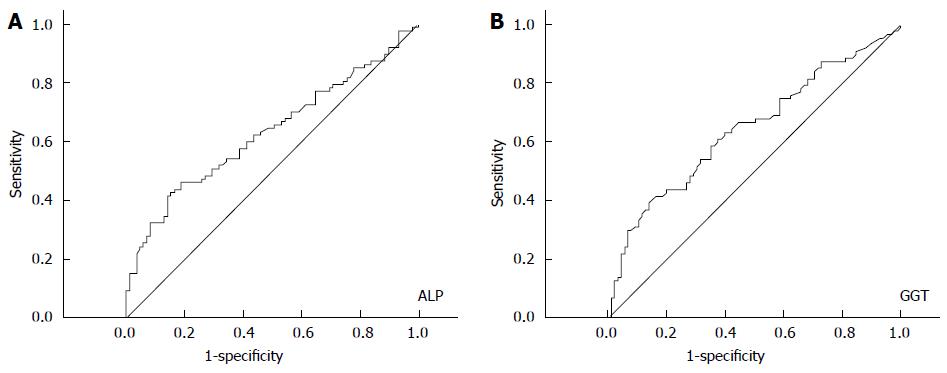
ROC curve analysis revealed an optimal cutoff of 121 U/L for ALP and 117 U/L for GGT in terms of predicting survival. As to ALP, the area under the ROC curve (AUC) was 0.631, with a 95%CI of 0.547-0.714 (Figure 2A), while the AUC for GGT was 0.643 (95%CI: 0.560-0.725) (Figure 2B). A cut-off value of 121 presented a sensitivity of 41.4% and a specificity of 85.9% for ALP, and a cut-off value of 117 presented a sensitivity of 39.1% and a specificity of 85.9% for GGT. In order to be utilized clinically, we chose a cutoff value of 120 for ALP and 115 for GGT, without significant impairment of the diagnostic accuracy of ALP and GGT.
Univariate and multivariate analysis of factors affecting OS and tumor-free survival (TFS) of HCC patients are shown in Table 1. Univariate analysis revealed that, tumor size, lymph-node metastasis, HBV infection, ALP and GGT were preoperative prognostic predictors of poor OS. Multivariate regression analysis was performed on all preoperative factors that were significant in univariate analysis, revealing tumor size, HBV infection, ALP and GGT as independent factors associated with OS (Table 1).
| Category | Subcategory | Overall survival | Tumor-free survival | ||||
| Univariate analysis | Multivariate analysis | HR (95%CI) | Univariate analysis | Multivariate analysis | HR (95%CI) | ||
| Gender | Male (139) | 0.935 | 0.916 | ||||
| Female (33) | |||||||
| Age | ≥ 60 yr (55) | 0.090 | 0.233 | ||||
| < 60 yr (117) | |||||||
| HBV | Yes (121) | 0.044 | 0.012 | 0.556 | 0.084 | ||
| No (51) | (0.353-0.878) | ||||||
| HCV | Yes (8) | 0.637 | 0.485 | ||||
| No (164) | |||||||
| Cirrhosis | Yes (59) | 0.321 | 0.141 | ||||
| No (113) | |||||||
| ALP | > 120 U/L (50) | < 0.001 | 0.008 | 1.866 | < 0.001 | 0.002 | 1.973 |
| ≤ 120 U/L(122) | (1.176-2.960) | (1.283-3.034) | |||||
| GGT | > 115 U/L (48) | < 0.001 | 0.030 | 1.674 | 0.001 | 0.676 | |
| ≤ 115 U/L (124) | (1.050-2.668) | ||||||
| AFP | ≥ 400 ng/mL (66) | 0.085 | 0.001 | 0.017 | 1.685 | ||
| < 400 ng/mL (106) | (1.099-2.583) | ||||||
| Tumor characteristics | |||||||
| Size | ≥ 5 cm (114) | < 0.001 | < 0.001 | 4.472 | < 0.001 | < 0.001 | 4.315 |
| < 5 cm (58) | (2.328-8.590) | (2.299-8.099) | |||||
| Number | > 1 (32) | 0.143 | 0.218 | ||||
| = 1 (140) | |||||||
| Invasion | Yes (54) | 0.319 | 0.379 | ||||
| No (118) | |||||||
| Lymphnode metastasis | Yes (6) | 0.001 | 0.242 | < 0.001 | 0.009 | 3.149 | |
| No (166) | (1.329-7.462) | ||||||
With regard to TFS, again, on univariate analysis, the presence of lymph-node metastasis, tumor size, ALP, GGT and AFP level were correlated with TFS. By further multivariate regression analysis, the presence of lymph-node metastasis, tumor size, ALP and AFP level were confirmed to be independent factors associated with the TFS of HCC patients.
