Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5505
Revised: February 2, 2014
Accepted: March 6, 2014
Published online: May 14, 2014
AIM: To investigate the changes in apoptosis in gastrointestinal cancer cells from patients with gastrointestinal cancers treated with arsenic trioxide (As2O3); and to study the possible molecular mechanisms of such changes by detecting the expression levels of p53 and Bcl-2.
METHODS: Twenty patients with gastrointestinal adenocarcinoma based on endoscopic and biopsy findings (ten patients with gastric cancer and ten patients with colorectal cancer) who received treatment in our hospital between August 2007 and December 2008 were included in this study. None of the patients had received anti-tumour agents prior to As2O3 treatment. As2O3 was administered intravenously at a dose of 0.01 g/d diluted with 5% glucose in normal saline for 2-3 h for 3 consecutive days before surgery. Morphological changes associated with apoptosis of gastrointestinal cancer cells were observed by light microscopy. Changes in the apoptotic index induced by As2O3 were investigated using the terminal deoxynucleotidyl transferase dUTP nick end labelling method. Expression levels of p53 and Bcl-2 proteins in gastrointestinal cancer tissues were determined by immunohistochemistry.
RESULTS: The apoptotic index of human gastrointestinal cancer cells was higher in cells from patients treated with As2O3 than in those not treated (P < 0.05). p53 protein expression in gastrointestinal tissues was unchanged by As2O3 (P > 0.05). However, Bcl-2 protein expression in gastrointestinal tissues was down-regulated by As2O3 (P < 0.01).
CONCLUSION: These results demonstrate that As2O3 treatment in patients with gastrointestinal cancers can induce apoptosis in gastrointestinal cancer cells and down-regulate Bcl-2 protein expression.
Core tip: In this study, patients with gastrointestinal cancer were treated with arsenic trioxide for the first time. The use of arsenic trioxide in neoadjuvant chemotherapy prior to surgery improved surgical results.
- Citation: Ma ZB, Xu HY, Jiang M, Yang YL, Liu LX, Li YH. Arsenic trioxide induces apoptosis of human gastrointestinal cancer cells. World J Gastroenterol 2014; 20(18): 5505-5510
- URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5505.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5505
Gastrointestinal cancer (GIC) is one of the most common malignant tumours in China. Although early GIC can be treated by surgical resection, the response of advanced-stage GIC to conventional chemotherapy or radiotherapy is usually unsatisfactory. Following studies in Harbin and Shanghai, arsenic trioxide (As2O3) was reported to be an effective drug for the treatment of patients with acute promyelocytic leukaemia (APL), by selectively inducing apoptosis in APL cells[1-3]; the effect of As2O3 on solid tumours has since been demonstrated in GIC cells in vitro and in vivo[4-7]. However, the clinical efficacy of As2O3 for the treatment of GIC remains unknown. Based on the above studies, we treated GIC patients with As2O3, observed its ability to induce apoptosis in GIC cells, and explored the possible molecular mechanism underlying this effect by detecting the expression levels of p53 and Bcl-2 in As2O3-treated GIC cells.
As2O3 for injection (0.01 g/10 mL) was produced by Harbin Yida Pharmaceutical Co., Ltd. (Harbin, China).
Twenty patients with gastrointestinal adenocarcinoma based on endoscopic and biopsy findings, including ten patients with gastric cancer and ten patients with colorectal cancer who received treatment in our hospital between August 2007 and December 2008, were included in this study. None of the patients had received anti-tumour agents. As2O3 was administered as an intravenous infusion at a dose of 0.01 g/d diluted with 5% glucose in normal saline for 2-3 h for 3 consecutive days before surgery.
Human gastrointestinal adenocarcinoma tissue specimens were obtained endoscopically from the same patients before and after As2O3 treatment and surgery.
The apoptotic cell morphology of GIC cells was examined by light microscopy.
