Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5263
Revised: January 9, 2014
Accepted: February 26, 2014
Published online: May 14, 2014
Helicobacter pylori (H. pylori) are resistant to hostile gastric environments and antibiotic therapy, reflecting the possibility that they are protected by an ecological niche, such as inside the vacuoles of human epithelial and immune cells. Candida yeast may also provide such an alternative niche, as fluorescently labeled H. pylori were observed as fast-moving and viable bacterium-like bodies inside the vacuoles of gastric, oral, vaginal and foodborne Candida yeasts. In addition, H. pylori-specific genes and proteins were detected in samples extracted from these yeasts. The H. pylori present within these yeasts produce peroxiredoxin and thiol peroxidase, providing the ability to detoxify oxygen metabolites formed in immune cells. Furthermore, these bacteria produce urease and VacA, two virulence determinants of H. pylori that influence phago-lysosome fusion and bacterial survival in macrophages. Microscopic observations of H. pylori cells in new generations of yeasts along with amplification of H. pylori-specific genes from consecutive generations indicate that new yeasts can inherit the intracellular H. pylori as part of their vacuolar content. Accordingly, it is proposed that yeast vacuoles serve as a sophisticated niche that protects H. pylori against the environmental stresses and provides essential nutrients, including ergosterol, for its growth and multiplication. This intracellular establishment inside the yeast vacuole likely occurred long ago, leading to the adaptation of H. pylori to persist in phagocytic cells. The presence of these bacteria within yeasts, including foodborne yeasts, along with the vertical transmission of yeasts from mother to neonate, provide explanations for the persistence and propagation of H. pylori in the human population. This Topic Highlight reviews and discusses recent evidence regarding the evolutionary adaptation of H. pylori to thrive in host cell vacuoles.
Core tip:Helicobacter pylori (H. pylori) have been observed within yeast vacuoles by light and fluorescence microscopy, and their presence has been confirmed by the detection of H. pylori-specific genes and proteins in yeast extracts, such as VacA subunits, UreA, peroxiredoxin and thiol peroxidase. Moreover, non-culturable H. pylori cells have been found in subsequent generations of yeasts, indicating the generational transmission of the bacteria is part of the transfer of vacuolar content. H. pylori are therefore well-equipped to establish in the vacuoles of yeast, which provide them with essential nutrients such as ergosterol for multiplication, as a pre-adaptation for invasion of human cells.
-
Citation: Siavoshi F, Saniee P. Vacuoles of
Candida yeast as a specialized niche forHelicobacter pylori . World J Gastroenterol 2014; 20(18): 5263-5273 - URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5263
It is currently unknown how Helicobacter pylori (H. pylori) persist in the environment and food supply. Moreover, the factors facilitating their entry to human gastric epithelium and the mechanism of their transmission from person to person are equally unclear. The ability to trace H. pylori from environmental sources to the human stomach is hampered by the fastidiousness of the bacterium, the chronic nature of bacterial infection, lack of outbreaks and a high prevalence of asymptomatic infected individuals. The failure to isolate H. pylori from environmental sources indicates the bacteria require a habitat that offers protection from environmental stresses, such as lack of nutrients and presence of biocides, for survival outside of the human stomach. Thus, the presence of an unknown environmental reservoir has been proposed, which could play a crucial role in the survival and spread of H. pylori[1]. Many bacteria, including pathogenic species, persist despite stressful conditions by establishing inside eukaryotic microorganisms. The intracellular life of prokaryotes inside eukaryotes is considered a significant evolutionary phenomenon that led to the adaptation of prokaryotes to a wide range of environmental niches, though details of this relationship have not been elucidated, largely due to the non-culturability of these intracellular bacteria[2].
Human gastric epithelial and immune cells have been recognized as the sole specialized eukaryotic cells that host H. pylori[3,4], with microscopic observations of H. pylori in the vacuoles of epithelial cells[5-7], macrophages[8] and dendritic cells[9]. Thus, H. pylori have been described as facultative intracellular bacteria, which have evolved to utilize the vacuoles of eukaryotic cells as a protective niche, allowing the bacteria to multiply and persist for a long time[3,4,6]. However, there are no reports documenting a stable intracellular association of H. pylori with a eukaryotic microorganism. This review presents the research works concerning H. pylori inside Candida yeast. As Candida yeast are remarkably resistant to stressful conditions[10], they could serve as an alternative host to shelter H. pylori against stressful conditions outside the human stomach, provide nutrients for its multiplication and act as a mediator for the spread of the bacterium in the environment and within human populations. In this regard, it is proposed that the yeast vacuole provides a unique and sophisticated niche for H. pylori that evolved when eukaryotic cells began to phagocytose prokaryotes as prey[11].
The intracellular establishment of bacteria inside fungi is regarded as an unusual evolutionary phenomenon, as the cell wall of fungi restricts endocytosis and bacterial uptake[12]. In contrast to other eukaryotes, such as protozoa, bivalves and insects[13], there are a limited number of examples of fungi harboring intracellular bacteria[14]. The most well-studied example involves the early evolutionary establishment of endobacteria in arbuscular mycorrhizal (AM) fungi[15], a plant root-associated fungus that dates back to 300-400 million years ago[16]. Fungal endobacteria have been localized inside membrane-bound vacuoles using light, electron[17,18], and confocal[15] microscopy, similar to other prokaryotic-eukaryotic endosymbioses[19]. The endosymbiotic bacteria of AM fungi have been identified as a new taxon based on ribosomal sequences, Candidatus Glomeribacter gigasporarum[17], which is related to Burkhoderia cepacia, a species of free-living bacteria with the potential to behave as saprophytes or pathogens. Similar to many other pathogenic bacteria, such as Legionella[20], Pseudomonas[21], and Mycobacteria[22], B. cepacia could survive in the vacuoles of eukaryotic cells, ranging from free-living amoebae[23], to macrophages[24], epithelial cells[25], and pneumocytes[26]. It is believed that this intracellular establishment protects the pathogenic bacteria against environmental stresses or the immune system of the host, and facilitates the transmission to a new host[27]. While the basis for the interaction between bacteria and AM fungi is not clear[16], it is proposed that fungal vacuoles provide a nourishing and protective niche for the endosymbiotic bacterium, facilitating its replication and transmission to the next generation[28].