In these preoperative factors, our multivariate analysis showed that the hazard ratio (HR) of HBV infection for OS was 0.556, which was contrary to the accepted consensus that HBV infection is a risk factor for prognosis of HCC patients, so we excluded this factor in the further analysis. We also excluded lymph-node metastasis and AFP level in the following analysis, because they were not independent factors in OS, which was more important in predicting the prognosis of HCC patients who underwent liver resection. In summary, we chose the easily accessible indices such as ALP, GGT, and tumor size as preoperative predictive factors, which were all independently associated with OS, and played important roles in the regression analysis of TFS.
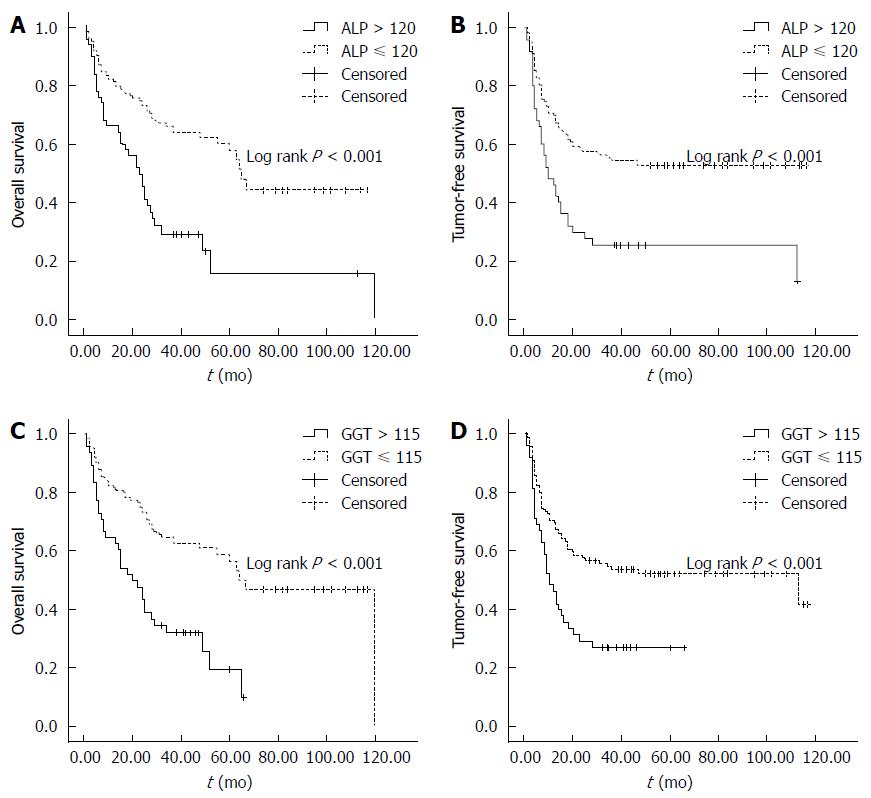
ALP was elevated at 50 in 172 patients (29.1%) and GGT was elevated at 48 in 172 patients (27.9%). For both ALP and GGT, a significant difference was observed in the OS and TFS between patients with normal and elevated levels. In terms of ALP, the 1-, 3- 5-year OS and TFS in patients with normal ALP level were 81.1%, 65.8% and 60.3%, and 70.0%, 54.1% and 52.8%, respectively, compared with 66.2%, 29.1% and 15.6% (OS) and 45.4%, 25.2% and 25.2% (TFS) in patients with elevated ALP (P < 0.05, Figure 3A, B). In terms of GGT, the 1-, 3- and 5-year OS and TFS in patients with normal GGT level were 80.7%, 61.7% and 56.4%, and 70.0%, 53.2% and 51.8%, respectively, compared with 64.5%, 32.9% and 19.1% (OS), and 45.3%, 25.9% and 25.9% (TFS) in patients with elevated GGT (P < 0.05, Figure 3C, D).
