Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9898
Revised: January 27, 2014
Accepted: March 12, 2014
Published online: August 7, 2014
Treatment of Helicobacter pylori (H. pylori) infection is paramount for the management of prevalent gastrointestinal disorders including peptic ulcer disease and gastric cancer. Due to the wide increase in prevalence of H. pylori resistance to antibiotics, clarithromycin-based triple therapies are not any more suitable for unconditional empiric use, and should not be recommended, unless local resistance to this antibiotic is low (< 20%). Alternative strategies have been proposed to overcome the issue of increasing clarithromycin resistance, and some of them are already implemented in clinical practice. These comprise: (1) adoption of novel, more effective, empirical treatments: bismuth quadruple, sequential, non-bismuth quadruple (concomitant), dual-concomitant (hybrid), and levofloxacin-based regimens, the latter mainly designated as second-line/rescue options; (2) perspectives for a susceptibility-guided (tailored) therapeutic approach based on culture-free molecular testing methods; and (3) adjunct use of probiotics to improve eradication rates. The present article is aimed to provide a comprehensive overview of current and emerging strategies in the treatment of H. pylori infection, focusing on the challenge of antimicrobial resistance.
Core tip: Rising clarithromycin resistance has accounted for a dramatic decline in the efficacy of standard therapies for Helicobacter pylori (H. pylori) infection. Bismuth-quadruple, sequential, non-bismuth quadruple (concomitant), dual-concomitant (hybrid), and levofloxacin-based regimens are now recommended as preferred empirical treatments (> 90% efficacy). However, empiric treatment of H. pylori is likely to become more challenging as even these improved regimens are prone to the effect of antibiotic resistance. Individualized therapy appears as a reasonable future alternative, currently limited by the shortcomings of performing culture. Advances in the genotypic characterization of H. pylori therapeutic susceptibility are likely to revolutionize our approach to tailored treatment.
-
Citation: Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of
Helicobacter pylori infection: Meeting the challenge of antimicrobial resistance. World J Gastroenterol 2014; 20(29): 9898-9911 - URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9898.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9898
Helicobacter pylori (H. pylori) is an ubiquitous human pathogen, infecting approximately one half of the world’s population and up to 80% in developing countries[1]. After colonizing the gastric mucosa, usually during childhood, H. pylori play a causal role in the development of chronic gastritis and peptic ulcer disease[2,3]. Moreover, H. pylori is a well-recognized carcinogen, primarily involved in the development of gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma (MALT)[4]. In parallel with accumulating evidence on its pathogenicity, the ability to reliably eradicate H. pylori has been established as a major step in the management of prevalent gastrointestinal disorders, including peptic ulcer disease, functional dyspepsia and low-grade MALT[2,3,5,6]. Nonetheless, eliminating the infection represents the most consistent strategy to prevent gastric cancer[3,7]. Apart from gastrointestinal disorders, extra-digestive conditions are now included as indications to treat H. pylori: idiopathic thrombocytopenic purpura, vitamin B12 deficiency and unexplained iron deficiency anemia[8]. Additional associations are emerging, including colorectal disease and cancer, ischemic heart disease and neurological disorders, although no clear therapeutic link is as yet available for these conditions[9,10].
Culturing the pathogen is a common step in the treatment of bacterial infections, but this has not been the case for H. pylori, for which treatments have been routinely prescribed empirically. This is due to the fact that performing endoscopy and H. pylori culture is neither widely available, nor well-tolerated by all patients, and furthermore it is time-consuming and costly[11]. For the last 2 decades, standard triple therapies comprising of a proton pump inhibitor (PPI) bid, amoxicillin 1000 mg bid and clarithromycin (CAM) 500 mg bid or metronidazole (MNZ) 500 mg bid, all given for 7-14 d, represented the standard of care regimens to empirically eradicate H. pylori. The high eradication rates (> 90%) provided by these treatments during the 90’s, together with their relative simplicity and optimal safety profile, have accounted for their enthusiastic acceptance in expert panels and consensus recommendations worldwide[12-15]. However, in following years, the efficacy of legacy triple regimens has been seriously challenged and eradication rates lower than 70% are now reported in many countries[16-19]. These elusive success rates preclude acceptability under Maastricht consensus [80% in intention to treat (ITT) analysis] and fall short of what it should be expected for an infectious disease, for which a 95% per protocol (PP) efficacy is warranted[20]. This will avoid exposing the patient in repeated treatment courses resulting in both multiple side effects (and therefore poor patient adherence and quality of life) and spreading of secondary antibiotic resistance. Although a series of both host- and pathogen-related factors may affect performance of legacy treatments[21-23], a worldwide increase in the levels of H. pylori resistance to antibiotics, especially that to CAM, is the most important determinant of failure of standard triple therapies[24].
The present review provides a comprehensive overview of H. pylori eradication focusing on current and emerging approaches to the issue of increasing antimicrobial resistance.
A class-wide resistance to macrolides is the result of point mutations in three adjacent nucleotide positions (A2143G, A2142G and A2142C) in the peptidyl transferase loop of the 23SrRNA gene[25,26]. Although these three point mutations account for 90% of cases of primary CAM resistance in Western countries, each of them is individually associated with different minimal inhibitory concentration (MIC) values for CAM resistance (assessed by H. pylori culture in vitro), suggesting a different impact on the determination of phenotypic CAM resistance[27]. Indeed, a lowest eradication rate (30.7%) has been observed when the phenotypic bacterial resistance was genetically linked to A2143G, suggesting this mutation, rather than the A2142G and A2142C, may significantly affect the therapeutic outcome[28]. In addition to 23SrRNA point mutations, an active multidrug efflux mechanism, responsible for rapidly transferring the drug out of the bacterial cell, is associated with the development of CAM resistance[29].
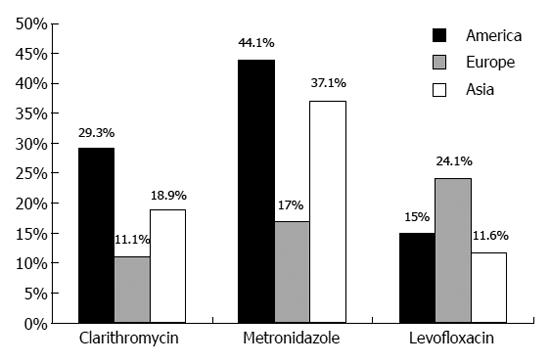
In a recent systematic review, the global incidence of primary H. pylori resistance to CAM has been reported to be as high as 17.2%, showing an increasing trend worldwide[30]. An overview of the continental (America, Europe and Asia) distribution of H. pylori antibiotic resistance is shown in Figure 1. Epidemiology of H. pylori susceptibility remains scarcely documented in Africa, with some studies suggesting extremely high rates[31] in contrast to rates as low as 1% of CAM resistance recorded by other[32]. Indiscriminate consumption of macrolides is likely the main reason for the consistent increase in CAM resistance rates[33,34]. Congruently, a positive anamnesis of respiratory tract infections was identified as an independent predictor of CAM resistance in a Bulgarian study[34], and different antibiotic consumption policies may, at least partially, explain geographical variations in H. pylori antimicrobial resistance. For instance, a 49% of CAM resistance has been reported in some Spanish areas, but only 1% in the Netherlands, reflecting a stricter Northern European policy for antibiotic use[33]. Such significant variation in the CAM resistance rates between Northern (< 10%) and Southern, or Western/Central European countries (> 20%), has been recently confirmed in a prospective assessment of H. pylori antimicrobial resistance including 18 European countries (2008-2009)[33]. In this same study (2204 patients), CAM-resistance was determined to 17.5% and was significantly associated with the use of long-acting macrolides only. Similarly, prevalence of CAM resistance has increased for 12.8% to 23.8% between 2000 and 2009 in China, whereas a consistent increase from 7% to 15.2% has been recorded in Japan[35,36]. Contrarily, prevalence of CAM resistance is still low (< 10%) in some developing countries (e.g., Bangladesh, Malaysia), and elsewhere (e.g., Sweden, Taiwan, Croatia), probably due to the low macrolide consumption or later introduction of newer macrolides in these areas[37]. However, as antibacterial resistance is a strictly local phenomenon, a wide geographic variation has been observed and a patchy distribution is possible even within the confines of a single country. For instance, resistance to CAM was found to vary from 0% to 25% among Italian regions[38]. This wide variation is clearly challenging efforts for standardization of anti-H. pylori regimens. Additional factors such as phylogeographic features of H. pylori strains may contribute to the significant geographical variation in prevalence of antibiotic resistance as well as differences in the virulence of H. pylori infection[39].