The fungal vacuole is an acidic storage compartment with certain similarities to plant vacuoles and mammalian lysosomes. The various functions of vacuoles include glycoprotein turnover and hydrolysis, storage of Ca2+, phosphate and amino acids, pH and osmotic regulation, ion homeostasis and cytoplasmic detoxification[29,30]. Vacuoles also incorporate membranes from biosynthetic, endocytotic and autophagic cellular pathways[31]. Ergosterol in unicellular invertebrates, akin to cholesterol in vertebrates, is an important constituent of membrane lipids, and is implicated in several fungal cell processes, including plasma membrane fusion during mating and endocytosis[32]. Accordingly, a considerable amount of ergosterol is found in the membranes of vacuoles and other intracellular organelles[33]. Interestingly, a unique property of members of the genus Helicobacter is the incorporation of a large amount of ergosterol in their cell membrane. Ergosterol comprises up to 70% of cellular neutral lipids in H. pylori, much higher than in Escherichia coli (E. coli) (17%), which may have developed as a consequence of the symbiotic association with eukaryotic hosts[34]. The incorporation of cholesterol is important for H. pylori colonization of the host[35], pathogenicity[36] and antibiotic resistance[37].
The relationship between bacteria and yeast has largely been focused on extracellular associations, such as those occurring in food materials[38] and human mucosal surfaces[39,40]. Although bacteria and yeast have co-existed for billions of years, the biological relevance of this inter-domain microbial interaction remains largely unknown[41]. The most well-studied interactions are in polymicrobial biofilms of Candida albicans (C. albicans) and bacterial species within the human host environment that exhibit resistance to the immune system and antimicrobials[42]. Shedding light on the details of such interactions could provide important information for the management of infectious diseases, especially those that exhibit resistance to antimicrobial therapy[43].
Yeasts are highly sophisticated microorganisms with a remarkable ability for rapid change and adaptation to environmental stresses[44], including antimicrobials, the host immune system and a change in body location or host physiology[45]. C. albicans mostly occur in association with humans, thriving on the mucosal surfaces of the gastrointestinal (GI) and genitourinary tracts and skin[39,40,45]. H. pylori are also found in the human GI tract, indicating that both microorganisms are well-adapted to this unique niche[46,47]. The interaction between these two microorganisms may have begun long ago and led to the intracellular establishment of H. pylori inside the yeast vacuole, as a pre-adaptation for the invasion of and persistence within human epithelial and immune cells. The resistance of intracellular bacteria to destruction in the phagosome may have originally occurred as a adaptation for survival within free-living amoebae[20], reflecting a long co-evolutionary process that began more than one billion years ago[48].
An association between H. pylori and Candida was first proposed in 1998 when yeast colonies were found as contaminants in gastric biopsy cultures on blood agar plates. Light microscopy revealed the presence of fast-moving bacterium-like bodies (BLBs) inside the vacuoles of 18 gastric yeasts cells that were purified and identified as Candida species based on their morphology and formation of blastoconidia on Sabouraud dextrose agar. The recruited yeasts were sub-cultured on yeast extract-glucose agar containing chloramphenicol several times to ensure the absence of bacterial contamination. Since BLBs from disrupted yeasts were not culturable, polymerase chain reaction (PCR) was used to reveal their bacterial nature. The H. pylori-specific ureA gene product, similar in size to control H. pylori, was amplified from 12/18 (67%) gastric yeast extracts. Furthermore, yeasts and pure cultures of control H. pylori were tested for tolerance to elevated temperatures, desiccation, acidic pH and biocides. The control H. pylori were inactivated upon exposure to stresses, however, yeasts were not inactivated and the intracellular H. pylori showed active movement[49]. There are many reports that indicate the tolerance of yeasts to stressful conditions[50,51]. Accordingly, it was proposed that concurrence of H. pylori and Candida in the human GI tract indicates the existence of a more intimate relationship, with the yeast serving to protect H. pylori from environmental stresses[49].
There are discrepancies concerning the permanence or transience of H. pylori in the oral cavity, and it is not clear whether the oral cavity creates sufficiently favorable conditions for H. pylori growth[52]. Bacterial DNA has been detected in the oral cavity[53,54], dental plaque[55] and saliva[56], thus implicating oral-oral and fecal-oral transmission modes of H. pylori[57]. However, the failure to cultivate H. pylori from the oral cavity and feces[58,59] indicates unfavorable survival conditions, likely due to antagonistic materials secreted by the microfloras of the oral cavity[60] and intestine[58]. On the other hand, persistence of H. pylori in the oral cavity after eradication therapy for gastric infection[61], as well as the resistance of the bacteria against antimicrobial products of oral microflora, suggest that H. pylori must somehow be protected in the oral cavity[62]. Accordingly, it remains unclear whether the oral cavity serves as a potent reservoir for H. pylori gastric reinoculation[63].
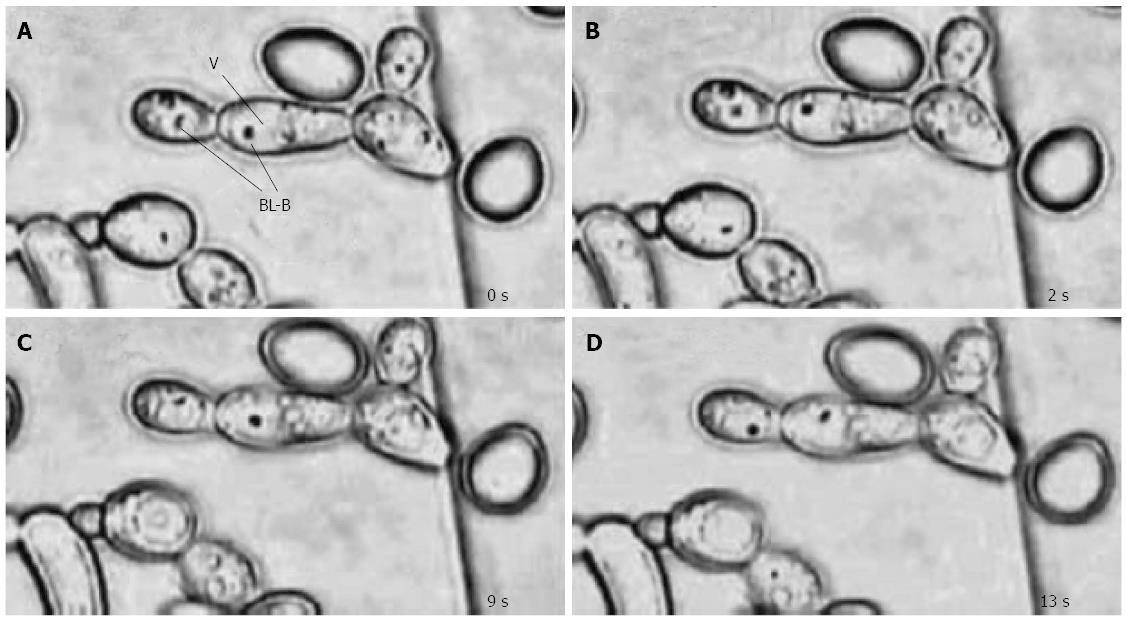
Candida spp. yeasts isolated from oral samples of dyspeptic patients were shown to contain fast-moving and non-culturable BLBs inside their vacuoles (Figure 1)[64]. H. pylori-specific 16S rRNA and cagA[64], as well as vacuolating cytotoxin A (vacA) and ureAB[65], were amplified from these yeasts, showing 98% homology with those of the control H. pylori. These findings indicated that H. pylori are accommodated within the vacuoles of the Candida that thrive on the mucosa of the human oral cavity, and may explain the persistence of H. pylori in the oral cavity, increased risk for reinoculation of the stomach and spread of the bacterium from person-to-person. Accordingly, oral hygiene was suggested as a way to effectively reduce the yeast content in the oral cavity and control H. pylori transmission[64,65].