The baseline characteristics in patients with normal or elevated ALP and GGT are shown in Table 2. There were no significant differences in sex, age, HBV, HCV, cirrhosis, AFP, and tumor characteristics such as tumor number, invasion, and lymph-node metastasis between the different groups according to ALP and GGT. The only significantly different factor in both the ALP and GGT groups was tumor size (P < 0.05), which might partially explain the varied outcomes of prognosis in patients with different ALP and GGT levels.
| Factors | ALP > 120(n = 50) | ALP ≤ 120(n = 122) | P value | GGT > 115(n = 48) | GGT ≤ 115(n = 124) | P value |
| Gender | 40 | 99 | 0.835 | 40 | 99 | 0.671 |
| Age | 19 | 36 | 0.286 | 18 | 37 | 0.365 |
| HBV | 34 | 87 | 0.714 | 35 | 86 | 0.712 |
| HCV | 2 | 6 | 1.000 | 3 | 5 | 0.687 |
| Cirrhosis | 23 | 36 | 0.051 | 20 | 39 | 0.215 |
| AFP | 17 | 49 | 0.493 | 21 | 45 | 0.386 |
| Tumor characteristics | ||||||
| Size | 39 | 75 | 0.050 | 42 | 72 | < 0.001 |
| Number | 10 | 22 | 0.830 | 11 | 21 | 0.387 |
| Invasion | 9 | 45 | 0.018 | 16 | 38 | 0.719 |
| Lymphnode metastasis | 3 | 3 | 0.358 | 4 | 2 | 0.052 |
Inspired by the preoperative prognostic score published by Wang et al[14] in HCC patients who underwent transplantation, we established a preoperative model using the three preoperative factors, namely, ALP, GGT, and tumor size, which were found to be significant by multivariate regression analysis.
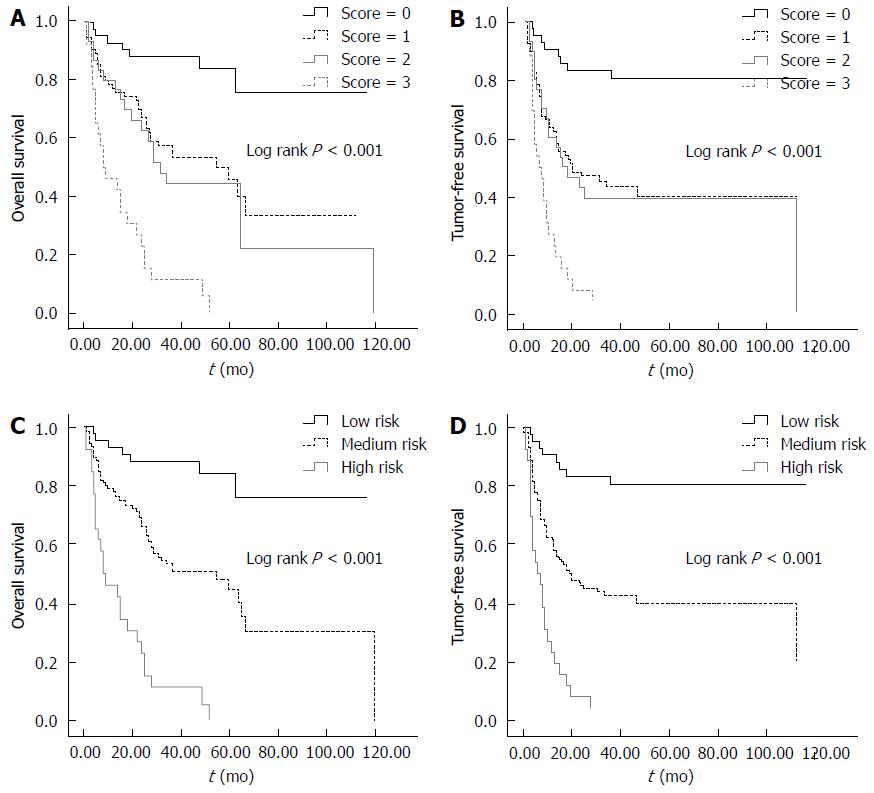
We defined each positive factor as a score of 1, and accordingly divided the patients into four groups, namely, a score of 0, 1, 2 or 3. Varied outcomes in OS and TFS stratified by different scores are shown in Figure 4. The 5-year OS for patients with a score of 0, 1, 2 or 3 was 84.0%, 45.9%, 44.1% or 0%, respectively (P < 0.05, Figure 4A). With respect to TFS, the 5-year survival for patients with a score of 0, 1, 2 or 3 was 80.6%, 40.0%, 38.8% or 0%, respectively (P < 0.05, Figure 4B). Although the OS and TFS of the patients with a score of 3 decreased sharply compared with those with a score < 3, and patients with a score of 0 had the longest survival, no significant difference was seen between the patients with a score of 1 or 2. Therefore, we combined the patients with a score of 1 and 2 as medium risk, and defined the patients with a score of 0 and 3 as low and high risk, respectively. Thus, the postoperative prognosis could be easily predicted by the Kaplan-Meier curves stratified by high, medium and low risks (P < 0.05, Figure 4C, D).