Apoptosis was assessed via transferase dUTP nick end labelling (TUNEL) assay according to the instructions of the In Situ Cell Death Detection Kit (Boehringer-Mannheim, Germany). Briefly, after three washes with phosphate-buffered saline (PBS), 400 μL TUNEL reaction solution was added to the well, followed by incubation at 37 °C for 1 h. Stained cells were examined under the light microscope. Apoptotic cells were scored as the number of positively stained cells per 1000 cells (n = 5). The apoptotic index (AI) was calculated as follows: AI = (number of apoptotic cells/total number of cells) × 100%.
The streptavidin-peroxidase (S-P) method was used to detect the expression levels of p53 and Bcl-2 proteins in human gastrointestinal adenocarcinoma tissues. Monoclonal rabbit anti-human p53 and rabbit anti-human Bcl-2 antibodies (antiserum titre, 1:50) were provided by Zhongshan Bio-Tech Co., Ltd. (Beijing, China). The expression levels of p53 and Bcl-2 proteins were determined using an UltraSensitive S-P (Mouse/Rabbit) kit (Zhongshan Bio-Tech Co., Ltd.) according to the manufacturer’s protocol. The percentage of positive cells was calculated by examination of the images under the light microscope.
Results were analysed using the SPSS13.0 statistical software package. All values were expressed as mean ± SD. Comparisons of values before and after As2O3 treatment were performed using one-sample t tests, and correlation analysis was performed using linear correlation. Values of P < 0.05 were considered statistically significant.
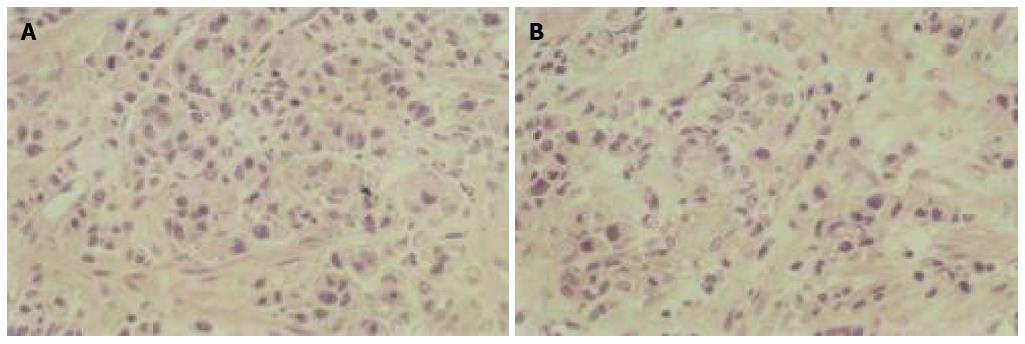
The number of apoptotic cells increased after As2O3 treatment as determined by light microscopy. Typical apoptotic morphological features were detected in GIC cells after treatment, including cellular shrinkage, cell nuclear fragmentation, chromatin condensation, and the production of apoptotic bodies (Figure 1).
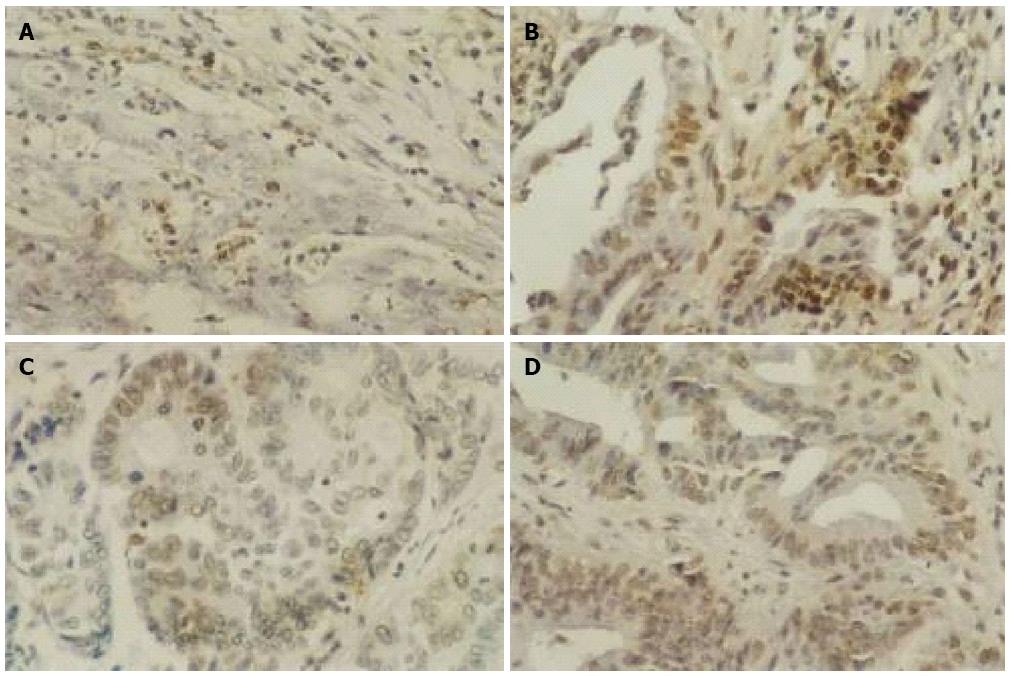
As shown in Figure 2, the number of apoptotic GIC cells as indicated by brown staining was increased after 3 days of As2O3 treatment. Correspondingly, the AI of GIC cells increased after As2O3 treatment relative to that before treatment (P < 0.05) (Table 1).
| Group | Index | Before As2O3 treatment | After As2O3 treatment | P value |
| Gastric cancer | Apoptotic index (%) | 6.88 ± 1.80 | 15.53 ± 4.51 | < 0.05 |
| Colon cancer | 7.11 ± 1.48 | 16.76 ± 4.16 | < 0.05 | |
| Gastric cancer | p53 protein (%) | 38.08 ± 18.52 | 35.42 ± 17.19 | > 0.05 |
| Colon cancer | 31.58 ± 14.55 | 30.83 ± 19.52 | > 0.05 | |
| Gastric cancer | Bcl-2 protein (%) | 37.16 ± 18.94 | 23.80 ± 3.30 | < 0.05 |
| Colon cancer | 28.69 ± 13.00 | 16.90 ± 5.74 | < 0.05 |
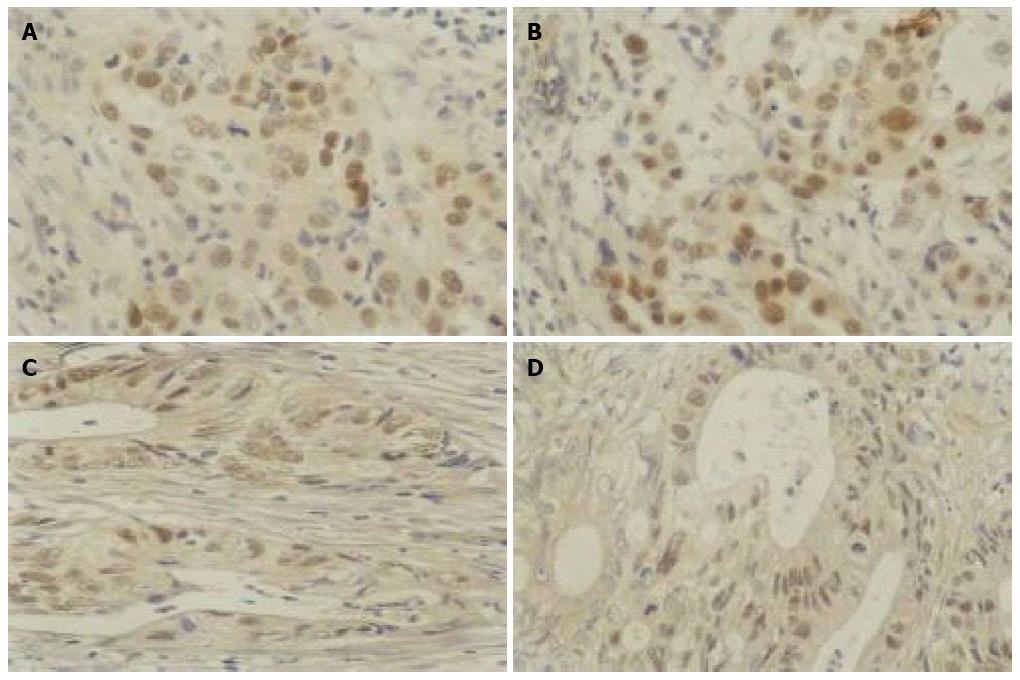
There was no significant change in p53 protein expression in either gastric or colon cancer tissues after As2O3 treatment, suggesting that the anti-apoptotic p53 gene was not sensitive to As2O3 at a dose of 0.01 g/d for 3 d (Table 1, Figure 3).
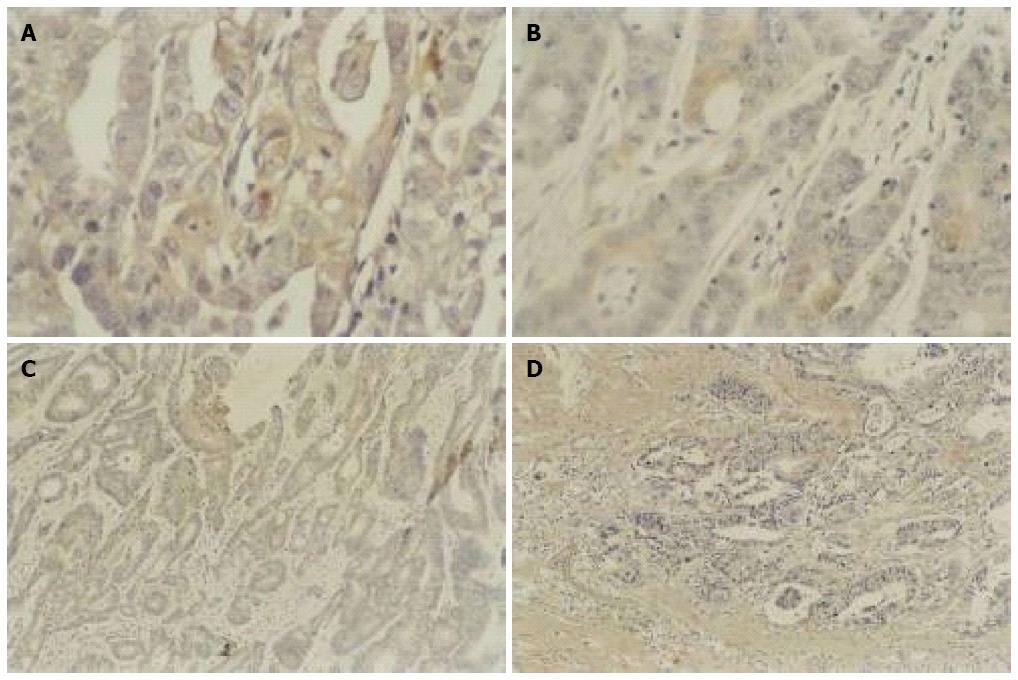
As shown in Figure 4, Bcl-2 protein expression was reduced in gastric cancer tissues after As2O3 treatment, and similar results were seen in colon cancer tissues, suggesting that As2O3 had down-regulated the expression of Bcl-2 protein (Table 1).
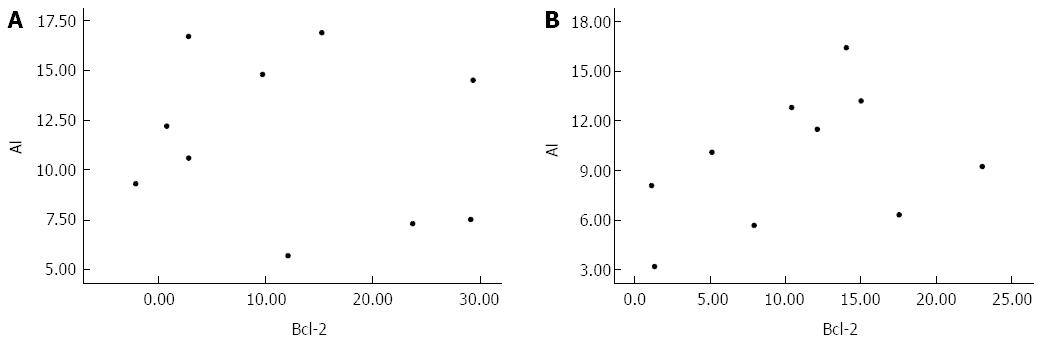
According to the above observations, the AI of GIC cells increased following the down-regulation of Bcl-2 protein expression. The correlation between apoptosis and Bcl-2 expression was examined to determine whether changes in Bcl-2 expression represented the molecular mechanism responsible for As2O3-induced apoptosis of GIC cells. The results showed no significant correlation between these measures in either gastric or colon cancer cells (Figure 5).
Two patients experienced vomiting after As2O3 treatment, which resolved after symptomatic treatment. Second-degree atrioventricular block occurred during As2O3 treatment in one patient, who recovered after treatment. There were no other secondary effects in any other patients.
The use of arsenic compounds as drugs has a long history in traditional Chinese medicine. As2O3 has been identified as an effective drug for the treatment of APL, with induction of apoptosis representing the main mechanism of action. Numerous clinical trials on As2O3 are therefore underway for the treatment of haematopoietic malignancies and solid tumours[8-10].
Recent studies[11-15] reported that As2O3 could induce apoptosis in various solid tumour cell lines, including GIC cell lines, as well as in animal models of GIC, especially gastric cancer. However, no clinical trial on As2O3 in patients with gastric cancer has been reported.
In the present study, we treated GIC patients with As2O3 and examined its effect on GIC cells. Light microscopy showed morphological changes characteristic of apoptosis in GIC cells in As2O3-treated patients. TUNEL assay showed that AI was also significantly increased in GIC cells of treated patients. These results suggest that As2O3 induces apoptosis of GIC cells not only in cell lines and animal models, but also in the clinical setting. The induction of apoptosis in GIC cells from GIC patients may indicate a new potential strategy for the treatment of these tumours. Gu et al[16] found that the induction of apoptosis was time- and concentration-dependent. In line with previous clinical reports, we chose a relatively safe dose of As2O3 in this study. However, the optimal dose required to produce the maximal clinical effect with minimal toxicity remains to be determined.
Although the effect of As2O3 on the induction of GIC cell apoptosis has been addressed, its precise mechanism remains unanswered. In APL cells, As2O3 was shown to inhibit cell proliferation by direct induction of apoptosis through down-regulation of Bcl-2 expression and modulation of the PML/RARα protein in in vitro studies[2]. Liu et al[17] reported that the PIG11 gene may be involved in As2O3-induced apoptosis in HepG2 cells, and suggested that the adaptive response of PIG11 expression was an important factor in enhancing cell sensitivity to As2O3-induced apoptosis. As2O3 has been shown to induce apoptosis in different GCC lines through up-regulation of p53 expression and down-regulation of Bcl-2 expression[18]. Many chemotherapy agents can induce apoptosis in gastric cancer and colon cancer cells by modulating the p53 gene[19]. Jiang et al[20] found that As2O3 induced apoptosis in the GCC lines AGS and MKN-28 through up-regulation of p53. Akao et al[21] also reported that As2O3 induced apoptosis through the down-regulation of Bcl-2 protein and activation of caspases in B-cell leukaemia cell lines, while As2O3-induced apoptosis was related to p53 and Bcl-2 gene expression in studies of colon cancer cell lines and in an animal model[6,7].
To further confirm the possible mechanism of As2O3-induced apoptosis, we determined the effect of As2O3 on p53 and Bcl-2 expression. In contrast to the previous reports, our immunohistochemical analysis found no significant change in p53 protein expression in either gastric or colon cancer tissues following As2O3 treatment. Possible reasons for this discrepancy are as follows: (1) As2O3 treatment was only administered for a short period (3 d); (2) the dose of As2O3 used in the current study may have been too low; (3) the effect of As2O3 on p53 expression may be related to the stage of GIC; or (4) the internal environment in GIC patients may be more dynamic than that in cell lines. Further studies are needed to clarify the effect of As2O3 on p53 protein expression in human GIC cells.
Bcl-2 is considered a survival gene that plays an important role in inhibiting tumour cell apoptosis[22]. Our experimental results revealed that As2O3 down-regulated the expression of Bcl-2 protein in human GIC tissues. However, the change in Bcl-2 protein expression was not correlated with AI in either gastric or colon cancers, suggesting that other genes may be involved in the process of As2O3-induced apoptosis in human GIC.
In conclusion, the results of this study indicate that clinical treatment with As2O3 induced apoptosis of GIC cells without causing serious adverse effects. In addition, although Bcl-2 gene expression was down-regulated by As2O3, this did not seem to be the mechanism responsible for As2O3-induced apoptosis. Similar to P53 and Bcl-2 genes, SIRT6 is a new tumor suppressor gene in colorectal cancer as shown in recent studies, and it is possible that the glycolytic pathway limits the growth of tumors. We also used SIRT6 antibody on the tissue samples before and after As2O3 treatment, and SIRT6 was found to be higher after treatment than before treatment. Further comprehensive clinical studies are needed to clarify the significance and precise mechanism of action of As2O3 in the treatment of GIC patients.
Gastrointestinal cancer is a common clinical malignancy, and is often diagnosed when it is unresectable. Therapeutic options are required to improve patient condition prior to surgery and this is a research hotspot.
This study assessed the effects of arsenic trioxide on gastrointestinal cancer cell apoptosis.
Arsenic trioxide was administered properatively in gastrointestinal cancer patients and its efficacy as neoadjuvant chemotherapy was assessed.
The use of arsenic trioxide as neoadjuvant chemotherapy may be extended in to other types of tumors.
The experimental design is reasonable and practical, with patients giving fully informed consent, and complies with legal requirements, with satisfactory results.
P- Reviewers: Hasegawa Y, Masmoudi K, Savarese G S- Editor: Wang JL L- Editor: Webster JR E- Editor: Liu XM
| 1. | Sun HD, Li YS, Ma L, Zhang TD. AiLing No1 treated 32 cases of acute promyelocytic leukemia. Zhongguo Zhongxiyi Jiehe Zazhi. 1992;12:170-171. [Cited in This Article: ] |
| 2. | Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052-1061. [PubMed] [Cited in This Article: ] |
| 3. | Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102-2111. [PubMed] [Cited in This Article: ] |
| 4. | Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532-539. [PubMed] [Cited in This Article: ] |
| 5. | Chowdhury R, Chowdhury S, Roychoudhury P, Mandal C, Chaudhuri K. Arsenic induced apoptosis in malignant melanoma cells is enhanced by menadione through ROS generation, p38 signaling and p53 activation. Apoptosis. 2009;14:108-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Wei L, Wang XW, Zuo WS, Song XR, Li M. The study of arsenic trioxide on inhibition of the growth of colonic cancer cells. Zhonghua Weichang Waike Zazhi. 2005;8:370-372. [Cited in This Article: ] |
| 7. | Liu L, Zhao W, Qin SK, Li SY, Qiu SM, Wang NY, Chen HY. The Studies on Anti-tumor Effect and Its Mechanism of Arsenic Trioxide for Human Colon Carcinoma in Nude Mouse. Zhongguo Zhongliu Linchuang. 2005;32:1067-1070. [Cited in This Article: ] |
| 8. | Zhang TC, Cao EH, Li JF, Ma W, Qin JF. Induction of apoptosis and inhibition of human gastric cancer MGC-803 cell growth by arsenic trioxide. Eur J Cancer. 1999;35:1258-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Miller WH, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893-3903. [PubMed] [Cited in This Article: ] |
| 10. | Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6 Suppl 2:22-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Shen ZY, Tan LJ, Cai WJ, Shen J, Chen C, Tang XM, Zheng MH. Arsenic trioxide induces apoptosis of oesophageal carcinoma in vitro. Int J Mol Med. 1999;4:33-37. [PubMed] [Cited in This Article: ] |
| 12. | Deng YP, Lin C, Zhang XY, Chen JP, Xiao PG, Wu W. Studies on arsenic trioxide induced Human pulmonary adenocarcinoma GLC-82 cell apoptosis and its mol ecular mechanisms. Aizheng. 1999;18:545-546. [Cited in This Article: ] |
| 13. | Zheng J, Deng YP, Lin C, Fu M, Xiao PG, Wu M. Arsenic trioxide induces apoptosis of HPV16 DNA-immortalized human cervical epithelial cells and selectively inhibits viral gene expression. Int J Cancer. 1999;82:286-292. [PubMed] [Cited in This Article: ] |
| 14. | Liang T, Liu TF, Zhaung LW, Gao GQ, Ma ZJ. Study on the apoptosis of hum an liver cancer cells induced by arsenic trioxide. Haerbing Yike Daxue Xuebao. 1999;33:117-119. [Cited in This Article: ] |
| 15. | Akao Y, Nakagawa Y, Akiyama K. Arsenic trioxide induces apoptosis in neuroblastoma cell lines through the activation of caspase 3 in vitro. FEBS Lett. 1999;455:59-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Gu QL, Li NL, Zhu ZG, Yin HR, Lin YZ. A study on arsenic trioxide inducing in vitro apoptosis of gastric cancer cell lines. World J Gastroenterol. 2000;6:435-437. [PubMed] [Cited in This Article: ] |
| 17. | Liu XM, Xiong XF, Song Y, Tang RJ, Liang XQ, Cao EH. Possible roles of a tumor suppressor gene PIG11 in hepatocarcinogenesis and As2O3-induced apoptosis in liver cancer cells. J Gastroenterol. 2009;44:460-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Tu SP, Jiang SH, Tan JH, Qiao MM, Wang LF, Jiang XH, Wu YL. Effect of Arsenic trioxide on apoptosis and apoptosis related genes in gastic cancer cells. Zhongliu. 2001;21:327-330. [Cited in This Article: ] |
| 19. | Müller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 587] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 20. | Jiang XH, Wong BC, Yuen ST, Jiang SH, Cho CH, Lai KC, Lin MC, Kung HF, Lam SK. Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of p53 and activation of caspase-3. Int J Cancer. 2001;91:173-179. [PubMed] [Cited in This Article: ] |
| 21. | Akao Y, Mizoguchi H, Kojima S, Naoe T, Ohishi N, Yagi K. Arsenic induces apoptosis in B-cell leukaemic cell lines in vitro: activation of caspases and down-regulation of Bcl-2 protein. Br J Haematol. 1998;102:1055-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2650] [Cited by in F6Publishing: 2857] [Article Influence: 84.0] [Reference Citation Analysis (1)] |