Although it is well known that H. pylori are gastric colonizers that likely enter the human GI tract through intake of contaminated water or food[66], culturable forms of H. pylori have not been recovered from water[56] or food[67,68] sources. There is no indication for long-term survival or growth of H. pylori in water[69] or food materials such as beef[70], raw chicken, lettuce, milk[71] and yogurt[71,72], due to the presence of oxidation and desiccation[71,73], acidic pH[72], unfavorable temperatures[74] and H. pylori-inhibitory products secreted by food microbiota[71]. Furthermore, it is not known whether the coccoid or non-culturable forms of H. pylori remain viable in food[75] or are able to infect humans[73,76]. Thus, the presence of H. pylori DNA in food and water sources has been thought to result from contamination with either naked DNA or dead bacteria[74,77]. However, reports have indicated that certain pathogenic bacteria, such as L. pneumophila[48], Vibrio cholera[78], Listeria monocytogenes[79], E. coli[80], Campylobacter jejuni[81] and H. pylori[82], have evolved to establish in the vacuoles of free-living amoebae, the inhabitants of water, soil and air, which resist environmental stresses by encysting[83]. Amoebae can protect bacteria against the stressful conditions, provide them with nutrients and serve as mediators for their spread in the environment and within hosts[84,85]. Accordingly, the presence of amoebae in water or the environment could be an important marker of contamination with these pathogenic bacteria[48].
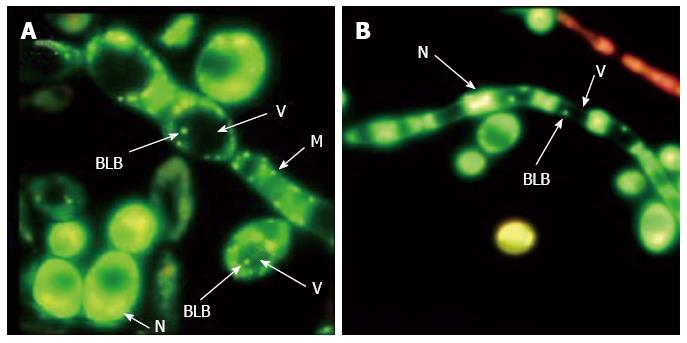
Yeast, another free-living microorganism that exhibits a remarkable resistance to environmental stresses[10,86], can similarly accommodate pathogenic bacteria within vacuoles[87]. In this regard, foodborne yeasts, which are frequently recruited as primary tools for preparation of fermented foods or enter food as environmental contaminants[88,89], may play a crucial role in the transmission of H. pylori. Foodborne yeasts that occur in a variety of food materials such as dairy products[89], fermented foods[90,91] and fruits[92] are able to withstand the stressful treatments applied in food processing, such as high temperatures, desiccation, acidic pH, high salt concentration and sanitization[10,93]. Accordingly, it was proposed that the intracellular establishment of H. pylori in the vacuoles of foodborne yeasts could protect the bacteria against these stressful conditions and play a crucial role in bacterial survival in food[87]. Indeed, H. pylori-specific genes ureAB and babAB were detected in Candida yeasts from Iranian traditional breads (Sangak, Taftoon and Barbary), yogurt, banana skin, grape juice and quince jam, which carried vacuolar fast-moving and non-culturable BLBs (Figure 2)[87]. Thus, foodborne yeasts originating from the environment, which were once considered as harmless microorganisms when ingested through fermented foods such as dairy products[38,89], including kefir and kumis[94,95], could now be pinpointed as a public health problem source. In this regard, occurrence of yeast in food and environment can be considered as an important indicator of contamination with H. pylori and other pathogenic bacteria. Therefore, a key approach for the control of H. pylori infection may be to reduce the yeast content of foods through proper hygienic practice, especially by food handlers and during food processing[87].
Vertical transmission from mother to neonate during vaginal delivery has been considered to be one important mechanism for H. pylori transmission. Although there are no published reports to show vaginal isolation of H. pylori[96], it is plausible to propose that since H. pylori can inhabit the squamous epithelium of the oral cavity, it may therefore be able to survive on the vaginal mucosa[97]. The vagina supports the growth of a number of microaerophilic organisms, suggesting that a coexisting infection could provide conditions favorable for the growth of H. pylori[98-100]. In this regard, a mother’s oral and vaginal yeasts were proposed to play a crucial role in the transmission of H. pylori to the neonate[101].
The mucosal surfaces of the human oral cavity and vulva-vagina are the areas that are the first and second most frequently colonized with yeasts, respectively[46]. A yeast oral carriage rate of up to 75% has been reported in healthy individuals[102], and a considerable proportion (5%-35%) of asymptomatic healthy women have positive vaginal cultures for C. albicans[103], with the highest level of colonization in the vagina compared to other body sites[104]. As the incidence of vaginal yeast colonization during pregnancy can be up to 46%, Candida spp. in infants may be acquired vertically from the mother when passing through the birth canal via cutaneous contact or swallowing of fungi[105,106]. When examining yeasts from pregnant woman, vaginal yeasts were twice as likely to contain the H. pylori-specific genes 16S rRNA and vacA s1 when compared to oral yeasts[101]. Furthermore, the carriage rate of oral yeast was found to be significantly higher in normally delivered neonates compared to those from a cesarean delivery. Moreover, a significant correlation was found between the frequency of H. pylori genes in vaginal yeasts and in oral yeasts of normally delivered neonates, indicating a common source[101].