The deleterious impact of CAM resistance on the efficacy of standard treatments has been well-documented in clinical trials. In the most consistent pooled data analysis (20 studies, 1975 patients), standard treatments were only 18% effective against CAM-resistant strains[40]. In a meta-analysis by Fischbach and Evans, the success of triple therapy was decreased by 66.2% (95%CI: 58.2%-74.2%) when strains of H. pylori were resistant vs susceptible to CAM[41]. A more recent analysis revealed similar results: including antimicrobial susceptibility data from 4 randomized clinical trials (RCTs)[18,42-44], standard triple therapies successfully eradicated 88% and 14% of CAM-sensitive and CAM-resistant H. pylori strains respectively (risk difference = 0.75, 95%CI: 0.63-0.87)[45].
MNZ is another agent frequently included in regimens to eradicate H. pylori and, if so, presence of MNZ-resistance may also affect the therapeutic outcome. Mechanisms of MNZ resistance are complex and are largely associated with inactivating mutations of the rdxA and frxA genes encoding reductases which are required for the activation of MNZ[46]. However, development of MNZ resistance is known to be possible independently to these mutations, suggesting the involvement of alternative resistance mechanisms[47]. Recently, prevalence of H. pylori resistance to MNZ has been estimated to 17%-44% for Europe and America, it is highest in Africa (up to 80%-92.4%, probably due to the wide use of MNZ for parasitic infections), whereas lower rates have been reported in Japan (9%-12%)[30,40]. Contrarily, in China, an explosive increase in MNZ resistance from 23.8% to 56.6% has been recorded in the last decade[35]. It has been postulated that resistance to MNZ accounts for a drop in efficacy of up to 50% for either bismuth- and PPI-containing triple therapies[48]. However, in contrast to CAM-resistance, resistance to MNZ can be largely overcome by increasing dose and prolonging duration of therapy, thus it is generally considered less important clinically[49]. Critically, evaluation of MNZ resistance by relying on Etest® (AB bioMerieux; Solna, Sweden) is inaccurate leading to overestimation of the true levels[50]. Confirmation by using the more laborious agar dilution method is therefore required to avoid misclassifications.
Less data is available with respect to quinolone resistance. As for CAM, development of levofloxacin resistance reflects use of the drug, frequently for the treatment of urinary tract infections[34]. It involves point mutations in the quinolone resistance-determining region in the gyrase A (gyrA) gene preventing binding between the antibiotic and the enzyme[51]. Recently, prevalence of levofloxacin resistance has been reported to exceed 20% in Europe and even 15% in America (Figure 1). On the contrary, levofloxacin resistance seems to be lower in Asia (11.6%), despite there is a substantial variation from a highest of 14.9% in Japan to a lowest of 2.6% in Hong Kong[30]. Age may be a useful predictor of resistance presence, as levofloxacin resistance is less frequently encountered in subjects < 45 years old[52]. Interestingly, Dutch investigators reported a trovafloxacin resistance rate of 4.7%, in spite of the fact that this agent was not yet introduced in the Netherlands[53]. Such finding is suggestive of cross-resistance between different quinolone agents[51,54].
Fortunately, resistance to amoxicillin is exceptional and generally do not constitute a relevant clinical problem.
As outlined by the recent European Maastricht IV/Florence consensus report, standard triple therapies are not any more recommended for unconditional empiric use[8]. Instead, use of standard regimens should be adapted to the local resistance pattern (i.e., used only if local CAM resistance is < 20%), or rely on susceptibility testing provided that pre-treatment culture is available (i.e., used as tailored treatments). Alternative strategies have been proposed, and some of them are already implemented in clinical practice, aiming to overcome the problem of antimicrobial resistance. These comprise: (1) development of novel, more effective, empirical treatments; (2) perspectives for a tailored therapeutic approach based on pre-treatment determination of H. pylori therapeutic susceptibility; and (3) adjunct use of probiotics to improve eradication regimens. Each of these developments and advances in the field of H. pylori infection therapies will be discussed below.
Although based on the same key antibiotics (CAM, MNZ and levofloxacin), these improved regimens are largely validated (PP eradication > 90%) in settings of high CAM resistance and are now recommended as first-line empirical therapies. An overview of currently recommended regimens to eradicate H. pylori is shown in Table 1.
| Treatment | Regimen | Comment |
| First-line therapies | ||
| Standard triple therapy | A PPI (standard dose, bid), amoxicillin (1 g, bid) and clarithromycin (500 mg, bid) for 14 d | Widely used option |
| Only suitable for areas with < 20% incidence of cam resistance or as tailored treatment. | ||
| Bismuth-containing quadruple therapy | A PPI (standard dose, bid), bismuth (standard dose, qid) tetracycline (500 mg, qid) and metronidazole (500 mg, qid) for 10-14 d | Works independently to CAM and largely overcome MNZ resistance |
| Valuable second-line treatment after failure of CAM-based regimens | ||
| Patient-friendly monocapsule available | ||
| Suitable for patients with penicillin allergy | ||
| Non-availability of bismuth and/or tetracycline in some countries | ||
| Sequential therapy | A 5-d dual therapy with a PPI (standard dose, bid) and amoxicillin (1 g, bid) followed by a 5-d triple therapy with a PPI (standard dose, bid), clarithromycin (500 mg, bid) and metronidazole (500 mg, bid) | Widely evaluated option |
| Probably effective in high resistance settings | ||
| Questionable efficacy against double-resistant strains | ||
| Less satisfactory results in more recent studies contacted outside Italy | ||
| Non-bismuth quadruple “Concomitant” therapy | A PPI (standard dose, bid), clarithromycin (500 mg, bid), amoxicillin (1 g, bid) and metronidazole (500 mg, bid) for 10 d | Probably effective in high resistance settings |
| Larger number of pills compared to sequential and hybrid therapies | ||
| Hybrid therapy | A 7-d dual therapy with a PPI (standard dose, bid) and amoxicillin (1 g, bid) followed by a 7-d quadruple therapy with a PPI (standard dose, bid), amoxicillin (1 g, bid), clarithromycin (500 mg, bid) and metronidazole (500 mg, bid) | Probably effective in high resistance settings |
| Few data available on its efficacy/safety | ||
| Second-line/rescue therapies | ||
| Levofloxacin-based triple therapy | A PPI (standard dose, bid), levofloxacin (500 mg, bid) and amoxicillin (1 g, bid) for 10 d | Works independently to CAM and MNZ |
| Ineffective for high quinolone resistance settings (> 10%) | ||
| Rapid development of quinolone resistance | ||
| Rifabutin-based triple therapy | A PPI (standard dose, bid), rifabutin (150 mg bid) and amoxicillin (1 g bid) for 14 d | Third or more rescue option |
| Significant safety issues | ||
| Development of mycobacterium resistance | ||
Bismuth quadruple therapy (BQT) works independently to CAM and levofloxacin, i.e., the two problematic, in terms of resistance development, compounds. It is not completely novel but rather represents an enhanced evolution of the old regimen comprising a bismuth salt, tetracycline and MNZ[55], in which addition of a PPI, increase MNZ dose (1500-1600 mg/d) and prolonged treatment duration (10-14 d) are successfully limiting the impact of MNZ resistance. BQT is now designated as a preferred first-line empirical treatment achieving > 90% eradication in presence of CAM resistance and > 85% in regions with a high MNZ resistance[56]. Increased efficacy against MNZ-resistant strains, which offsets the ability of standard therapies to overcome CAM resistance, is likely the key for the improved performance with BQT[57]. To solve the issue of taking a large number of pills, a galenic three-in-one formulation (containing bismuth, MNZ and tetracycline in a single capsule) has been proposed (Pylera®; Aptalis, Mont St Hilaire, QC, Canada)[58]. Efficacy of the monocapsule formulation was addressed in two large randomized control trials (RCTs), conducted in North America[43] and Europe[18], showing ITT eradication rates of 86% and 80% respectively. Contrarily, the superiority of BQT over standard triple therapy was questioned in a recent meta-analysis, in which both treatments yielded suboptimal results (ITT eradication: 77.8% for BQT and 77% for standard therapy), although there was a significant heterogeneity among studies, especially concerning MNZ dosing[57]. BQT is also a valid, and cost-effective, rescue option after failure of CAM-based regimens. A second-line, high MNZ-dose (2 g/d) BQT regimen yielded a 90.8% PP efficacy in a Taiwanese study[59], and 93.1% was obtained in yet another Asian study using standard MNZ dose (1600 mg/d) conducted in a high MNZ-resistance setting (96.8%)[60]. In Greece, a high MZN-resistance country, 84% of second-line efficacy was recorded by using BQT[61]. In a meta-analysis, the average ITT eradication using BQT after failure of standard triple therapy was 77%, even though in 19 out of the 30 studies duration of treatment was inherently short (7 d)[62]. Notably, retrying BQT after its own failure may be worthy[63]. The potential toxicity of bismuth as well as non-availability of bismuth salts or tetracycline in some countries are the main shortcomings related to BQT. Attempts to substitute tetracycline by either amoxicillin or doxycycline yielded elusive results[64,65]. Of note, a recent meta-analysis (4763 patients) questioned a suboptimal safety of bismuth showing a comparable tolerability between bismuth-containing vs non-bismuth regimens, except from dark stools being more common in the bismuth group[66]. In a recent multicenter study from Spain, BQT was an acceptable third line option (65% in ITT and 67% in PP analysis) after two previous eradication failures with CAM- and levofloxacin-containing triple therapies. Adverse events were reported in 22% of patients, nausea (12%), abdominal pain (11%) and metallic taste (8.5%), but none of them severe[67].
Proposed by Italian investigators, sequential therapy (ST) is another novelty recommended as first-line therapy for high CAM-resistance settings[68]. It involves the same key antibiotics used in standard treatments, but given sequentially. By disrupting the bacterial wall, initial administration of amoxicillin has been suggested to prevent the development of efflux channels which rapidly transfer CAM out of the bacteria[69]. Several RCTs and meta-analyses reported on the superiority of ST over standard treatments in settings of high resistance to either CAM or MNZ[70-73]. Including 15 RCTs (3346 patients), ST displayed an overall eradication rate of 91.7% (95%CI: 90%-93%, ITT analysis) vs 76.7% (95%CI: 75%-79%) yielded by standard therapy. By pooling data from 4 studies, the efficacy of ST against CAM-resistant H. pylori strains (n = 55) was 75%[73]. However, later studies conducted outside Italy (where most of the initial trials were coming from) provided discouraging results: in a large South-American RCT[74], the probability of successful eradication was 5.6% (95%CI: -0.04%-11.6%) higher using a 14-d ST (82.2%) vs 10-d ST (76.5%). Similarly, eradication rates < 80% were shown in studies conducted in Iran and South Korea[75,76], contrarily to a RCT from Taiwan (90.7% and 87% of eradication success with a 14- and 10-d ST)[77], which however is known to be a country of low (< 20%) CAM resistance. In a very recent Asian meta-analysis (17 RCTs, 3419 participants), a 10-d ST appeared superior to legacy treatment [81.8% (95%CI: 78.9%-84.6%) vs 74.3% (95%CI: 69.6%-78.8%)], although the pooled efficacy was lower than results from earlier European studies[78]. Despite ST seems fairly effective against mono-resistant strains, a decreased efficacy against double-resistant (CAM and imidazole) H. pylori strains may compromise use of ST in high-resistance areas[79]. Including 8 studies with antibiotic susceptibility data, Gatta et al[80] analyzed the effect of antimicrobial resistance on the eradication rates provided by ST. They found that ST was able to eradicate 72.8% (range: 61.6%-82.8%) of CAM-resistant strains and 86.4% (range 78%-93%) of MNZ-resistant strains, but only 37% (range 16.2%-60.7%) of dual-resistant H. pylori strains. However, as stated by the same authors, these results should be interpreted with caution due to low number of patients with antimicrobial susceptibility (91/192/34 with CAM/MNZ/double-resistant strains treated with ST). Crucially, in this consistent meta-analysis (including 46 RCTs), a 10-d ST (overall eradication rate 84.3%, 95%CI: 82.1%-86.4%) was proven superior to 7-d triple therapy (RR = 1.21, 95%CI: 1.17-1.25), marginally superior to 10-d triple therapy (RR = 1.11, 95%CI: 1.04-1.19), but not superior to either a 14-d triple therapy (RR = 1, 95%CI: 0.94-1.06) or bismuth-based therapy (RR = 0.99, 95%CI: 0.94-1.05).
A non-bismuth quadruple therapy (NBQT), also called “concomitant” therapy, of short (3-5 d) duration was originally proposed in 1988 by German and Japanese investigators[81,82]. It returns nowadays as an effective first-line option in areas harboring high CAM resistance, but with prolonged treatment duration (10-d), as this seems a reasonable strategy to maximize cure rates at no cost in terms of safety[83]. Data on its efficacy (> or close to 90%) and safety, as well as superiority over legacy triple therapy, has been provided in several trials[84-88] and proven on a meta-analytic base. In a 2012 meta-analysis by Gisbert et al[83] (19 studies, 2070 patients), the overall cure rate provided by NBQT was 88% (95%CI: 85%-91%), increased to 91% when 3 outlier studies were excluded. Worthy of note, in many of the included trials duration of treatment was 3-5 d. However, as in a previous meta-analysis[89], the authors noted a trend toward better results with longer (7 or more days) treatments. Evaluation of 5 RCTs comparing NBQT vs standard therapy revealed superiority of the quadruple regimen with a pooled eradication difference of about 11%[89]. Crucially, a combination of extra-prolonging treatment duration (14 d) and use of a high PPI dose (omeprazole 40 mg × 2) may significantly boost cure rates as demonstrated in a non-inferiority Spanish multi-center trial were > 95% (PP) efficacy was obtained[90]. Comparison between ST and NBQT is paramount, as both treatments are relevant competitors. In a recent back to back comparison (338 patients from 11 hospitals) performed in a country with high CAM resistance (Spain), NBQT showed a non-significant advantage over ST (OR = 1.5, 95%CI: 0.9-2.8)[91]. Crucially, although both regimens seem reasonably effective against mono-resistant strains, NBQT has been suggested to be more effective against double resistance[79]. In yet another Spanish report, a 10-d concomitant treatment successfully eradicated 100% and 75% of CAM- and dual-resistant H. pylori strains vs 75% and 60% respectively with sequential therapy, although the small numbers of dual-resistant strains (4 treated with NBQT, 5 with ST) precludes drawing conclusions[92]. However, a significant clinical impact of dual resistance on the outcome of NBQT was recently showed by Georgopoulos et al[93]: including 106 patients with susceptibility testing, eradication rates were significantly higher in single CAM- and MNZ-resistant strains (100% and 91% respectively) than in dual-resistant H. pylori strains (55%), with dual antibiotic resistance remaining as the only predictor of treatment failure by multivariate analysis. Finally, in the most extensive analysis of ST compared to NBQT (evaluating a total of 6 comparative RCTs, 2070 patients), the two regimens performed comparably (81.7% vs 81.3%; respectively). However, in 2 out of 6 RCTs, NBQT lasted 5 d only and only one trial was at low risk of bias[80]. Thus, further comparative data is awaited to a definitively address the issue of concomitant vs sequential administration. Another promising first-line alternative and a relevant competitor for both ST and NBQT is a two-step hybrid (dual-concomitant) therapy. Originally proposed by Hsu et al[94], this treatment yielded high efficacy either against CAM- and dual resistant H. pylori strains, demonstrating optimal eradication rates of 97% in ITT and 99% in PP analysis. Evaluation of two RCTs[76,95] comparing hybrid (86.6%) with ST (81%) revealed no statistically significant difference[80]. Similarly, comparison with a 14-d NBQT did not show any significant difference although, interestingly, fewer treatment-related adverse events occurred in those treated with hybrid therapy[90]. Prolonged (14-d) exposure to amoxicillin is likely the key for the improved eradication with hybrid therapy. In line with this hypothesis, no benefit seems to be obtained by prolonging to 14-d duration of ST, in which amoxicillin is discontinued at midpoint[80,96].
Rising prevalence of CAM resistance has prompted use of levofloxacin, a broad spectrum quinolone agent, as a substitute for CAM. A levofloxacin-based triple therapy (LTT) has been proposed as a suitable alternative in settings with a high CAM resistance but < 10% prevalence of levofloxacin resistance achieving 72%-90% cure rates, and even > 95% has been reported provided that 500 mg of levofloxacin bid (vs 250 mg bid) are used[97-99]. Notably, a 5-d levofloxacin-based non-bismuth quadruple regimen was as effective as a 10-d duration same treatment allowing a substantial reduction in treatment costs[100]. In a study by Romano et al[101], use of levofloxacin in a 10-d sequential regimen displayed > 95% efficacy (vs 80.8% yielded by a CAM-based sequential regimen), although the low levofloxacin resistance rate in this study (3.7%) should be acknowledged. Thus, it may not be feasible to reproduce these good results in most countries, as quinolone resistance currently exceeds 40% in America, 20% in Europe and 10% in Asia[30]. Indeed, rapid development of quinolone resistance has discouraged first-line use of quinolones which are currently reserved as second-line/rescue options after failure of CAM- and/or MNZ-based regimens[102]. In 2006 two meta-analyses confirmed better second-line efficacy with LTT (cure rates 81%-87%) than BQT[103,104]. Similar efficacy (88.7%) was shown by an updated analysis including RCTs up to October 2010, whereas performance of a second-line LTT has been reported to be 74%-81% with stable efficacy over the period 2006-2011 in a Spanish study with 1000 patients[105]. By using LTT after failure of ST (second-line efficacy 75%) Italian investigators obtained a 97.8% of cumulative eradication[106], and the same cure rate (75%) has been reported when using LTT after failure of either first-line ST or NBQT[107]. An impressive 95.8% eradication (ITT, 95%CI: 87.8%-103.8%) was recently reported by using a 10-d quadruple bismuth- and levofloxacin-containing regimen after failure of ST in a small study (24 subjects)[108]. Critically, use of daily levofloxacin doses higher than 500 mg does not seem to confer any therapeutic advantage while it increases the rate of adverse events[109]. Moxifloxacin and Sitafloxacin are newer (and thus more expensive) quinolones which probably encounter less resistance problems. They have shown encouraging results in both first-line and rescue regimens, although no evidence of superiority over levofloxacin is as yet available[110-113].
Susceptibility testing is currently recommended after two consecutive treatment failures. However, when culture of H. pylori is not locally available, rifabutin (an anti-tuberculous agent active against H. pylori) may be implemented together with a PPI and amoxicillin (all bid) as a third-or-more line rescue option providing good results[114]. Using results from 11 studies (2982 patients) the mean rifabutin resistance ranged from 0.6% in treatment-naïve to 1.6% in treatment-experienced patients[115]. Overall, the eradication rates for second- (223 patients), third- (342 patients) and fourth/fifth-line (95 patients) rifabutin-based therapies were 79%, 66% and 70% respectively. Concerning optimal rifabutin dose and treatment duration, most studies recommend 300 mg/d for a total of 10-12 d of treatment. However, clinicians should bear in mind the shortcomings related to rifabutin use, including myelotoxicity and development of resistant Mycobacterium species.
The continuous rise in prevalence of antibiotic resistance is likely to bring to a deadlock today’s efforts to optimize empirical treatments. Even the novel, more effective, empirical regimens discussed above are to some (although lesser as compared to standard therapies) degree prone to the deleterious impact of increasing resistance to their individual key antibiotics. Indeed, results > 95% are infrequently achieved, and even eradication rates > 90% (PP) are disputable[74,77,116]. Tailored therapy of H. pylori (as for any other bacterial infection in which near 100% of therapeutic efficacy is obtainable) seems to represent the reasonable next step. This is currently limited by the shortcomings of systematically performing H. pylori culture which, as mentioned, is invasive, time-consuming, costly (mainly due to the associated endoscopy costs) and do not 100% reflects in vivo susceptibility[11]. However, molecular testing methods may allow for a rapid and noninvasive characterization H. pylori susceptibility to antibiotics revolutionizing our approach to tailored treatment. Nonetheless, a future refinement on tailoring H. pylori treatments may be delivered from the field of pharmacogenomics.
Due to the aforementioned limitations of systematically performing culture, this procedure is currently reserved for cases with at least two empirical treatment failures. However, this recommendation has not always yield full consensus among experts. By analyzing 5 RCTs, Wenzhen et al[117] showed that a culture-guided triple therapy was more effective and also (based on one RCT focusing on costs) more cost-effective as compared to empirical standard triple therapy. The authors concluded that culture with antimicrobial susceptibility testing should be carried out before first-line treatment. Critically, similar cost-effectiveness evaluations should include the novel BQT, ST and NBQT as, by achieving > 90% and > 80% of first- and second-line efficacy respectively, these regimens are deemed to definitively displace any debate for performing culture systematically.
Conventional methods to detect macrolide resistance are time-consuming as culture requires 3-10 d and further susceptibility testing (e.g., by Etest, AB bioMerieux; Solna, Sweden) will require additional 3-4 d. These culture-based methods can be now replaced by rapid molecular techniques relying on the measurement of the 3 point mutations in the 23SrRNA gene (namely A2143G, A2142G and A2142C) which account for 90% of cases of primary CAM resistance in Western countries[25,26]. They include a standard polymerase chain reaction (PCR) and other PCR-based methods including PCR-restriction fragment length polymorphism (RFLP), PCR-DNA enzyme immunoassay (DEIA), PCR oligonucleotide ligation assay (OLA) and PCR-line probe assay[118,119]. Real-time PCR assays, representing a powerful advancement of the basic PCR method, have been also developed and are commercially available[120]. These PCR-based methods can be directly applied on gastric specimens, offering fast and highly accurate results (reportedly > 80%-90% of both sensitivity and specificity) including detection of the heteroresistant status (defined as the co-existence of strains susceptible and resistant to the same antibiotic in the same patient), which is not detectable by conventional culture-based methods accounting for a significant rate of treatment failures[28,121,122]. Importantly, no additional gastric mucosal specimens are required as samples stored in rapid urease test, in room temperature, for up to 30 d, may be successfully used for PCR obviating the necessity to freeze specimens for off-site testing[123,124]. Genotypic detection of macrolide resistance without PCR is also possible by using fluorescence in-situ hybridization (FISH). It uses fluorescent probes specific to mutations associated with resistance and has the advantage that it can be applied on paraffin-embedded tissue samples, thus it can be offered by pathology services[122,125,126]. A great application of molecular methods is non-invasive (i.e., without need for endoscopy) determination of H. pylori susceptibility to antibiotics. Indeed, minimally-invasive techniques (including oro-gastric brushing and gastric wash) or use of stool specimens can be used to obtain H. pylori DNA for molecular testing[127-130]. Few available studies have assessed the benefits of a PCR-based tailoring of treatment for H. pylori infection. In a Japanese study, tailored treatment using a dual PPI/MNZ regimen for CAM-resistant strains yielded 94.3% eradication (100% against CAM-resistant strains) vs 71.4% using empirical standard treatment[127]. In a larger Asian RCT, 218 patients were treated with a PCR-tailored triple therapy: if a CAM-resistant strain was detected, then CAM was replaced by MNZ in the triple regimen[131]. Eradication rates were 91.2% in the tailored group vs 79.1% and 75.9% by using empirical MNZ- and CAM-based triple therapies (n = 308 in each control group) respectively (P < 0.001). A molecular approach is also available to test for levofloxacin resistance based on the detections of gyrA mutations[132]. Contrarily, there is not such availability for MNZ resistance as molecular basis of that phenomenon remain not fully understood.
Apart from bacterial susceptibility to antimicrobial agents, magnitude of the acid suppression achieved by PPI’s is critical for the treatment of H. pylori infection; thus, an ideal tailored treatment should also target to optimize the PPI’s metabolism[133,134]. PPI’s are metabolized by cytochrome P450 (CYP) 2C19 (CYP2C19) in the liver[135]. However, there is substantial genetic variability in the activity of this enzyme. Three different CYP2C19 genotypes are recognized, influencing the PPI plasma levels and consequently the eradication of H. pylori: rapid metabolizer, intermediate metabolizer and poor metabolizer, with plasma PPI levels and intragastric pH’s being lowest in the rapid metabolizer group[136]. In a landmark study, 300 H. pylori-positive patients were randomized to either a 1 week standard regimen or to personalized therapy based on both CYP2C19 and CAM susceptibility status assessed by genetic testing[137]. The ITT eradication rates were significantly higher in the tailored group (96% vs 70%) without an increase of the final per-patient cost for successful eradication. Critically, in this study lansoprazole was used, which is known to be affected by CYP2C19 status[138]. In contrast, the metabolism of other PPI’s (e.g., esomeprazole or rabeprazole) has been reported to be independent to CYP2C19 status[139,140], thus choice of these agents could probably outplace the need for CYP2C19 genotyping. Further studies are warranted to clarify the role of PPI pharmacogenomics as a basis for tailoring H. pylori eradication therapies.
Adjunct use of probiotics has attracted growing attention in recent years as a strategy aimed to both improve eradication rates and prevent occurrence of H. pylori therapy-related side-effects. Although mechanisms of a possible inhibitory effect of probiotics on H. pylori remain largely unknown, some hypothesis have been put forward including production of an inhibitory substance, competition for adhesion, strength of the mucosal barrier, and modulation of the H. pylori-related immune cascade of the host[141]. To date, several trials supported co-administration of probiotics together with standard[142], sequential[143] or levofloxacin-based regimens[144], whereas others did not[145-147]. Both single-strain (mostly Lactobacillus spp., Saccharomyces spp., Bifidobacterium spp. and B. clausii) and multistrain[146] compounds have been evaluated. Lack of placebo control, and a substantial heterogeneity in treatment duration as well as choice of different probiotic strains may have accounted for the conflicting results between studies. In a systematic review, Wilhelm et al[148] concluded that probiotics may be beneficial in reducing adverse effects and increase tolerability of H. pylori eradication regimens, particularly in cases with recurrent infection or history of gastrointestinal antibiotic-related side-effects. To date, meta-analytic evidence has been provided for using either Saccharomyces boulardi (OR = 1.13, 95%CI: 1.05-1.21) or Lactobacillus spp. (OR = 1.78, 95%CI: 1.2-2.6) supplementation adjunctively to standard triple therapy[149,150]. A recent meta-analysis (10 trials, 1469 patients) assessed the effect of Lactobacillus-containing and Bifidobacterium-containing supplementation during H. pylori therapy: the pooled odds ratio (ITT) with probiotic supplementation was 2.066 (95%CI: 1.398-3.055) for eradication and 0.305 (95%CI: 0.117-0.793) for the incidence of total side effects[151]. In conclusion, although increasing evidence supports probiotic supplementation, further studies are required to better characterize the magnitude and mechanisms of a beneficial effect of probiotics, standardize administration, and assess cost-effectiveness, as these agents are not inexpensive.
Due to the wide-spread consumption of antibiotics, H. pylori resistance to CAM is now exceeding 20% in many parts of the world. Conventional triple therapies, which for many years have represented the backbone of treating H. pylori, are not any more recommended for empiric use, and should not be prescribed, unless local CAM resistance is low or culture confirms susceptibility to CAM. More effective CAM-based regimens are now replacing standard triple therapies as empirical first-line treatments. These include the sequential, non-bismuth quadruple (concomitant) and dual-concomitant (hybrid) regimens. A bismuth-containing quadruple regimen can be effectively used either as first-line or rescue option when a CAM-based regimen fails. Rapidly growing quinolone resistance has precluded use of levofloxacin in first-line treatments. Thus, substitution of CAM with levofloxacin in triple, sequential or quadruple regimens should be reserved as a second-line/rescue option when CAM- and/or MNZ-based regimens fail. Critically, due to the steady increase in prevalence of antimicrobial resistance, empiric use of either CAM or levofloxacin may become no longer feasible in the future. Tailored treatment of H. pylori infection appears as the reasonable alternative to maintain high therapeutic efficacy, thus avoiding exposing the patient to repeated empirical antibiotic courses. A culture-based tailoring of therapy is currently recommended after at least two empirical treatment failures as it has obvious limitations, including it is invasive, time-consuming, and costly. Molecular PCR-based and FISH methods may allow for a rapid, non-invasive and highly accurate determination of H. pylori susceptibility to antibiotics, including detection of the heteroresistant status. Tailoring treatment according to the CYP2C19 status affecting the metabolism of PPIs may represent a further refinement delivered by the field of pharmacogenomics. Both practical and logistic issues should be addressed before a tailored approach based on the genotypic detection of H. pylori therapeutic susceptibility is ready for wide-spread implementation into routine practice. Until then, efforts to enhance performance of empirical treatments should continue, including use of probiotics in the therapeutic schemes.
P- Reviewer: Baryshnikova NV, Borgmann S, Yokota S S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
| 1. | Moayyedi P, Hunt RH. Helicobacter pylori public health implications. Helicobacter. 2004;9 Suppl 1:67-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 493] [Article Influence: 32.9] [Reference Citation Analysis (1)] |
| 3. | Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997;11 Suppl 1:71-88. [PubMed] [Cited in This Article: ] |
| 4. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] [Cited in This Article: ] |
| 5. | Zhao B, Zhao J, Cheng WF, Shi WJ, Liu W, Pan XL, Zhang GX. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Guo Q, Guo S, Zhang Y. Treatment of gastric MALT lymphoma with a focus on Helicobacter pylori eradication. Int J Hematol. 2013;97:735-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol. 2013;23:492-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1494] [Article Influence: 124.5] [Reference Citation Analysis (3)] |
| 9. | Banić M, Franceschi F, Babić Z, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2012;17 Suppl 1:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Georgopoulos SD, Polymeros D, Triantafyllou K, Spiliadi C, Mentis A, Karamanolis DG, Ladas SD. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion. 2006;74:42-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Gisbert JP. Is culture necessary before first-line treatment for Helicobacter pylori infection? Intern Med. 2011;50:2717; author reply 2719-2720. [PubMed] [Cited in This Article: ] |
| 12. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 819] [Cited by in F6Publishing: 792] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 13. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 14. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1286] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 15. | Malfertheiner P, Mégraud F, O’Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. [PubMed] [Cited in This Article: ] |
| 16. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [PubMed] [Cited in This Article: ] |
| 17. | Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931. [PubMed] [Cited in This Article: ] |
| 18. | Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, Mégraud F. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 19. | Vakil N, Lanza F, Schwartz H, Barth J. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20:99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Georgopoulos SD, Ladas SD, Karatapanis S, Mentis A, Spiliadi C, Artikis V, Raptis SA. Factors that may affect treatment outcome of triple Helicobacter pylori eradication therapy with omeprazole, amoxicillin, and clarithromycin. Dig Dis Sci. 2000;45:63-67. [PubMed] [Cited in This Article: ] |
| 22. | Kamada T, Haruma K, Komoto K, Mihara M, Chen X, Yoshihara M, Sumii K, Kajiyama G, Tahara K, Kawamura Y. Effect of smoking and histological gastritis severity on the rate of H. pylori eradication with omeprazole, amoxicillin, and clarithromycin. Helicobacter. 1999;4:204-210. [PubMed] [Cited in This Article: ] |
| 23. | Yang JC, Lin CJ. CYP2C19 genotypes in the pharmacokinetics/pharmacodynamics of proton pump inhibitor-based therapy of Helicobacter pylori infection. Expert Opin Drug Metab Toxicol. 2010;6:29-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Megraud F. Helicobacter pylori and antibiotic resistance. Gut. 2007;56:1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Versalovic J, Shortridge D, Kibler K, Griffy MV, Beyer J, Flamm RK, Tanaka SK, Graham DY, Go MF. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477-480. [PubMed] [Cited in This Article: ] |
| 26. | Taylor DE, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41:2621-2628. [PubMed] [Cited in This Article: ] |
| 27. | De Francesco V, Zullo A, Ierardi E, Vaira D. Minimal inhibitory concentration (MIC) values and different point mutations in the 23S rRNA gene for clarithromycin resistance in Helicobacter pylori. Dig Liver Dis. 2009;41:610-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | De Francesco V, Zullo A, Ierardi E, Giorgio F, Perna F, Hassan C, Morini S, Panella C, Vaira D. Phenotypic and genotypic Helicobacter pylori clarithromycin resistance and therapeutic outcome: benefits and limits. J Antimicrob Chemother. 2010;65:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Hirata K, Suzuki H, Nishizawa T, Tsugawa H, Muraoka H, Saito Y, Matsuzaki J, Hibi T. Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori. J Gastroenterol Hepatol. 2010;25 Suppl 1:S75-S79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409-414. [PubMed] [Cited in This Article: ] |
| 31. | Aboderin OA, Abdu AR, Odetoyin B’, Okeke IN, Lawal OO, Ndububa DA, Agbakwuru AE, Lamikanra A. Antibiotic resistance of Helicobacter pylori from patients in Ile-Ife, South-west, Nigeria. Afr Health Sci. 2007;7:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 15] [Reference Citation Analysis (0)] |
| 32. | Seck A, Burucoa C, Dia D, Mbengue M, Onambele M, Raymond J, Breurec S. Primary antibiotic resistance and associated mechanisms in Helicobacter pylori isolates from Senegalese patients. Ann Clin Microbiol Antimicrob. 2013;12:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 607] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 34. | Boyanova L, Ilieva J, Gergova G, Davidkov L, Spassova Z, Kamburov V, Katsarov N, Mitov I. Numerous risk factors for Helicobacter pylori antibiotic resistance revealed by extended anamnesis: a Bulgarian study. J Med Microbiol. 2012;61:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Iizuka Y, Fukuda K, Fujita Y, Katsurahara M, Ito T. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009;14:86-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010;8:59-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | De Francesco V, Giorgio F, Ierardi E, Zotti M, Neri M, Milano A, Varasano V, Luzza F, Suraci E, Marmo R. Primary clarithromycin resistance in Helicobacter pylori: the Multicentric Italian Clarithromycin Resistance Observational (MICRO) study. J Gastrointestin Liver Dis. 2011;20:235-239. [PubMed] [Cited in This Article: ] |
| 39. | Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077-1083. [PubMed] [Cited in This Article: ] |
| 40. | Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 648] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 41. | Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 42. | Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002;123:1763-1769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562-567. [PubMed] [Cited in This Article: ] |
| 44. | Zheng Q, Chen WJ, Lu H, Sun QJ, Xiao SD. Comparison of the efficacy of triple versus quadruple therapy on the eradication of Helicobacter pylori and antibiotic resistance. J Dig Dis. 2010;11:313-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Gerrits MM, van der Wouden EJ, Bax DA, van Zwet AA, van Vliet AH, de Jong A, Kusters JG, Thijs JC, Kuipers EJ. Role of the rdxA and frxA genes in oxygen-dependent metronidazole resistance of Helicobacter pylori. J Med Microbiol. 2004;53:1123-1128. [PubMed] [Cited in This Article: ] |
| 47. | Bereswill S, Krainick C, Stähler F, Herrmann L, Kist M. Analysis of the rdxA gene in high-level metronidazole-resistant clinical isolates confirms a limited use of rdxA mutations as a marker for prediction of metronidazole resistance in Helicobacter pylori. FEMS Immunol Med Microbiol. 2003;36:193-198. [PubMed] [Cited in This Article: ] |
| 48. | Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy--the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047-1055. [PubMed] [Cited in This Article: ] |
| 49. | Graham DY, Qureshi WA. Antibiotic-resistant H. pylori infection and its treatment. Curr Pharm Des. 2000;6:1537-1544. [PubMed] [Cited in This Article: ] |
| 50. | Osato MS, Reddy R, Reddy SG, Penland RL, Graham DY. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int J Antimicrob Agents. 2001;17:39-44. [PubMed] [Cited in This Article: ] |
| 51. | Wang LH, Cheng H, Hu FL, Li J. Distribution of gyrA mutations in fluoroquinolone-resistant Helicobacter pylori strains. World J Gastroenterol. 2010;16:2272-2277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 44] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | O’Connor A, Taneike I, Nami A, Fitzgerald N, Ryan B, Breslin N, O’Connor H, McNamara D, Murphy P, O’Morain C. Helicobacter pylori resistance rates for levofloxacin, tetracycline and rifabutin among Irish isolates at a reference centre. Ir J Med Sci. 2013;182:693-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Debets-Ossenkopp YJ, Herscheid AJ, Pot RG, Kuipers EJ, Kusters JG, Vandenbroucke-Grauls CM. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxycillin, tetracycline and trovafloxacin in The Netherlands. J Antimicrob Chemother. 1999;43:511-515. [PubMed] [Cited in This Article: ] |
| 54. | Bogaerts P, Berhin C, Nizet H, Glupczynski Y. Prevalence and mechanisms of resistance to fluoroquinolones in Helicobacter pylori strains from patients living in Belgium. Helicobacter. 2006;11:441-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Borody TJ, Carrick J, Hazell SL. Symptoms improve after the eradication of gastric Campylobacter pyloridis. Med J Aust. 1987;146:450-451. [PubMed] [Cited in This Article: ] |
| 56. | Fischbach LA, van Zanten S, Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004;20:1071-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 58. | de Boer WA. A novel therapeutic approach for Helicobacter pylori infection: the bismuth-based triple therapy monocapsule. Expert Opin Investig Drugs. 2001;10:1559-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Kuo CH, Hsu PI, Kuo FC, Wang SS, Hu HM, Liu CJ, Chuah SK, Chen YH, Hsieh MC, Wu DC. Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line Helicobacter pylori therapy: a randomized controlled trial. J Antimicrob Chemother. 2013;68:222-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, Xiao S, Lu H. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol. 2013;11:802-807.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 61. | Georgopoulos SD, Ladas SD, Karatapanis S, Triantafyllou K, Spiliadi C, Mentis A, Artikis V, Raptis SA. Effectiveness of two quadruple, tetracycline- or clarithromycin-containing, second-line, Helicobacter pylori eradication therapies. Aliment Pharmacol Ther. 2002;16:569-575. [PubMed] [Cited in This Article: ] |
| 62. | Gisbert JP. Rescue Therapy for Helicobacter pylori Infection 2012. Gastroenterol Res Pract. 2012;2012:974594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Lee SK, Lee SW, Park JY, Kwon BS, Kim SY, Hyun JJ, Kim JH, Jung SW, Koo JS, Yim HJ. Effectiveness and safety of repeated quadruple therapy in Helicobacter pylori infection after failure of second-line quadruple therapy: repeated quadruple therapy as a third-line therapy. Helicobacter. 2011;16:410-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Garcia N, Calvet X, Gené E, Campo R, Brullet E. Limited usefulness of a seven-day twice-a-day quadruple therapy. Eur J Gastroenterol Hepatol. 2000;12:1315-1318. [PubMed] [Cited in This Article: ] |
| 65. | Perri F, Festa V, Merla A, Quitadamo M, Clemente R, Andriulli A. Amoxicillin/tetracycline combinations are inadequate as alternative therapies for Helicobacter pylori infection. Helicobacter. 2002;7:99-104. [PubMed] [Cited in This Article: ] |
| 66. | Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol. 2008;14:7361-7370. [PubMed] [Cited in This Article: ] |
| 67. | Gisbert JP, Perez-Aisa A, Rodrigo L, Molina-Infante J, Modolell I, Bermejo F, Castro-Fernández M, Antón R, Sacristán B, Cosme A. Third-line rescue therapy with bismuth-containing quadruple regimen after failure of two treatments (with clarithromycin and levofloxacin) for H. pylori infection. Dig Dis Sci. 2014;59:383-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Zullo A, Vaira D, Vakil N, Hassan C, Gatta L, Ricci C, De Francesco V, Menegatti M, Tampieri A, Perna F. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther. 2003;17:719-726. [PubMed] [Cited in This Article: ] |
| 69. | Webber MA, Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother. 2003;51:9-11. [PubMed] [Cited in This Article: ] |
| 70. | Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069-3079; quiz 1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 71. | Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a meta-analysis. J Clin Pharm Ther. 2009;34:41-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353-1357. [PubMed] [Cited in This Article: ] |
| 73. | Gisbert JP, Calvet X, O’Connor A, Mégraud F, O’Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 2010;44:313-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 74. | Greenberg ER, Anderson GL, Morgan DR, Torres J, Chey WD, Bravo LE, Dominguez RL, Ferreccio C, Herrero R, Lazcano-Ponce EC. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011;378:507-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 75. | Lim JH, Lee DH, Choi C, Lee ST, Kim N, Jeong SH, Kim JW, Hwang JH, Park YS, Lee SH. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter. 2013;18:180-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013;18:129-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 77. | Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, Lee JY, Hsu SJ, Luo JC, Chang WH. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 78. | Yoon H, Lee DH, Kim N, Park YS, Shin CM, Kang KK, Oh DH, Jang DK, Chung JW. Meta-analysis: is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013;28:1801-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, Wang SS, Chen A, Hung WC, Graham DY. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36-41.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 80. | Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 193] [Article Influence: 17.5] [Reference Citation Analysis (95)] |
| 81. | Okada M, Oki K, Shirotani T, Seo M, Okabe N, Maeda K, Nishimura H, Ohkuma K, Oda K. A new quadruple therapy for the eradication of Helicobacter pylori. Effect of pretreatment with omeprazole on the cure rate. J Gastroenterol. 1998;33:640-645. [PubMed] [Cited in This Article: ] |
| 82. | Treiber G, Ammon S, Schneider E, Klotz U. Amoxicillin/metronidazole/omeprazole/clarithromycin: a new, short quadruple therapy for Helicobacter pylori eradication. Helicobacter. 1998;3:54-58. [PubMed] [Cited in This Article: ] |
| 83. | Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol. 2012;5:23-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 84. | Georgopoulos S, Papastergiou V, Xirouchakis E, Laoudi F, Lisgos P, Spiliadi C, Papantoniou N, Karatapanis S. Nonbismuth quadruple “concomitant” therapy versus standard triple therapy, both of the duration of 10 days, for first-line H. pylori eradication: a randomized trial. J Clin Gastroenterol. 2013;47:228-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 85. | Georgopoulos S, Papastergiou V, Xirouchakis E, Laudi F, Papantoniou N, Lisgos P, Spiliadi C, Fragou P, Skorda L, Karatapanis S. Evaluation of a four-drug, three-antibiotic, nonbismuth-containing “concomitant” therapy as first-line Helicobacter pylori eradication regimen in Greece. Helicobacter. 2012;17:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Kim SY, Lee SW, Hyun JJ, Jung SW, Koo JS, Yim HJ, Park JJ, Chun HJ, Choi JH. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple “concomitant” therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013;47:21-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Nagahara A, Miwa H, Ogawa K, Kurosawa A, Ohkura R, Iida N, Sato N. Addition of metronidazole to rabeprazole-amoxicillin-clarithromycin regimen for Helicobacter pylori infection provides an excellent cure rate with five-day therapy. Helicobacter. 2000;5:88-93. [PubMed] [Cited in This Article: ] |
| 88. | Nagahara A, Miwa H, Yamada T, Kurosawa A, Ohkura R, Sato N. Five-day proton pump inhibitor-based quadruple therapy regimen is more effective than 7-day triple therapy regimen for Helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15:417-421. [PubMed] [Cited in This Article: ] |
| 89. | Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 90. | Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145:121-128.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 91. | McNicholl AG, Marin AC, Molina-Infante J, Castro M, Barrio J, Ducons J, Calvet X, de la Coba C, Montoro M, Bory F. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut. 2014;63:244-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 92. | Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, Perez-Gallardo B, Dueñas-Sadornil C, Hernandez-Alonso M, Gonzalez-Garcia G, Mateos-Rodriguez JM, Fernandez-Bermejo M, Gisbert JP. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter. 2012;17:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 93. | Georgopoulos SD, Xirouchakis E, Martinez-Gonzalez B, Sgouras DN, Spiliadi C, Mentis AF, Laoudi F. Clinical evaluation of a ten-day regimen with esomeprazole, metronidazole, amoxicillin, and clarithromycin for the eradication of Helicobacter pylori in a high clarithromycin resistance area. Helicobacter. 2013;18:459-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 95. | Zullo A, Scaccianoce G, De Francesco V, Ruggiero V, D’Ambrosio P, Castorani L, Bonfrate L, Vannella L, Hassan C, Portincasa P. Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol. 2013;37:647-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | Hsu PI, Wu DC, Wu JY, Graham DY. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter. 2011;16:146-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Berning M, Krasz S, Miehlke S. Should quinolones come first in Helicobacter pylori therapy? Therap Adv Gastroenterol. 2011;4:103-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Di Caro S, Fini L, Daoud Y, Grizzi F, Gasbarrini A, De Lorenzo A, Di Renzo L, McCartney S, Bloom S. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastroenterol. 2012;18:5669-5678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Zullo A, De Francesco V, Hassan C, Ridola L, Repici A, Bruzzese V, Vaira D. Modified sequential therapy regimens for Helicobacter pylori eradication: a systematic review. Dig Liver Dis. 2013;45:18-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Federico A, Nardone G, Gravina AG, Iovene MR, Miranda A, Compare D, Pilloni PA, Rocco A, Ricciardiello L, Marmo R. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology. 2012;143:55-61.e1; quize e13-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | Romano M, Cuomo A, Gravina AG, Miranda A, Iovene MR, Tiso A, Sica M, Rocco A, Salerno R, Marmo R. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut. 2010;59:1465-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 102. | Carothers JJ, Bruce MG, Hennessy TW, Bensler M, Morris JM, Reasonover AL, Hurlburt DA, Parkinson AJ, Coleman JM, McMahon BJ. The relationship between previous fluoroquinolone use and levofloxacin resistance in Helicobacter pylori infection. Clin Infect Dis. 2007;44:e5-e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 104. | Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol. 2006;101:488-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Gisbert JP, Pérez-Aisa A, Bermejo F, Castro-Fernández M, Almela P, Barrio J, Cosme A, Modolell I, Bory F, Fernández-Bermejo M. Second-line therapy with levofloxacin after failure of treatment to eradicate helicobacter pylori infection: time trends in a Spanish Multicenter Study of 1000 patients. J Clin Gastroenterol. 2013;47:130-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Manfredi M, Bizzarri B, de’Angelis GL. Helicobacter pylori infection: sequential therapy followed by levofloxacin-containing triple therapy provides a good cumulative eradication rate. Helicobacter. 2012;17:246-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 107. | Gisbert JP, Molina-Infante J, Marin AC, Vinagre G, Barrio J, McNicholl AG. Second-line rescue triple therapy with levofloxacin after failure of non-bismuth quadruple “sequential” or “concomitant” treatment to eradicate H. pylori infection. Scand J Gastroenterol. 2013;48:652-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 108. | Hsu PI, Chen WC, Tsay FW, Shih CA, Kao SS, Wang HM, Yu HC, Lai KH, Tseng HH, Peng NJ. Ten-day Quadruple therapy comprising proton-pump inhibitor, bismuth, tetracycline, and levofloxacin achieves a high eradication rate for Helicobacter pylori infection after failure of sequential therapy. Helicobacter. 2014;19:74-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Di Caro S, Franceschi F, Mariani A, Thompson F, Raimondo D, Masci E, Testoni A, La Rocca E, Gasbarrini A. Second-line levofloxacin-based triple schemes for Helicobacter pylori eradication. Dig Liver Dis. 2009;41:480-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | Wenzhen Y, Kehu Y, Bin M, Yumin L, Quanlin G, Donghai W, Lijuan Y. Moxifloxacin-based triple therapy versus clarithromycin-based triple therapy for first-line treatment of Helicobacter pylori infection: a meta-analysis of randomized controlled trials. Intern Med. 2009;48:2069-2076. [PubMed] [Cited in This Article: ] |
| 111. | Wu C, Chen X, Liu J, Li MY, Zhang ZQ, Wang ZQ. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011;16:131-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 112. | Murakami K, Okimoto T, Kodama M, Tanahashi J, Fujioka T, Ikeda F, Muraoka H, Takigawa M, Saika T, Hasegawa M. Sitafloxacin activity against Helicobacter pylori isolates, including those with gyrA mutations. Antimicrob Agents Chemother. 2009;53:3097-3099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Suzuki H, Nishizawa T, Muraoka H, Hibi T. Sitafloxacin and garenoxacin may overcome the antibiotic resistance of Helicobacter pylori with gyrA mutation. Antimicrob Agents Chemother. 2009;53:1720-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 114. | Gisbert JP, Castro-Fernandez M, Perez-Aisa A, Cosme A, Molina-Infante J, Rodrigo L, Modolell I, Cabriada JL, Gisbert JL, Lamas E. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012;35:941-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 115. | Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35:209-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 116. | Vakil N, Vaira D. Treatment for H. pylori infection: new challenges with antimicrobial resistance. J Clin Gastroenterol. 2013;47:383-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 117. | Wenzhen Y, Yumin L, Quanlin G, Kehu Y, Lei J, Donghai W, Lijuan Y. Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern Med. 2010;49:1103-1109. [PubMed] [Cited in This Article: ] |
| 118. | Lehours P, Siffré E, Mégraud F. DPO multiplex PCR as an alternative to culture and susceptibility testing to detect Helicobacter pylori and its resistance to clarithromycin. BMC Gastroenterol. 2011;11:112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 119. | Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 459] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 120. | Schabereiter-Gurtner C, Hirschl AM, Dragosics B, Hufnagl P, Puz S, Kovách Z, Rotter M, Makristathis A. Novel real-time PCR assay for detection of Helicobacter pylori infection and simultaneous clarithromycin susceptibility testing of stool and biopsy specimens. J Clin Microbiol. 2004;42:4512-4518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 121. | Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 122. | Morris JM, Reasonover AL, Bruce MG, Bruden DL, McMahon BJ, Sacco FD, Berg DE, Parkinson AJ. Evaluation of seaFAST, a rapid fluorescent in situ hybridization test, for detection of Helicobacter pylori and resistance to clarithromycin in paraffin-embedded biopsy sections. J Clin Microbiol. 2005;43:3494-3496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 123. | Furuta T, Sagehashi Y, Shirai N, Sugimoto M, Nakamura A, Kodaira M, Kenmotsu K, Nagano M, Egashira T, Ueda K. Influence of CYP2C19 polymorphism and Helicobacter pylori genotype determined from gastric tissue samples on response to triple therapy for H pylori infection. Clin Gastroenterol Hepatol. 2005;3:564-573. [PubMed] [Cited in This Article: ] |
| 124. | Li Y, Rimbara E, Thirumurthi S, Trespalacios A, Reddy R, Sabounchi S, Attumi TA, Graham DY. Detection of clarithromycin resistance in Helicobacter pylori following noncryogenic storage of rapid urease tests for 30 days. J Dig Dis. 2012;13:54-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | Yilmaz O, Demiray E. Clinical role and importance of fluorescence in situ hybridization method in diagnosis of H pylori infection and determination of clarithromycin resistance in H pylori eradication therapy. World J Gastroenterol. 2007;13:671-675. [PubMed] [Cited in This Article: ] |
| 126. | Yilmaz O, Demiray E, Tümer S, Altungöz O, Yörükoğlu K, Soytürk M, Simşek I. Detection of Helicobacter pylori and determination of clarithromycin susceptibility using formalin-fixed, paraffin-embedded gastric biopsy specimens by fluorescence in situ hybridization. Helicobacter. 2007;12:136-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Kawai T, Yamagishi T, Yagi K, Kataoka M, Kawakami K, Sofuni A, Itoi T, Sakai Y, Moriyasu F, Osaka Y. Tailored eradication therapy based on fecal Helicobacter pylori clarithromycin sensitivities. J Gastroenterol Hepatol. 2008;23 Suppl 2:S171-S174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 128. | Graham DY, Kudo M, Reddy R, Opekun AR. Practical rapid, minimally invasive, reliable nonendoscopic method to obtain Helicobacter pylori for culture. Helicobacter. 2005;10:1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 129. | Baba S, Oishi Y, Watanabe Y, Oikawa R, Morita R, Yoshida Y, Hiraishi T, Maehata T, Nagase Y, Fukuda Y. Gastric wash-based molecular testing for antibiotic resistance in Helicobacter pylori. Digestion. 2011;84:299-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Lottspeich C, Schwarzer A, Panthel K, Koletzko S, Rüssmann H. Evaluation of the novel Helicobacter pylori ClariRes real-time PCR assay for detection and clarithromycin susceptibility testing of H. pylori in stool specimens from symptomatic children. J Clin Microbiol. 2007;45:1718-1722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 131. | Lee HJ, Kim JI, Cheung DY, Kim TH, Jun EJ, Oh JH, Chung WC, Kim BW, Kim SS, Park SH. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 2013;208:1123-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 132. | Cambau E, Allerheiligen V, Coulon C, Corbel C, Lascols C, Deforges L, Soussy CJ, Delchier JC, Megraud F. Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori. J Clin Microbiol. 2009;47:3600-3607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 133. | Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idström JP, Cederberg C, Spiller RC. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111:358-367. [PubMed] [Cited in This Article: ] |
| 134. | Grayson ML, Eliopoulos GM, Ferraro MJ, Moellering RC. Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1989;8:888-889. [PubMed] [Cited in This Article: ] |
| 135. | Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 549] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 136. | Furuta T, Shirai N, Takashima M, Xiao F, Hanai H, Sugimura H, Ohashi K, Ishizaki T, Kaneko E. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001;69:158-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 137. | Furuta T, Shirai N, Kodaira M, Sugimoto M, Nogaki A, Kuriyama S, Iwaizumi M, Yamade M, Terakawa I, Ohashi K. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007;81:521-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 138. | Zhao F, Wang J, Yang Y, Wang X, Shi R, Xu Z, Huang Z, Zhang G. Effect of CYP2C19 genetic polymorphisms on the efficacy of proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: a meta-analysis. Helicobacter. 2008;13:532-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 139. | Lee VW, Chau TS, Chan AK, Lee KK, Waye MM, Ling TK, Chan FK. Pharmacogenetics of esomeprazole or rabeprazole-based triple therapy in Helicobacter pylori eradication in Hong Kong non-ulcer dyspepsia Chinese subjects. J Clin Pharm Ther. 2010;35:343-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Pan X, Li Y, Qiu Y, Tang Q, Qian B, Yao L, Shi R, Zhang G. Efficacy and tolerability of first-line triple therapy with levofloxacin and amoxicillin plus esomeprazole or rabeprazole for the eradication of Helicobacter pylori infection and the effect of CYP2C19 genotype: a 1-week, randomized, open-label study in Chinese adults. Clin Ther. 2010;32:2003-2011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 141. | Lesbros-Pantoflickova D, Corthésy-Theulaz I, Blum AL. Helicobacter pylori and probiotics. J Nutr. 2007;137:812S-818S. [PubMed] [Cited in This Article: ] |
| 142. | Du YQ, Su T, Fan JG, Lu YX, Zheng P, Li XH, Guo CY, Xu P, Gong YF, Li ZS. Adjuvant probiotics improve the eradication effect of triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012;18:6302-6307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 54] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 143. | Efrati C, Nicolini G, Cannaviello C, O’Sed NP, Valabrega S. Helicobacter pylori eradication: sequential therapy and Lactobacillus reuteri supplementation. World J Gastroenterol. 2012;18:6250-6254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 144. | Ojetti V, Bruno G, Ainora ME, Gigante G, Rizzo G, Roccarina D, Gasbarrini A. Impact of Lactobacillus reuteri Supplementation on Anti-Helicobacter pylori Levofloxacin-Based Second-Line Therapy. Gastroenterol Res Pract. 2012;2012:740381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 145. | Manfredi M, Bizzarri B, Sacchero RI, Maccari S, Calabrese L, Fabbian F, De’Angelis GL. Helicobacter pylori infection in clinical practice: probiotics and a combination of probiotics + lactoferrin improve compliance, but not eradication, in sequential therapy. Helicobacter. 2012;17:254-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 146. | Shavakhi A, Tabesh E, Yaghoutkar A, Hashemi H, Tabesh F, Khodadoostan M, Minakari M, Shavakhi S, Gholamrezaei A. The effects of multistrain probiotic compound on bismuth-containing quadruple therapy for Helicobacter pylori infection: a randomized placebo-controlled triple-blind study. Helicobacter. 2013;18:280-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 147. | Navarro-Rodriguez T, Silva FM, Barbuti RC, Mattar R, Moraes-Filho JP, de Oliveira MN, Bogsan CS, Chinzon D, Eisig JN. Association of a probiotic to a Helicobacter pylori eradication regimen does not increase efficacy or decreases the adverse effects of the treatment: a prospective, randomized, double-blind, placebo-controlled study. BMC Gastroenterol. 2013;13:56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 148. | Wilhelm SM, Johnson JL, Kale-Pradhan PB. Treating bugs with bugs: the role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother. 2011;45:960-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. | Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 150. | Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 151. | Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |