Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9384
Revised: January 10, 2014
Accepted: February 17, 2014
Published online: July 28, 2014
A number of preclinical studies have demonstrated anticancer effects for curcumin in various types of tumors, including pancreatic cancer. Curcumin has anticancer effects both alone and in combination with other anticancer drugs (e.g., gemcitabine, 5-fluorouracil, and oxaliplatin), and it has been shown to modulate a variety of molecular targets in preclinical models, with more than 30 molecular targets identified to date. Of these various molecules, NF-κB is thought to be one of the primary targets of curcumin activity. Based on these promising preclinical results, several research groups, including our own, have progressed to testing the anticancer effects of curcumin in clinical trials; however, the poor bioavailability of this agent has been the major challenge for its clinical application. Despite the ingestion of gram-level doses of curcumin, plasma curcumin levels remain at low (ng/mL) levels in patients, which is insufficient to yield the anticancer benefits of curcumin. This problem has been solved by the development of highly bioavailable forms of curcumin (THERACURMIN®), and higher plasma curcumin levels can now be achieved without increased toxicity in patients with pancreatic cancer. In this article, we review possible therapeutic applications of curcumin in patients with pancreatic cancer.
Core tip: A growing body of evidence supports the idea that curcumin is a promising anticancer drug. Curcumin has anticancer effects, both alone and in combination with other anticancer drugs, through the modulation of a variety of molecular targets in preclinical models. However, the poor bioavailability of curcumin has been the major challenge to its clinical application. This problem has been overcome by the development of highly bioavailable forms of curcumin (THERACURMIN®), and higher plasma curcumin levels can now be achieved without increased toxicity. Further clinical trials will be necessary to test the therapeutic applications of this promising agent in patients with pancreatic cancer.
- Citation: Kanai M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J Gastroenterol 2014; 20(28): 9384-9391
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9384.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9384
Pancreatic cancer is one of the most lethal malignancies worldwide[1], and the majority of patients are diagnosed too late for curative resection. Even in patients who have undergone curative resection, the disease relapse rate within 2 years is greater than 80%[2]. Systemic gemcitabine-based chemotherapy has been a standard therapy for patients with advanced pancreatic cancer since 1997, when a randomized phase III study demonstrated that gemcitabine monotherapy significantly improved cancer-related symptoms compared with 5-fluorouracil[3]. Over the past decade, many efforts have been made to improve the overall survival of patients with this disease by combining gemcitabine with a second cytotoxic agent. However, most of these gemcitabine combination therapies have failed to show significant survival advantages over gemcitabine monotherapy[4-11]. Therefore, novel approaches - other than simply adding additional cytotoxic agents to gemcitabine - are warranted. In addition, it is important to consider the balance between efficacy and quality of life when choosing a palliative chemotherapy, as patients with pancreatic cancer often suffer from cancer-related symptoms, such as fatigue, appetite loss, and pain.
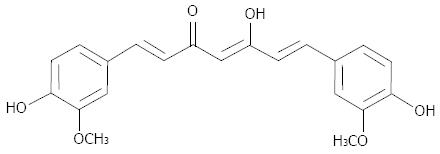
Curcumin is a natural polyphenol compound derived from turmeric (Curcuma longa). Constituting 1%-5% of turmeric preparations, curcumin has a molecular weight of 368.37 and the molecular formula C21H20O6 (Figure 1). Curcumin has long been used as a food (e.g., in the popular Indian curry), a coloring agent and in traditional medicine[12,13]. A number of preclinical studies have demonstrated that curcumin has anticancer effects against a variety of tumors, including pancreatic cancer, both in vitro and in vivo[14-32]. These promising results have attracted the interest of many researchers hoping to develop this agent as a chemopreventive as well as a chemotherapeutic drug[33,34]. In contrast with conventional cytotoxic drugs - which often have side effects such as nausea, vomiting or fatigue - curcumin has minimal toxicity. This is a great advantage when treating patients with pancreatic cancer, who generally show poor tolerance to intensive therapy due to their poor clinical conditions. Safety is another advantage of this agent. The safety of curcumin has been approved by the Food and Drug Administration and World Health Organization; In addition, its safety is strongly supported by the fact that this agent has been used in traditional Hindu and Chinese medicine for thousands of years.
In this article, we review possible therapeutic applications of curcumin for the treatment of patients with pancreatic cancer.
A PubMed search using the key words ‘‘curcumin’’ and ‘‘cancer’’ reveals that over 2000 articles have been published on this topic since 1983, with that number increasing rapidly year after year. Numerous preclinical studies have demonstrated anticancer effects for curcumin against not only pancreatic cancer[14,17,22,24,26-28,32,35] but also a variety of other malignancies, including breast[21], colon[23,29], gastric[30], head and neck[25], hepatic[15], ovarian[20], lung[31]and prostate cancers[19], as well as lymphoma and leukemia[16,18].
Li et al[14] were the first to report the anticancer effects of curcumin against pancreatic cancer cells. They demonstrated that curcumin can suppress tumor growth in pancreatic cancer cell lines in a time- and dose-dependent manner by inhibiting nuclear transcription factor-kappa B (NF-κB). The efficacy of curcumin has also been demonstrated using an orthotopic mouse model of pancreatic cancer[36]. Although treatment with either curcumin (1 g/kg orally) or gemcitabine (25 mg/kg via intraperitoneal injection) had modest antitumor effects, the combination of curcumin and gemcitabine suppressed tumor growth more effectively than either agent alone. In addition to gemcitabine, curcumin has also been shown to potentiate the effects of other cytotoxic agents, including cisplatin, oxaliplatin, and 5-fluorouracil, in preclinical models[25,29,37].
Curcumin can modulate the activity of a variety of molecules that play important roles in cancer progression, with more than 30 molecular targets identified to date[38]. Of these molecules, NF-κB appears to be one of the primary targets of curcumin[14,27,36]. Interestingly, recent studies have demonstrated that changes in microRNA (miRNA) expression levels following treatment with curcumin or a curcumin analog are involved in the anticancer effects of these agents[28,39]. For example, curcumin can upregulate the expression of miR-200[28], which plays important roles in regulating the epithelial-to-mesenchymal transition (EMT) and cancer progression[40]. Conversely, curcumin can downregulate the expression of miR-21[28], which is overexpressed in a variety of tumors, including pancreatic cancer, and is considered to be an oncogenic miRNA[41]. Representative preclinical studies of the anticancer effects of curcumin against pancreatic cancer are summarized in Table 1.
| Reported molecular targets | Curcumin dose required for the reported effects | |
| in vitro(μmol/L) | in vivo | |
| NF-κB↓ (Ref. 14) | ≥ 5.4 | NA |
| NF-κB↓, cyclin-D1↓ | ≥ 25 | 1 g/kg per day, po |
| c-myc↓, Bcl-2↓ | ||
| Bcl-xL↓, cIAP-1↓ | ||
| MMP↓, COX2↓ | ||
| VEGF↓ (Ref. 36) | ||
| NF-κB↓, Sp-1, Sp-3, Sp4↓ | ≥ 25 | 100 mg/kg per day, intraperitoneal injection |
| cyclin-D1↓, survivin↓ | ||
| VEGF↓ (Ref. 27) | ||
| NF-κB↓, PGE2↓ | ≥ 4 | NA |
| VEGF↓, miR-21↓ | ||
| miR-200↑ (Ref. 28) | ||
Based on these promising preclinical results, several researcher groups, including our own, have progressed to testing the anticancer effects of curcumin in clinical trials.
Despite numerous published preclinical studies, relatively few clinical trials have been reported so far. Several phase I and pharmacokinetic studies have been conducted using curcumin, and they found no dose-limiting toxicity (DLT) up to at least 12 g/d when administered orally to both healthy volunteers[42,43] and cancer patients[44-46]. The minor toxicities of Grade 1-2 diarrhea and nausea have been reported, although these were likely due to the ingestion of large volumes of curcumin at one time. Due to poor bioavailability, curcumin doses greater than 8 g/d do not lead to further increases in plasma curcumin levels; therefore, daily oral doses of 8 g or less have been most commonly used in clinical trials.
Dhillon et al[47] were the first to report a phase II clinical trial of the effects of curcumin against pancreatic cancer. Twenty-five patients, including 3 chemo-naive patients, were enrolled in this study. Of the 22 patients that could be evaluated for responses, one patient showed a stable disease course for over 18 mo and another patient showed a partial response in a liver metastasis (73% decrease in size), although this effects lasted for only 1 month. Furthermore, curcumin treatment was found to be safe in patients with pancreatic cancer, and no toxicity was associated with curcumin intake.
Our group conducted a phase I/II clinical trial of curcumin in patients with pancreatic cancer who had become resistant to gemcitabine-based chemotherapy[48]. In contrast with the study by Dhillon et al[47], which tested the safety and efficacy of curcumin monotherapy, our study evaluated the efficacy of combined gemcitabine-based chemotherapy and curcumin treatment, which we tested based on the preclinical results showing that curcumin could potentiate the anticancer effects of gemcitabine[36]. As no previous studies had demonstrated the safety and feasibility of this drug combination in cancer patients, we began with a phase I study involving an 8-g daily oral dose of curcumin in combination with gemcitabine-based chemotherapy. The first 3 patients that could be assessed completed their first treatment cycle without a predefined DLT. Therefore, we selected this dose for the following phase II study. In total, 21 patients who showed disease progression during previous gemcitabine-based chemotherapy were enrolled in the study. The addition of an 8-g daily oral curcumin dose did not increase the risk of clinically relevant toxicity, and the toxicity profile of the combined drugs was comparable with that observed in pancreatic cancer patients treated with gemcitabine-based chemotherapy alone. Cumulative toxicity from curcumin was not observed, and 4 patients were able to continue this intake regimen for over 6 mo, indicating that this agent is safe for long-term use. Even though the preliminary results were from a small sample, the observed median survival time (MST) of 5.4 (95%CI 3.6-7.4) mo and a 1-year survival rate of 19% (95%CI 4.4%-41.4%) are promising results, particularly considering the poor prognosis of patients with pancreatic cancer with resistance to gemcitabine-based chemotherapy.
Epelbaum et al[49] reported the results from another clinical trial testing the efficacy and feasibility of curcumin in combination with gemcitabine monotherapy in chemo-naive patients with advanced pancreatic cancer. Seventeen patients were enrolled in the study, and they received the standard dose and schedule of gemcitabine in combination with an 8-g daily oral dose of curcumin. In contrast to the previous 2 studies that showed low toxicity for 8-g daily oral doses of curcumin[47,48], this study reported that 5 patients (29%) discontinued the curcumin regimen after a period of several days to 2 wk due to intractable abdominal fullness and/or pain. Indeed, the dose of curcumin was eventually reduced to 4 g/d due abdominal complaints in 2 other patients. The researchers discussed the possibility that increased gastrointestinal toxicity could be caused by the combination of curcumin and gemcitabine, and they concluded that 8 g oral curcumin is not a viable treatment dose when combined with gemcitabine in patients with pancreatic cancer. One possible explanation for the discrepancy between our results and those of Epelbaum et al[49] is that the baseline clinical condition of the patients was poorer in the Epelbaum et al[49] study than in ours, and therefore, the abdominal fullness or pain experienced by these patients may have been primarily attributable to cancer-related symptoms.
Table 2 summarizes the published clinical trials that have tested the effects of curcumin in patients with pancreatic cancer.
| Dhillon et al[47] | Kanai et al[48] | Epelbaum et al[49] | Kanai et al[69] | |
| Sample size | 25 | 21 | 17 | 14 |
| Study design | Phase II | Phase I/II | Phase II | Phase I |
| Study period | 20081 | 2008-2009 | 2004-2006 | 2011-2012 |
| Dose of curcumin | 8 g/d | 8 g/d | 8 g/d | 200 mg/d2 (n = 9) 400 mg/d2 (n = 5) |
| Prior history of chemotherapy | Yes (n = 22) | Yes (n = 21) | None | yes (n = 14) |
| Concomitant use of anticancer drug | No | Yes | Yes | Yes |
| Major toxicity associated with curcumin | None | None | Abdominal discomfort (n = 5) | Abdominal pain (n = 2) |
| Median survival time (mo) | NA | 5.4 | 5 | 4.4 |
Several investigators, including ourselves, have tested plasma curcumin levels in clinical trials, and most studies have reported that plasma curcumin levels remained at low (ng/mL) levels, despite multi-gram doses of curcumin[42,45,46,48]. As described in the previous section, the intake of oral doses of curcumin greater than 8 g did not lead to further increases in plasma curcumin levels in human subjects[42-44]. Therefore, the poor bioavailability of curcumin has been the primary challenge to its clinical application. As a result, many efforts have been made to improve the bioavailability of this agent using a variety of approaches, including innovative drug delivery systems (nanoparticles, liposomes and phospholipids)[50-65] and the development of new curcumin analogs[66,67]. For example, a nanoparticle-based drug delivery system has been shown to improve the water solubility of hydrophobic agents such as curcumin, and several different types of nanoparticle-based curcumin have been published[52,56-59,61,62,64,65].
Of these new varieties of nanoparticle-based curcumin, we chose THERACURMIN® for further study, as it showed a greater than 30-fold increase in bioavailability compared with conventional curcumin in rat models[64]. THERACURMIN® was prepared as follows[64,68]. First, gum ghatti - which primarily consists of polysaccharides obtained from ghatti tree exudates - was dissolved in water to make a gum ghatti solution. Curcumin powder was mixed into this solution, and water and glycerin were added to adjust the final weight. This mixture was ground using a wet grinding mill (DYNO-MILL®KDL, Willy A Bachofen AG) and then dispersed with a high-pressure homogenizer (Homogenizer 15MR-8TA, APV Gaulin). Stable THERACURMIN® is obtained from this procedure.
To verify the improved bioavailability of THERACURMIN® in human subjects, we conducted a dose-escalation and pharmacokinetic study[68]. Six healthy human volunteers were recruited and given THERACURMIN®via a single oral dose of 150 mg. Following an interval of 2 wk, the same subjects were then given THERACURMIN®via a single oral dose of 210 mg. The Cmax values for THERACURMIN® at the 150 and 210 mg doses were 189 ± 48 and 275 ± 67 ng/mL (mean ± SEM), respectively. No toxicity associated with THERACURMIN® intake was observed in this study.
These results indicate that the ingestion of THERACURMIN® can lead to higher plasma curcumin levels than those achieved with conventional curcumin (Table 3). Therefore, we considered this new form of curcumin to be a promising tool for testing the potential anticancer effects of curcumin in clinical trials, and we conducted a phase I study testing the safety of THERACURMIN® in patients with pancreatic cancer[69].
A total of 16 patients (14 patients with pancreatic cancer and 2 patients with biliary tract cancer) who failed standard gemcitabine-based chemotherapy were enrolled in the study. Based on our previous pharmacokinetic study, we chose to use THERACURMIN® containing 200 mg curcumin (Level 1) as the starting dose. THERACURMIN® was administered orally every day in combination with standard gemcitabine-based chemotherapy.
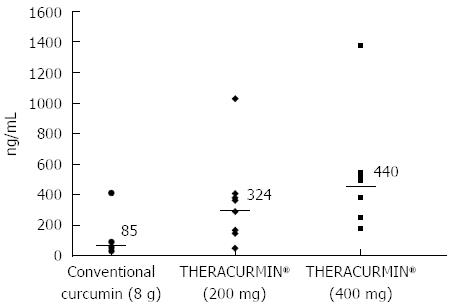
Ten patients were assigned to the Level 1 group and six to the Level 2 group (THERACURMIN® containing 400 mg curcumin). Peak plasma curcumin levels (median) following THERACURMIN® administration were 324 ng/mL (range = 47-1029 ng/mL) for Level 1 and 440 ng/mL (range = 179-1380 ng/mL) for Level 2. Importantly, these values were significantly higher than the median value (85 ng/mL) observed in our previous study using 8-g doses of conventional curcumin (Figure 2). With respect to safety, two patients reported increased abdominal pain following THERACURMIN® administration. Computed tomography scans performed prior to THERACURMIN® administration in these patients revealed dilated colons, which could have been due to intestinal obstructions caused by peritonitis carcinomatosa. As described in the previous section, Epelbaum et al[49] reported abdominal fullness or pain following curcumin administration in patients with pancreatic cancer. We speculate that curcumin may irritate the intestine, potentially increasing abdominal pain in patients with intestinal obstructions due to peritonitis carcinomatosa or other complications. In future clinical trials, we advise caution when administering curcumin to these types of patients.
Other observed toxicities were comparable to those for gemcitabine-based chemotherapy alone, and repetitive exposure to high concentrations of curcumin did not cause any unexpected serious adverse events, nor did they increase the incidence of adverse events in patients with pancreatic cancer receiving gemcitabine-based chemotherapy. In fact, three patients safely continued THERACURMIN® treatment for > 9 mo. With respect to efficacy, no responses were observed in this study based on RECIST; however, the MST was 4.4 mo (95% confidence interval: 1.8-7.0 mo) for the 14 patients with pancreatic cancer, and three patients (21%) survived for > 12 mo following initiation of THERACURMIN®.
Interestingly, fatigue- and functioning-associated quality of life (QOL) scores scaled by EORTC QLQ-C30 significantly improved following THERACURMIN® administration. In five patients, the fatigue score improved by > 20, which was interpreted as a significant and clinically relevant change[70]. Preclinical and clinical studies demonstrating the benefits of curcumin on heart disease, depression, and fatigue, also support these findings[71-73]. As improved QOL has been demonstrated to contribute to better outcomes in cancer patients[74], it is tempting to speculate that THERACURMIN® may prolong the overall survival of patients with pancreatic cancer through QOL improvements. A randomized placebo-controlled clinical trial is now underway to verify this hypothesis (UMIN000010326).
A growing body of evidence supports the idea that curcumin is a promising anticancer drug. In preclinical models, curcumin has been shown to have anticancer effects, both alone and in combination with other anticancer drugs, through the modulation of a variety of molecular targets. However, the poor bioavailability of curcumin has been the major challenge to its clinical application. This problem has now been solved by the development of highly bioavailable forms of curcumin (THERACURMIN®), which can induce higher plasma curcumin levels without increased toxicity. Further clinical trials will be necessary to test the therapeutic applications of this promising agent in patients with pancreatic cancer.
P- Reviewer: Chen CY, Tocharus J S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9215] [Cited by in F6Publishing: 9719] [Article Influence: 883.5] [Reference Citation Analysis (3)] |
| 2. | Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 603] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 3. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] [Cited in This Article: ] |
| 4. | Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 521] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 5. | Rocha Lima CM, Green MR, Rotche R, Miller WH, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776-3783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 418] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 6. | Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-3516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 681] [Cited by in F6Publishing: 704] [Article Influence: 37.1] [Reference Citation Analysis (1)] |
| 7. | Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, Zimmermann A, John W, Von Hoff D, Arning M. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639-1645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946-3952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 482] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 9. | Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212-2217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 422] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 10. | Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778-3785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 11. | Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 442] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 12. | Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 898] [Cited by in F6Publishing: 840] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 13. | Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 420] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 14. | Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351-2362. [PubMed] [Cited in This Article: ] |
| 15. | Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D’Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Tomita M, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Takasu N, Matsuda T, Ohta T, Tanaka Y, Ohshiro K. Curcumin (diferuloylmethane) inhibits constitutive active NF-kappaB, leading to suppression of cell growth of human T-cell leukemia virus type I-infected T-cell lines and primary adult T-cell leukemia cells. Int J Cancer. 2006;118:765-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503-2513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Everett PC, Meyers JA, Makkinje A, Rabbi M, Lerner A. Preclinical assessment of curcumin as a potential therapy for B-CLL. Am J Hematol. 2007;82:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988-1996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423-3430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 21. | Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Höhneke C, Jochum M, Nerlich AG, Pfeffer U. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008;29:779-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128-2136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283-7292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Cancer. 2009;100:1425-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Duarte VM, Han E, Veena MS, Salvado A, Suh JD, Liang LJ, Faull KF, Srivatsan ES, Wang MB. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKβ protein of the NFκB pathway. Mol Cancer Ther. 2010;9:2665-2675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Glienke W, Maute L, Wicht J, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest. 2010;28:166-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. J Biol Chem. 2010;285:25332-25344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606-3617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 354] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 29. | Howells LM, Sale S, Sriramareddy SN, Irving GR, Jones DJ, Ottley CJ, Pearson DG, Mann CD, Manson MM, Berry DP. Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo. Int J Cancer. 2011;129:476-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Yu LL, Wu JG, Dai N, Yu HG, Si JM. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol Rep. 2011;26:1197-1203. [PubMed] [Cited in This Article: ] |
| 31. | Yang CL, Liu YY, Ma YG, Xue YX, Liu DG, Ren Y, Liu XB, Li Y, Li Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One. 2012;7:e37960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 32. | Youns M, Fathy GM. Upregulation of extrinsic apoptotic pathway in curcumin-mediated antiproliferative effect on human pancreatic carcinogenesis. J Cell Biochem. 2013;114:2654-2665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 34. | Kanai M, Guha S, Aggarwal BB. The potential role of curcumin for treatment of pancreatic cancer. Pancreatic Cancer-Molecular Mechanisms and Targets. Croatia: InTech 2012; 213-224. [DOI] [Cited in This Article: ] |
| 35. | Li Y, Revalde JL, Reid G, Paxton JW. Modulatory effects of curcumin on multi-drug resistance-associated protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol. 2011;68:603-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853-3861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 37. | Tsai MS, Weng SH, Kuo YH, Chiu YF, Lin YW. Synergistic effect of curcumin and cisplatin via down-regulation of thymidine phosphorylase and excision repair cross-complementary 1 (ERCC1). Mol Pharmacol. 2011;80:136-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 488] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 39. | Soubani O, Ali AS, Logna F, Ali S, Philip PA, Sarkar FH. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis. 2012;33:1563-1571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704-6712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 538] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 41. | Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171-2176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 42. | Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 796] [Cited by in F6Publishing: 856] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 43. | Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 44. | Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895-2900. [PubMed] [Cited in This Article: ] |
| 45. | Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847-6854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 835] [Cited by in F6Publishing: 858] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 46. | Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120-125. [PubMed] [Cited in This Article: ] |
| 47. | Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491-4499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 880] [Cited by in F6Publishing: 932] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 48. | Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 49. | Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62:1137-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 50. | Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 436] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 51. | Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006;40:720-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 52. | Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 682] [Article Influence: 40.1] [Reference Citation Analysis (1)] |
| 53. | Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 54. | Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J Pharm Sci. 2008;70:445-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 55. | Sahu A, Bora U, Kasoju N, Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4:1752-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 56. | Sou K, Inenaga S, Takeoka S, Tsuchida E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int J Pharm. 2008;352:287-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Gupta V, Aseh A, Ríos CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomedicine. 2009;4:115-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 58. | Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867-3875. [PubMed] [Cited in This Article: ] |
| 59. | Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 637] [Cited by in F6Publishing: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 60. | Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem. 2009;57:9141-9146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 61. | Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79:330-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 377] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 62. | Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine. 2010;6:153-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 63. | Koppolu B, Rahimi M, Nattama S, Wadajkar A, Nguyen KT. Development of multiple-layer polymeric particles for targeted and controlled drug delivery. Nanomedicine. 2010;6:355-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34:660-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 65. | Yallapu MM, Ebeling MC, Khan S, Sundram V, Chauhan N, Gupta BK, Puumala SE, Jaggi M, Chauhan SC. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol Cancer Ther. 2013;12:1471-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 66. | Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 67. | Sato A, Kudo C, Yamakoshi H, Uehara Y, Ohori H, Ishioka C, Iwabuchi Y, Shibata H. Curcumin analog GO-Y030 is a novel inhibitor of IKKβ that suppresses NF-κB signaling and induces apoptosis. Cancer Sci. 2011;102:1045-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Kanai M, Imaizumi A, Otsuka Y, Sasaki H, Hashiguchi M, Tsujiko K, Matsumoto S, Ishiguro H, Chiba T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother Pharmacol. 2012;69:65-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 69. | Kanai M, Otsuka Y, Otsuka K, Sato M, Nishimura T, Mori Y, Kawaguchi M, Hatano E, Kodama Y, Matsumoto S. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol. 2013;71:1521-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 70. | Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139-144. [PubMed] [Cited in This Article: ] |
| 71. | Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K. Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology. 2009;214:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Morimoto T, Sunagawa Y, Fujita M, Hasegawa K. Novel heart failure therapy targeting transcriptional pathway in cardiomyocytes by a natural compound, curcumin. Circ J. 2010;74:1059-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Sugawara J, Akazawa N, Miyaki A, Choi Y, Tanabe Y, Imai T, Maeda S. Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. Am J Hypertens. 2012;25:651-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4817] [Cited by in F6Publishing: 4598] [Article Influence: 328.4] [Reference Citation Analysis (0)] |