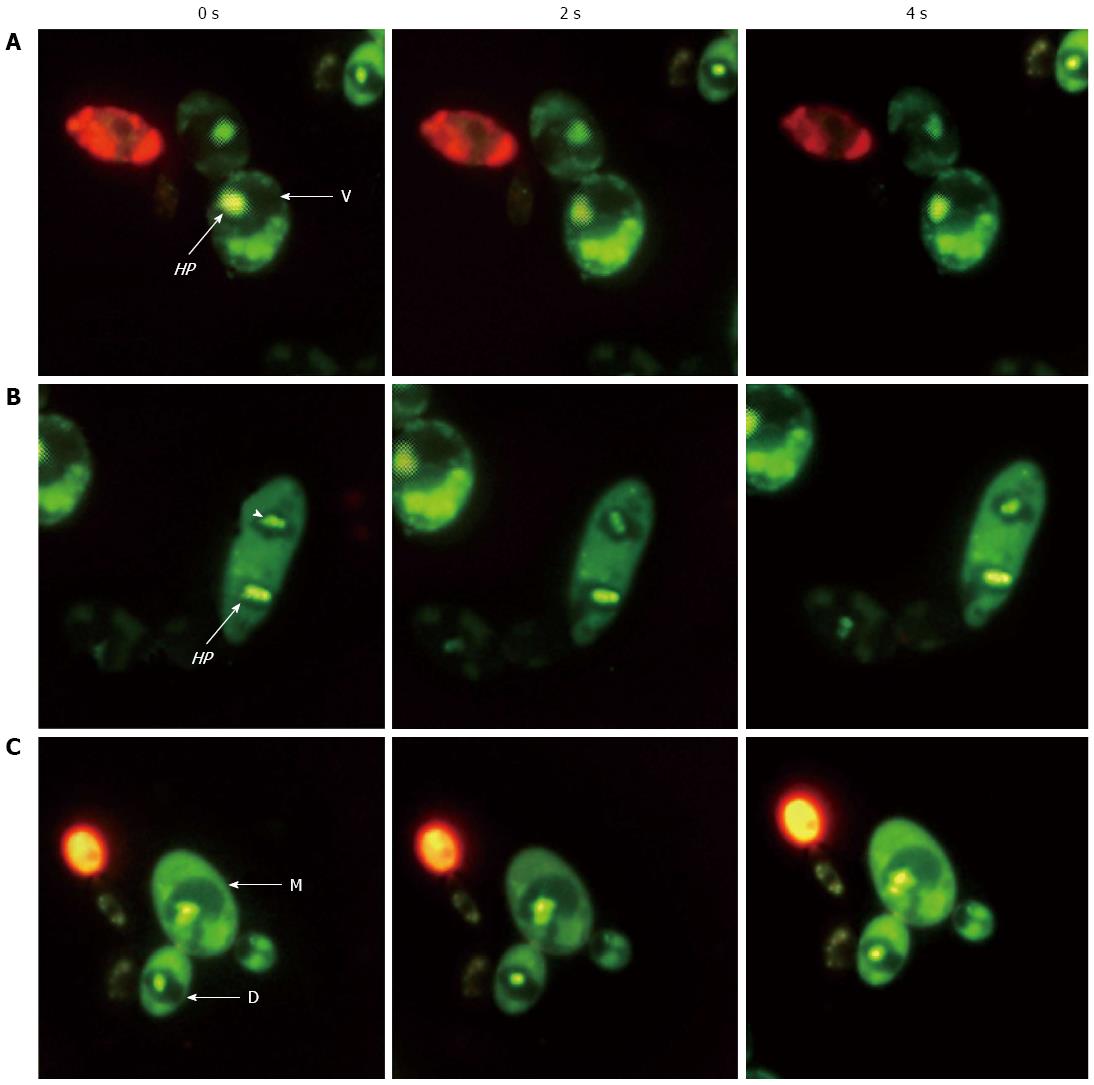
The inheritance of vacuoles by yeast daughter cells is a highly regulated process[107]. Fluorescence microscopy revealed the occurrence of H. pylori cells inside the vacuoles of mother and daughter cells in consecutive subcultures of yeasts (Figure 3)[108]. Some yeasts contained more than one H. pylori cell, indicating the potential for endobacterial cells to multiply and transmit within the vacuole to the next generation of yeast. As a hallmark of endosymbiosis is that both partners need to be alive[109], the mutual adaptation of intracellular bacteria and their eukaryotic hosts would often allow both partners to survive the entire lifespan with the endobacteria transmitted to the next generation[17,110]. Many intracellular bacteria have evolved to recruit certain proteins for protecting the membrane-bound vacuole and promoting intracellular partnership[111]. H. pylori-specific genes, encoding proteins such as VacA, urease and peroxiredoxin, have been detected in the extracts of oral and gastric yeasts[112]. Furthermore, western blotting performed on yeast extracts with antibodies raised against H. pylori revealed the presence of proteins with molecular weights of 56, 36, 32, 26 and 21 kDa, corresponding to the VacA large subunit, VacA small subunit[113,114], urease A subunit[115], peroxiredoxin and thiol peroxidase[116], respectively. These proteins were detected with antibodies such as the IgY-Hp polyclonal antibody, which is used as a powerful tool for detection of H. pylori immunodominant proteins, such as the urease A and B-subunits, Hsp60, peroxiredoxin and thiol peroxidase[116]. Peroxiredoxin and thiol peroxidase may allow the bacteria to detoxify oxygen metabolites formed during processes such as the respiratory burst of immune cells[111]. The bacterial urease and VacA have been recognized as the two important H. pylori virulence factors that influence phago-lysosome fusion and bacterial survival in macrophages[46,117].
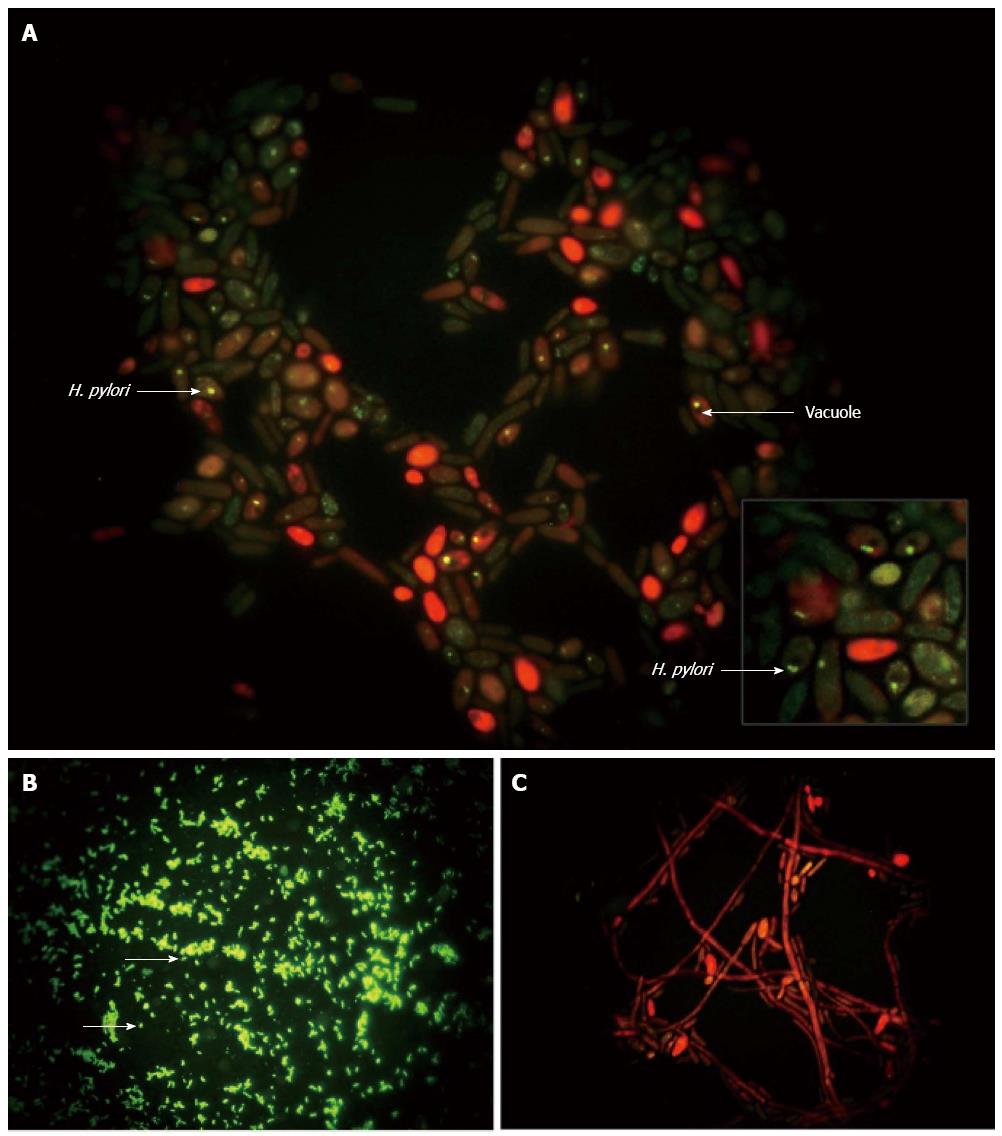
The IgY-Hp antibody has also been used as a marker for localization of H. pylori inside vacuoles of yeast (Figure 4)[108]. Additionally, whole cell H. pylori and H. pylori-immunoreactivity have been observed in lamina propria of gastric biopsy specimens[118]. Furthermore, the specific identity and localization of immunoreactive H. pylori within defined membrane-bound vacuoles has been revealed with confocal[119] and ultrastructural[120] microscopy. The preserved ultrastructural morphology and presence of H. pylori-specific mRNA, detected with fluorescence in situ hybridization, indicated the viability of these intracellular bacteria[119,120].
Taken together, the data suggest that H. pylori are well-equipped for invasion of eukaryotic cells and survival within their vacuoles[108,112]. The intimate relationship between these two organisms suggests that yeasts are the unknown non-human source from which H. pylori were able to infect humans as early as 100000 years ago[121]. Establishment of H. pylori inside the ubiquitous yeast might explain why such a fastidious bacteria is able to survive outside the human stomach and remain highly prevalent in certain populations, with yeast acting as a Trojan horse, ferrying the potentially infectious H. pylori into the GI tract environment. It is possible that the unique property of ergosterol dependence in H. pylori evolved as the result of adaptation to life inside the yeast vacuole, showing the crucial role of this organelle in the evolution and persistence of H. pylori. Further studies will elucidate how the intracellular life of H. pylori inside yeast influenced its adaptation for existence in the human stomach and long-term colonization of gastric epithelium, as well as provide insight regarding the remarkable heterogeneity of virulence determinants, and resistance to antibiotics and the immune system. Furthermore, examination of individuals within a population, with consideration of their yeast carriage, consumption of foods containing live yeast and H. pylori infection status, will guide understanding of the spread of H. pylori among humans.
P- Reviewers: Arino J, Galgoczy L, Gurevich VV, Krishnan T S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Loster BW, Majewski SW, Cześnikiewicz-Guzik M, Bielanski W, Pierzchalski P, Konturek SJ. The relationship between the presence of Helicobacter pylori in the oral cavity and gastric in the stomach. J Physiol Pharmacol. 2006;57 Suppl 3:91-100. [PubMed] [Cited in This Article: ] |
| 2. | Ruiz-Lozano JM, Bonfante P. Identification of a putative P-transporter operon in the genome of a Burkholderia strain living inside the arbuscular mycorrhizal fungus Gigaspora margarita. J Bacteriol. 1999;181:4106-4109. [PubMed] [Cited in This Article: ] |
| 3. | Dubois A, Borén T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 2007;9:1108-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Petersen AM, Krogfelt KA. Helicobacter pylori: an invading microorganism? A review. FEMS Immunol Med Microbiol. 2003;36:117-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Wyle FA, Tarnawski A, Schulman D, Dabros W. Evidence for gastric mucosal cell invasion by C. pylori: an ultrastructural study. J Clin Gastroenterol. 1990;12 Suppl 1:S92-S98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Chu YT, Wang YH, Wu JJ, Lei HY. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010;78:4157-4165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Noach LA, Rolf TM, Tytgat GN. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Wang YH, Wu JJ, Lei HY. The autophagic induction in Helicobacter pylori-infected macrophage. Exp Biol Med (Maywood). 2009;234:171-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Wang YH, Gorvel JP, Chu YT, Wu JJ, Lei HY. Helicobacter pylori impairs murine dendritic cell responses to infection. PLoS One. 2010;5:e10844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Fleet GH. Yeasts in dairy products. J Appl Bacteriol. 1990;68:199-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 242] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Cavalier-Smith T. The neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification. Int J Syst Evol Microbiol. 2002;52:7-76. [PubMed] [Cited in This Article: ] |
| 12. | Gehrig H, Schüssler A, Kluge M. Geosiphon pyriforme, a fungus forming endocytobiosis with Nostoc (cyanobacteria), is an ancestral member of the Glomales: evidence by SSU rRNA analysis. J Mol Evol. 1996;43:71-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Douglas AE. Symbiotic interactions. New York, NY: Oxford University Press 1994; . [Cited in This Article: ] |
| 14. | Scannerini S, Bonfante P. Bacteria and bacteria like objects in endomycorrhizal fungi (Glomaceae). Symbiosis as source of evolutionary innovation: Speciation and morphogenesis. Cambridge: MIT Press 1991; 273-287. [Cited in This Article: ] |
| 15. | Bianciotto V, Lumini E, Lanfranco L, Minerdi D, Bonfante P, Perotto S. Detection and identification of bacterial endosymbionts in arbuscular mycorrhizal fungi belonging to the family Gigasporaceae. Appl Environ Microbiol. 2000;66:4503-4509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Simon L, Bousquet J, Lévesque RC, Lalonde M. Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature. 1993;363:67-69. [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 545] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | Bianciotto V, Bandi C, Minerdi D, Sironi M, Tichy HV, Bonfante P. An obligately endosymbiotic mycorrhizal fungus itself harbors obligately intracellular bacteria. Appl Environ Microbiol. 1996;62:3005-3010. [PubMed] [Cited in This Article: ] |
| 18. | MacDonald RM, Chandler MR, Mosse B. The occurrence of bacterium-like organelles in vesicular-arbascular mycorizal fungi. New Phytol. 1982;90:659-663. [DOI] [Cited in This Article: ] |
| 19. | Moran NA, Degnan PH, Santos SR, Dunbar HE, Ochman H. The players in a mutualistic symbiosis: insects, bacteria, viruses, and virulence genes. Proc Natl Acad Sci USA. 2005;102:16919-16926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Bozue JA, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668-673. [PubMed] [Cited in This Article: ] |
| 21. | Michel R, Burghardt H, Bergmann H. [Acanthamoeba, naturally intracellularly infected with Pseudomonas aeruginosa, after their isolation from a microbiologically contaminated drinking water system in a hospital]. Zentralbl Hyg Umweltmed. 1995;196:532-544. [PubMed] [Cited in This Article: ] |
| 22. | Laochumroonvorapong P, Paul S, Manca C, Freedman VH, Kaplan G. Mycobacterial growth and sensitivity to H2O2 killing in human monocytes in vitro. Infect Immun. 1997;65:4850-4857. [PubMed] [Cited in This Article: ] |
| 23. | Marolda CL, Hauröder B, John MA, Michel R, Valvano MA. Intracellular survival and saprophytic growth of isolates from the Burkholderia cepacia complex in free-living amoebae. Microbiology. 1999;145:1509-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Saini LS, Galsworthy SB, John MA, Valvano MA. Intracellular survival of Burkholderia cepacia complex isolates in the presence of macrophage cell activation. Microbiology. 1999;145:3465-3475. [PubMed] [Cited in This Article: ] |
| 25. | Martin DW, Mohr CD. Invasion and intracellular survival of Burkholderia cepacia. Infect Immun. 2000;68:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Keig PM, Ingham E, Kerr KG. Invasion of human type II pneumocytes by Burkholderia cepacia. Microb Pathog. 2001;30:167-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Berk SG, Ting RS, Turner GW, Ashburn RJ. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol. 1998;64:279-286. [PubMed] [Cited in This Article: ] |
| 28. | Bianciotto V, Genre A, Jargeat P, Lumini E, Bécard G, Bonfante P. Vertical transmission of endobacteria in the arbuscular mycorrhizal fungus Gigaspora margarita through generation of vegetative spores. Appl Environ Microbiol. 2004;70:3600-3608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Klionsky DJ, Herman PK, Emr SD. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990;54:266-292. [PubMed] [Cited in This Article: ] |
| 30. | Kane PM. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev. 2006;70:177-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 326] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 31. | Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 897] [Cited by in F6Publishing: 909] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 32. | Jin H, McCaffery JM, Grote E. Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J Cell Biol. 2008;180:813-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Schneiter R, Brügger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J Cell Biol. 1999;146:741-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 400] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Haque M, Hirai Y, Yokota K, Mori N, Jahan I, Ito H, Hotta H, Yano I, Kanemasa Y, Oguma K. Lipid profile of Helicobacter spp.: presence of cholesteryl glucoside as a characteristic feature. J Bacteriol. 1996;178:2065-2070. [PubMed] [Cited in This Article: ] |
| 35. | Hildebrandt E, McGee DJ. Helicobacter pylori lipopolysaccharide modification, Lewis antigen expression, and gastric colonization are cholesterol-dependent. BMC Microbiol. 2009;9:258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, Zähringer U, Mollenkopf HJ, Heinz E, Meyer TF. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | McGee DJ, George AE, Trainor EA, Horton KE, Hildebrandt E, Testerman TL. Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37. Antimicrob Agents Chemother. 2011;55:2897-2904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Welthagen JJ, Viljoen BC. Yeast profile in Gouda cheese during processing and ripening. Int J Food Microbiol. 1998;41:185-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Odds FC. Candida infections: an overview. Crit Rev Microbiol. 1987;15:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 201] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Soll DR, Galask R, Schmid J, Hanna C, Mac K, Morrow B. Genetic dissimilarity of commensal strains of Candida spp. carried in different anatomical locations of the same healthy women. J Clin Microbiol. 1991;29:1702-1710. [PubMed] [Cited in This Article: ] |
| 41. | Hogan DA, Kolter R. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science. 2002;296:2229-2232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 402] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 42. | Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1314] [Cited by in F6Publishing: 1204] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 43. | Pittet D, Li N, Wenzel RP. Association of secondary and polymicrobial nosocomial bloodstream infections with higher mortality. Eur J Clin Microbiol Infect Dis. 1993;12:813-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Gasch AP, Werner-Washburne M. The genomics of yeast responses to environmental stress and starvation. Funct Integr Genomics. 2002;2:181-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 45. | Soll DR. Candida commensalism and virulence: the evolution of phenotypic plasticity. Acta Trop. 2002;81:101-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Kalogeropoulos NK, Whitehead R. Campylobacter-like organisms and Candida in peptic ulcers and similar lesions of the upper gastrointestinal tract: a study of 247 cases. J Clin Pathol. 1988;41:1093-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Ansorg R, Schmid EN. Adhesion of Helicobacter pylori to yeast cells. Zentralbl Bakteriol. 1998;288:501-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17:413-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 727] [Cited by in F6Publishing: 699] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 49. | Siavoshi F, Nourali-Ahari F, Zeinali S, Hashemi-Dogaheh MH, Malekzadeh R, Massarrat S. Yeast protects Helicobacter pylori against the environmental stress. Arch Iran Med. 1998;1:2-8. [Cited in This Article: ] |
| 50. | Jones MV, Johnson MD, Herd TM. Sensitivity of yeast vegetative cells and ascospores to biocides and environmental stress. Lett Appl Microbiol. 1991;12:254-257. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Phaff HJ, Starmer WT. Yeasts associated with plants, insects and soil. The yeasts. London: Academic Press 1987; 181-205. [Cited in This Article: ] |
| 52. | Bielański W. Epidemiological study on Helicobacter pylori infection and extragastroduodenal disorders in Polish population. J Physiol Pharmacol. 1999;50:723-733. [PubMed] [Cited in This Article: ] |
| 53. | Song Q, Spahr A, Schmid RM, Adler G, Bode G. Helicobacter pylori in the oral cavity: high prevalence and great DNA diversity. Dig Dis Sci. 2000;45:2162-2167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Umeda M, Kobayashi H, Takeuchi Y, Hayashi J, Morotome-Hayashi Y, Yano K, Aoki A, Ohkusa T, Ishikawa I. High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J Periodontol. 2003;74:129-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Banatvala N, Lopez CR, Owen R, Abdi Y, Davies G, Hardie J, Feldman R. Helicobacter pylori in dental plaque. Lancet. 1993;341:380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Mapstone NP, Lynch DA, Lewis FA, Axon AT, Tompkins DS, Dixon MF, Quirke P. Identification of Helicobacter pylori DNA in the mouths and stomachs of patients with gastritis using PCR. J Clin Pathol. 1993;46:540-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 171] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Bussac G. Helicobacter pylori and the oral environment. Pract Periodontics Aesthet Dent. 1999;11:918, 920, 922. [PubMed] [Cited in This Article: ] |
| 58. | Sahay P, Axon AT. Reservoirs of Helicobacter pylori and modes of transmission. Helicobacter. 1996;1:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Sahay P, West AP, Hawkey PM, Axon AT. Isolation of Helicobacter pylori from faeces. J Infect. 1995;30:262-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Okuda K, Ishihara K, Miura T, Katakura A, Noma H, Ebihara Y. Helicobacter pylori may have only a transient presence in the oral cavity and on the surface of oral cancer. Microbiol Immunol. 2000;44:385-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Pytko-Polonczyk J, Konturek SJ, Karczewska E, Bielański W, Kaczmarczyk-Stachowska A. Oral cavity as permanent reservoir of Helicobacter pylori and potential source of reinfection. J Physiol Pharmacol. 1996;47:121-129. [PubMed] [Cited in This Article: ] |
| 62. | Majka J, Róg T, Konturek PC, Konturek SJ, Bielański W, Kowalsky M, Szczudlik A. Influence of chronic Helicobacter pylori infection on ischemic cerebral stroke risk factors. Med Sci Monit. 2002;8:CR675-CR684. [PubMed] [Cited in This Article: ] |
| 63. | Cześnikiewicz-Guzik M, Karczewska E, Bielański W, Guzik TJ, Kapera P, Targosz A, Konturek SJ, Loster B. Association of the presence of Helicobacter pylori in the oral cavity and in the stomach. J Physiol Pharmacol. 2004;55 Suppl 2:105-115. [PubMed] [Cited in This Article: ] |
| 64. | Siavoshi F, Salmanian AH, Akbari F, Malekzadeh R, Massarrat S. Detection of Helicobacter pylori-specific genes in the oral yeast. Helicobacter. 2005;10:318-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Salmanian AH, Siavoshi F, Akbari F, Afshari A, Malekzadeh R. Yeast of the oral cavity is the reservoir of Heliobacter pylori. J Oral Pathol Med. 2008;37:324-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Thomas E, Jiang C, Chi DS, Li C, Ferguson DA. The role of the oral cavity in Helicobacter pylori infection. Am J Gastroenterol. 1997;92:2148-2154. [PubMed] [Cited in This Article: ] |
| 67. | Mapstone NP, Lynch DA, Lewis FA, Axon AT, Tompkins DS, Dixon MF, Quirke P. PCR identification of Helicobacter pylori in faeces from gastritis patients. Lancet. 1993;341:447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | van Duynhoven YT, de Jonge R. Transmission of Helicobacter pylori: a role for food? Bull World Health Organ. 2001;79:455-460. [PubMed] [Cited in This Article: ] |
| 69. | Hegarty JP, Dowd MT, Baker KH. Occurrence of Helicobacter pylori in surface water in the United States. J Appl Microbiol. 1999;87:697-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 70. | Stevenson TH, Bauer N, Lucia LM, Acuff GR. Attempts to isolate Helicobacter from cattle and survival of Helicobacter pylori in beef products. J Food Prot. 2000;63:174-178. [PubMed] [Cited in This Article: ] |
| 71. | Poms RE, Tatini SR. Survival of Helicobacter pylori in ready-to-eat foods at 4 degrees C. Int J Food Microbiol. 2001;63:281-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Midolo PD, Lambert JR, Hull R, Luo F, Grayson ML. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J Appl Bacteriol. 1995;79:475-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Kusters JG, Gerrits MM, Van Strijp JA, Vandenbroucke-Grauls CM. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997;65:3672-3679. [PubMed] [Cited in This Article: ] |
| 74. | Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1446] [Cited by in F6Publishing: 1411] [Article Influence: 78.4] [Reference Citation Analysis (1)] |
| 75. | Enroth H, Wreiber K, Rigo R, Risberg D, Uribe A, Engstrand L. In vitro aging of Helicobacter pylori: changes in morphology, intracellular composition and surface properties. Helicobacter. 1999;4:7-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Bode G, Mauch F, Malfertheiner P. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol Infect. 1993;111:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 151] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Enroth H, Engstrand L. Immunomagnetic separation and PCR for detection of Helicobacter pylori in water and stool specimens. J Clin Microbiol. 1995;33:2162-2165. [PubMed] [Cited in This Article: ] |
| 78. | Thom S, Warhurst D, Drasar BS. Association of Vibrio cholerae with fresh water amoebae. J Med Microbiol. 1992;36:303-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Ly TM, Müller HE. Ingested Listeria monocytogenes survive and multiply in protozoa. J Med Microbiol. 1990;33:51-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 111] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Alsam S, Jeong SR, Sissons J, Dudley R, Kim KS, Khan NA. Escherichia coli interactions with Acanthamoeba: a symbiosis with environmental and clinical implications. J Med Microbiol. 2006;55:689-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Axelsson-Olsson D, Waldenström J, Broman T, Olsen B, Holmberg M. Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni. Appl Environ Microbiol. 2005;71:987-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Winiecka-Krusnell J, Wreiber K, von Euler A, Engstrand L, Linder E. Free-living amoebae promote growth and survival of Helicobacter pylori. Scand J Infect Dis. 2002;34:253-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Rodríguez-Zaragoza S. Ecology of free-living amoebae. Crit Rev Microbiol. 1994;20:225-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 295] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 84. | Rowbotham TJ. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 718] [Cited by in F6Publishing: 664] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 85. | Winiecka-Krusnell J, Linder E. Free-living amoebae protecting Legionella in water: the tip of an iceberg? Scand J Infect Dis. 1999;31:383-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Attfield PV. Stress tolerance: the key to effective strains of industrial baker’s yeast. Nat Biotechnol. 1997;15:1351-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 325] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 87. | Salmanian AH, Siavoshi F, Beyrami Z, Latifi-Navid S, Tavakolian A, Sadjadi A. Foodborne yeasts serve as reservoirs of Helicobacter pylori. J Food Saf. 2012;32:152-160. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Viljoen BC, Greyling T. Yeasts associated with Cheddar and Gouda making. Int J Food Microbiol. 1995;28:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Viljoen BC. The interaction between yeasts and bacteria in dairy environments. Int J Food Microbiol. 2001;69:37-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 90. | Ingram M. Yeasts in food spoilage. Chemistry and Biology of Yeasts. London: Academic Press 1958; 603-633. [Cited in This Article: ] |
| 91. | Kosikowski FV. Cheese and Fermented Milk Foods. Ann Arbor: Edwards Bros 1977; . [Cited in This Article: ] |
| 92. | Heard GM, Fleet GH. The effects of temperature and pH on the growth of yeast species during the fermentation of grape juice. J Appl Bacteriol. 1988;65:23-8. [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | Laubsher PJ, Viljoen BC. The resistance of dairy yeasts against commercially available cleaning compounds and sanitizers. Food Technology and Biotechnology. 1999;37:281-286. [Cited in This Article: ] |
| 94. | Fleet G. Spoilage yeasts. Crit Rev Biotechnol. 1992;12:1-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 247] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 95. | Gadaga TH, Mutukumira AN, Narvhus JA. Growth characteristics of Candida kefyr and two strains of Lactococcus lactis subsp. lactis isolated from Zimbabwean naturally fermented milk. Int J Food Microbiol. 2001;70:11-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Martín-De-Argila C, García Arata I, Boixeda D, Sancha M, Cantón R, Baquero F. Failure to detect Helicobacter pylori in vaginal secretions. Clin Microbiol Infect. 1998;4:412-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 97. | Eslick GD. Helicobacter pylori infection transmitted sexually via oral-genital contact: a hypothetical model. Sex Transm Infect. 2000;76:489-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Ellis JE, Yarlett N, Cole D, Humphreys MJ, Lloyd D. Antioxidant defences in the microaerophilic protozoan Trichomonas vaginalis: comparison of metronidazole-resistant and sensitive strains. Microbiology. 1994;140:2489-2494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 99. | Pine L, Malcolm GB, Curtis EM, Brown JM. Demonstration of Actinomyces and Arachnia species in cervicovaginal smears by direct staining with species-specific fluorescent-antibody conjugate. J Clin Microbiol. 1981;13:15-21. [PubMed] [Cited in This Article: ] |
| 100. | Reid G, McGroarty JA, Tomeczek L, Bruce AW. Identification and plasmid profiles of Lactobacillus species from the vagina of 100 healthy women. FEMS Immunol Med Microbiol. 1996;15:23-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Siavoshi F, Taghikhani A, Malekzadeh R, Sarrafnejad A, Kashanian M, Jamal AS, Saniee P, Sadeghi S, Sharifi AH. The role of mother’s oral and vaginal yeasts in transmission of Helicobacter pylori to neonates. Arch Iran Med. 2013;16:288-294. [PubMed] [Cited in This Article: ] |
| 102. | Martin MV, Wilkinson GR. The oral yeast flora of 10-year-old schoolchildren. Sabouraudia. 1983;21:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Linares LM, de Marin C. Frequency of yeasts of the genus Candida in humans, as pathogens and as part of normal flora. Brazil: Pan American Health Organization 1987; 124-133. [Cited in This Article: ] |
| 104. | Lee JC, King RD. Characterization of Candida albicans adherence to human vaginal epithelial cells in vitro. Infect Immun. 1983;41:1024-1030. [PubMed] [Cited in This Article: ] |
| 105. | Whyte RK, Hussain Z, deSa D. Antenatal infections with Candida species. Arch Dis Child. 1982;57:528-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 98] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Maudsley RF, Brix GA, Hinton NA, Robertson EM, Bryans AM, Haust MD. Placental inflammation and infection. A prospective bacteriologic and histologic study. Am J Obstet Gynecol. 1966;95:648-659. [PubMed] [Cited in This Article: ] |
| 107. | Seeley ES, Kato M, Margolis N, Wickner W, Eitzen G. Genomic analysis of homotypic vacuole fusion. Mol Biol Cell. 2002;13:782-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 108. | Saniee P, Siavoshi F, Nikbakht Broujeni G, Khormali M, Sarrafnejad A, Malekzadeh R. Localization of H.pylori within the vacuole of Candida yeast by direct immunofluorescence technique. Arch Iran Med. 2013;16:705-710. [PubMed] [Cited in This Article: ] |
| 109. | Lumini E, Ghignone S, Bianciotto V, Bonfante P. Endobacteria or bacterial endosymbionts? To be or not to be. New Phytol. 2006;170:205-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Ewald PW. Evolution of infectious disease. USA: Oxford University Press 1996; . [Cited in This Article: ] |
| 111. | Mauël J. [Mechanisms of intracellular microbicide]. Bull Eur Physiopathol Respir. 1983;19:115-122. [PubMed] [Cited in This Article: ] |
| 112. | Saniee P, Siavoshi F, Nikbakht Broujeni G, Khormali M, Sarrafnejad A, Malekzadeh R. Immunodetection of Helicobacter pylori-specific proteins in oral and gastric Candida yeasts. Arch Iran Med. 2013;16:624-630. [PubMed] [Cited in This Article: ] |
| 113. | Ye D, Willhite DC, Blanke SR. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J Biol Chem. 1999;274:9277-9282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Torres VJ, Ivie SE, McClain MS, Cover TL. Functional properties of the p33 and p55 domains of the Helicobacter pylori vacuolating cytotoxin. J Biol Chem. 2005;280:21107-21114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 115. | Filipec Kanizaj T, Katicić M, Presecki V, Gasparov S, Colić Cvrlje V, Kolarić B, Mrzljak A. Serum antibodies positivity to 12 Helicobacter pylori virulence antigens in patients with benign or malignant gastroduodenal diseases--cross-sectional study. Croat Med J. 2009;50:124-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 116. | Shin JH, Nam SW, Kim JT, Yoon JB, Bang WG, Roe IH. Identification of immunodominant Helicobacter pylori proteins with reactivity to H. pylori-specific egg-yolk immunoglobulin. J Med Microbiol. 2003;52:217-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Zwolinska-Wcisło M, Budak A, Bogdał J, Trojanowska D, Stachura J. Fungal colonization of gastric mucosa and its clinical relevance. Med Sci Monit. 2001;7:982-988. [PubMed] [Cited in This Article: ] |
| 118. | Andersen LP, Holck S. Possible evidence of invasiveness of Helicobacter (Campylobacter) pylori. Eur J Clin Microbiol Infect Dis. 1990;9:135-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187:1165-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 120. | Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 121. | Moodley Y, Linz B, Bond RP, Nieuwoudt M, Soodyall H, Schlebusch CM, Bernhöft S, Hale J, Suerbaum S, Mugisha L. Age of the association between Helicobacter pylori and man. PLoS Pathog. 2012;8:e1002693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 210] [Article Influence: 17.5] [Reference Citation Analysis (1)] |