The predictive value of the prognostic scoring model, compared with the traditional prognostic markers such as AFP level, tumor size, and presence of cirrhosis, by univariate Cox proportional hazards analysis, is summarized in Table 3. The prognostic score was superior to the traditional prognostic markers of AFP and cirrhosis, and more accurate than tumor size as prognostic markers, with medium and high risks having HRs of 4.250 and 15.560, respectively.
| P value | HR | 95%CI | |
| Low risk | - | - | - |
| Middle risk | < 0.001 | 4.250 | 1.927-9.370 |
| High risk | < 0.001 | 15.56 | 6.181-39.175 |
| AFP | 0.085 | 1.454 | 0.950-2.227 |
| Cirrhosis | 0.321 | 0.790 | 0.495-1.259 |
| Tumor size | < 0.001 | 5.139 | 2.722-9.703 |
Among appropriately selected patients with HCC, liver resection provides excellent outcomes, with a 5-year survival rate of 70%[15]. However, even under strict screening, 68% of HCC patients still develop tumor recurrence in 5 years after liver resection[15]. According to our results, the 1-, 3- and 5-year OS rates for all patients included in this study were 74.1%, 54.4% and 46.6%, respectively; a little lower than the survival rates reported by Roayaie et al[15], which could be explained by the fact that patients in their study with tumor size < 2 cm. In contrast, our patients had an average tumor size of 6.95 cm. Thus, to develop novel noninvasive biomarkers for patients suitable for liver resection is urgently needed, to avoid tumor recurrence and surgical complications.
Despite the fact that several markers have been intented to guide prognosis in HCC, few were of significant prognostic value, or too inconvenient to implement clinically. Inflammatory markers have long been linked with malignancy, and Virchow first observed leukocytes in neoplastic tissue in the mid-1800s, suggesting an important role for inflammation in the development of malignancies[16]. Inflammation contributes to the development of at least 15% of all cancers, especially of the digestive system[17]. For example, patients with HBV infection experience chronic inflammation, which increases the risk of liver cancer[18].
With respect to hepatitis virus, as one of the most common etiologies of HCC, the estimated risk of HCC is 15-20-fold higher in persons infected with hepatitis virus than in uninfected persons. HBV is predominant in the east and HCV in the west, therefore, carriers of both viruses have a substantial risk of HCC-related death. However, we failed to demonstrate HBV or HCV as an independent risk factor; probably due to the development of antiviral therapy in recent years, or because of the small number of patients in our cohort.
ALP and GGT are liver enzymes that are routinely tested clinically for liver function evaluation. ALP is a hydrolase enzyme, which is present in all tissues throughout the entire body, but particularly concentrated in the liver, bile duct, kidney, bone, and placenta[19]. Clinically, as a stable serum marker, high levels of serum ALP are indicative of hepatic or bile-tract-associated disease. The ALP level also increases if bone formation occurs, because ALP is a byproduct of osteoblast activity. In addition, ALP has already been included in the Chinese University Prognostic Index, an HCC staging system that assigns a score of 3 when ALP is > 200 IU/L, indicating the potential roles of ALP in predicting the prognosis of HCC patients[20].
GGT is a nearly ubiquitous epithelial enzyme, which initiates the degradation of extracellular glutathione and its conjugates and correlates with biotransformation, nucleic acid metabolism, and tumorigenesis[21]. Moreover, elevation of serum GGT was detected in a series of clinical conditions other than hepatobiliary disorders, including pancreatic disease, myocardial infarction, renal failure, and diabetes[10]. With respect to cancer risk, significant associations have been reported between elevated GGT and increased risk of cancer. Furthermore, a previous study showed that GGT was a potential predictor in liver-specific diseases in both HBV patients and the general population in western countries[22].
However, preoperative liver function tests, specifically ALP and GGT, and their values in long-term follow-up of HCC patients have seldom been systematically explored. We extensively evaluated the association between the liver enzymes ALP and GGT and prognosis of HCC patients undergoing liver resection. We found significant elevation of ALP and GGT levels before surgery, which independently predicted prognosis in HCC patients. In addition, this effect was also significantly increased when we combined the two serum markers with tumor size, and we successfully constructed a preoperative prognostic scoring model.
In this study, we systematically explored the cut-off value of ALP and GGT by using ROC curve analysis in predicting prognosis in HCC patients. We found that the cut-off value of ALP and GGT was 121 U/L and 117 U/L, respectively, which were a little higher than the those reported by others when utilized in prediction of prognosis[23,24]. Utilizing the ALP and GGT values with relatively high sensitivity and specificity in multivariate regression analysis, our results showed that ALP, GGT and tumor size were independent prognostic predictors of poor OS and TFS. Further analysis showed patients with elevated ALP and GGT had significantly higher risks of death and tumor recurrence by Kaplan-Meier analysis, indicating the potential predictive roles of ALP and GGT in the prognosis of HCC patients undergoing liver resection. Although the specific mechanism is still unclear, there are several possible hypotheses.
Previous studies have shown that ALP is a differentiation marker for embryonic and other stem cells derived from the bone and adipose tissue. In addition, ALP was found to indicate cancer cell proliferation in nucleolar localization in an electron microscopic cytochemistry study[25]. Cancer cells showed higher ALP activity in the nucleolus and changes in localization during the cell cycle, which revealed the roles of ALP in tumor proliferation and progression, besides its common correlation with cholestasis and hepatitis. With respect to GGT, its impact on tumorigenesis might be mediated by the functions of the oxidative stress pathways in cellular responses[26]. There is extensive evidence that GGT and glutathione (GSH), the degradation of which is catalyzed by GGT, can cooperatively generate free radicals and thus lead to lipid peroxidation. On the other hand, lipid peroxidation is significantly implicated in the tumorigenesis of many malignancies including HCC, which might also partially explain the GGT-HCC association[27,28].
Although we demonstrated the prognostic roles of GGT and ALP in predicting the prognosis of HCC, however, there was no significant correlation with respect to Child score, when we explored the potential mechanisms of GGT and ALP in cancer prognosis. We speculated that it was the tumor features represented by GGT and ALP, rather than the traditional values of GGT and ALP in liver function reserve, that affect OS of HCC patients.
Based on multivariate analysis, we further established a simple prognostic model with an AUC of 0.745. When we divided the patients into different groups by giving each positive factor as a score of 1, the 5-year OS for patients with a score of 0, 1, 2 or 3 was 84.0%, 45.9%, 44.1% or 0%, respectively, while the TFS was 80.6%, 40.0%, 38.8% or 0%, respectively. Considering the similarities in prognosis of patients with scores of 1 and 2, we combined these patients into the medium-risk group, while patients with scores of 0 and 3 were allocated into the low-risk and high-risk group, respectively. On this basis, varied outcomes were significantly divided by risk groups. When compared with the traditional prognostic markers such as AFP level, tumor size and presence of cirrhosis, the predictive value of the prognostic model was significantly more accurate by univariate analysis, with HRs of the medium- and high-risk groups of 4.25 and 15.56, respectively[6,29,30].
It is worth noting that although elevated ALP and GGT might predict prognosis of HCC in some way, we should not be totally dependent on these markers. Many other factors affecting ALP and GGT, such as hepatobiliary disorders and bone formation, could impair the accuracy of prognostic prediction. In addition, HBV or HCV infection, tumor number, and lymph-node metastasis, might also affect the prognosis of HCC, although the effect was not significant in our study, probably due to the limited number of patients[31]. Thus, further studies are still needed to confirm and update our preoperative scoring model to predict the prognosis of HCC.
Although α-fetoprotein is frequently used to predict post-hepatectomy outcomes in patients with hepatocellular carcinoma (HCC), contradictory results have been reported from different studies, and the predictive accuracy was far from satisfactory. In this respect, building a scoring model combining the imaging findings and serum parameters to predict the prognosis of HCC patients undergoing liver resection is useful in choosing the best treatment.
Alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT) have long been recognized to play potential roles in the diagnosis of cancer. However, few studies have systematically explored the prognostic roles of these liver enzymes. In the current study, the authors sought to evaluate the effects of ALP and GGT on the long-term prognosis of patients with HCC undergoing liver resection.
Recent reports have highlighted the importance of ALP and GGT in cancer prognosis. The authors of the manuscript successfully demonstrated the prognostic roles of ALP and GGT in HCC. Furthermore, they combined these serum markers such as ALP and GGT, and tumor characteristics such as tumor number and tumor size, to establish a scoring model consisting of comprehensive features of tumors to better predict the prognosis of HCC.
By establishing the novel prognostic scoring model consisting of comprehensive features of tumors, this study may represent a future strategy for cancer prediction in the follow-up of patients with hepatocellular carcinoma.
Serum liver enzymes such as ALP, and GGT, are routinely tested in clinical patients. These enzymes are commonly elevated in patients with liver diseases and thus may reflect the status of liver injury. Recently, ALP and GGT have also been recognized to play potential roles in the diagnosis of cancer, highlighting the potential prognostic roles in hepatocellular carcinoma.
The goal of this clinical study was to evaluate the predictive values of alanine aminotransferase and γ-glutamyltransferase on the prognosis of patients with HCC and liver resection. To this aim, 172 HCC patients were enrolled with complete follow-up for 10-years and it was concluded that elevated ALP and GGT levels were risk predictors for this population. This is a well-conducted study and well-written paper with interesting information to the readers. The authors have provided a very even handed and documented discussion why these biochemical markers are valuable tools for addressing this important and relevant question.
P- Reviewer: Boros M, Golfieri R, Gong ZJ, Tarnawski AS S- Editor: Qi Y L- Editor: Kerr C E- Editor: Wang CH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 2. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 666] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 4991] [Article Influence: 178.3] [Reference Citation Analysis (0)] |
| 4. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1594] [Cited by in F6Publishing: 1583] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 5. | Witjes CD, Polak WG, Verhoef C, Eskens FA, Dwarkasing RS, Verheij J, de Man RA, Ijzermans JN. Increased alpha-fetoprotein serum level is predictive for survival and recurrence of hepatocellular carcinoma in non-cirrhotic livers. Dig Surg. 2012;29:522-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Shim JH, Yoon DL, Han S, Lee YJ, Lee SG, Kim KM, Lim YS, Lee HC, Chung YH, Lee YS. Is serum alpha-fetoprotein useful for predicting recurrence and mortality specific to hepatocellular carcinoma after hepatectomy? A test based on propensity scores and competing risks analysis. Ann Surg Oncol. 2012;19:3687-3696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, Tanemura M, Tomokuni A, Takemasa I, Umeshita K. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 8. | Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798-9807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 9. | Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B, Hood L, Wang H, Yang S, Gu J. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol. 2009;50:948-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 710] [Cited by in F6Publishing: 643] [Article Influence: 26.8] [Reference Citation Analysis (3)] |
| 11. | Xu K, Meng XY, Wu JW, Shen B, Shi YC, Wei Q. Diagnostic value of serum gamma-glutamyl transferase isoenzyme for hepatocellular carcinoma: a 10-year study. Am J Gastroenterol. 1992;87:991-995. [PubMed] [Cited in This Article: ] |
| 12. | Hann HW, Wan S, Myers RE, Hann RS, Xing J, Chen B, Yang H. Comprehensive analysis of common serum liver enzymes as prospective predictors of hepatocellular carcinoma in HBV patients. PLoS One. 2012;7:e47687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Lopez JB, Balasegaram M, Thambyrajah V, Timor J. The value of liver function tests in hepatocellular carcinoma. Malays J Pathol. 1996;18:95-99. [PubMed] [Cited in This Article: ] |
| 14. | Wang GY, Yang Y, Li H, Zhang J, Jiang N, Li MR, Zhu HB, Zhang Q, Chen GH. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS One. 2011;6:e25295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M, Mazzaferro V. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57:1426-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 16. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5245] [Cited by in F6Publishing: 5425] [Article Influence: 235.9] [Reference Citation Analysis (0)] |
| 17. | Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 521] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 18. | Xu C, Zhou W, Wang Y, Qiao L. Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 2014;345:216-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Weiss MJ, Ray K, Henthorn PS, Lamb B, Kadesch T, Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1988;263:12002-12010. [PubMed] [Cited in This Article: ] |
| 20. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [PubMed] [Cited in This Article: ] |
| 21. | Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 735] [Cited by in F6Publishing: 760] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 22. | Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477-485.e11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 23. | Yu MC, Chan KM, Lee CF, Lee YS, Eldeen FZ, Chou HS, Lee WC, Chen MF. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15:1440-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Zhang JB, Chen Y, Zhang B, Xie X, Zhang L, Ge N, Ren Z, Ye SL. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2011;23:787-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Yamamoto K, Awogi T, Okuyama K, Takahashi N. Nuclear localization of alkaline phosphatase in cultured human cancer cells. Med Electron Microsc. 2003;36:47-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F. Gamma-glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol. 2007;7:360-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44:1125-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 474] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 28. | Zhao J, Zhao Y, Wang H, Gu X, Ji J, Gao C. Association between metabolic abnormalities and HBV related hepatocelluar carcinoma in Chinese: a cross-sectional study. Nutr J. 2011;10:49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Faber W, Sharafi S, Stockmann M, Denecke T, Sinn B, Puhl G, Bahra M, Malinowski MB, Neuhaus P, Seehofer D. Long-term results of liver resection for hepatocellular carcinoma in noncirrhotic liver. Surgery. 2013;153:510-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Wang Q, Fiel MI, Blank S, Luan W, Kadri H, Kim KW, Manizate F, Rosenblatt AG, Labow DM, Schwartz ME. Impact of liver fibrosis on prognosis following liver resection for hepatitis B-associated hepatocellular carcinoma. Br J Cancer. 2013;109:573-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35:858-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |