Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4345
Revised: December 18, 2013
Accepted: January 19, 2014
Published online: April 21, 2014
AIM: To investigate hepcidin expression, interleukin-6 (IL-6) production and iron levels in the rat colon in the presence of trinitrobenzene sulfonic acid (TNBS)-induced colitis.
METHODS: In rats, we evaluated the severity of colitis induced by repeated TNBS administration using macroscopic and microscopic scoring systems and myeloperoxidase activity measurements. The colonic levels of hepcidin, tumor necrosis factor alpha (TNF-α), IL-10 and IL-6 were measured by Enzyme-Linked Immunosorbent Assay, and hepcidin-25 expression and iron deposition were analyzed by immunohistochemistry and the Prussian blue reaction, respectively. Stat-3 phosphorylation was assessed by Western blot analysis. Hematological parameters, iron and transferrin levels, and transferrin saturation were also measured. Additionally, the ability of iron, pathogen-derived molecules and IL-6 to induce hepcidin expression in HT-29 cells was evaluated.
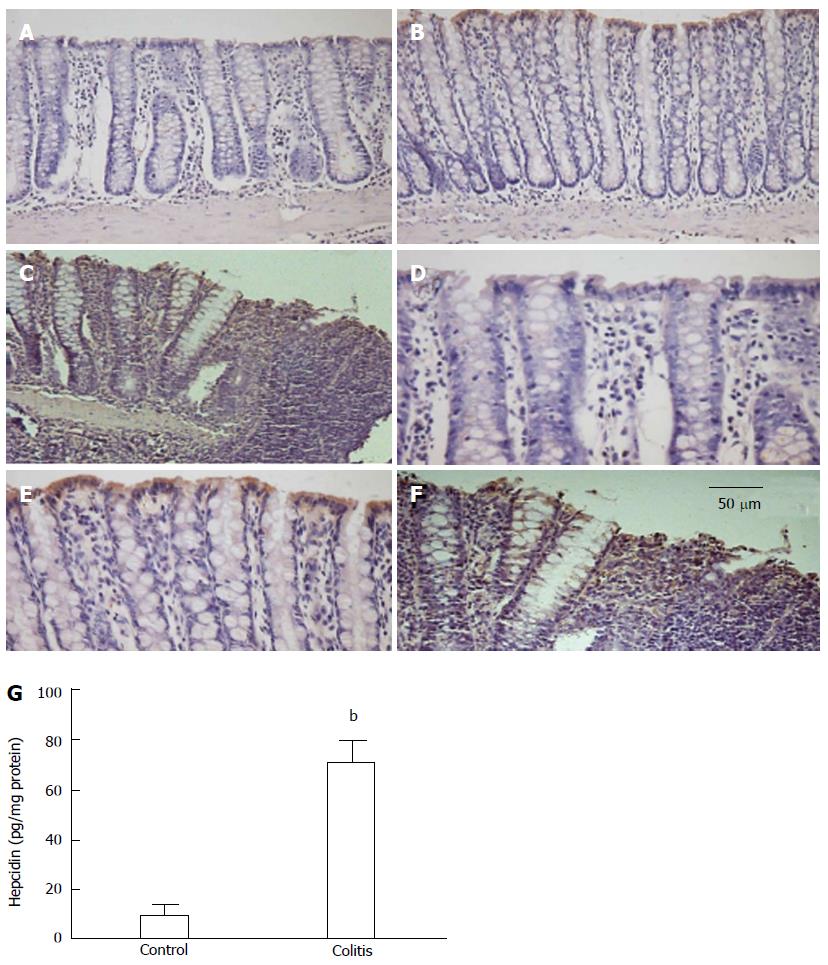
RESULTS: Repeated TNBS administration to rats resulted in macroscopically and microscopically detectable colon lesions and elevated colonic myeloperoxidase activity. Hepcidin-25 protein levels were increased in colonic surface epithelia in colitic rats (10.2 ± 4.0 pg/mg protein vs 71.0 ± 8.4 pg/mg protein, P < 0.01). Elevated IL-6 levels (8.2 ± 1.7 pg/mg protein vs 14.7 ± 0.7 pg/mg protein, P < 0.05), TNF-α levels (1.8 ± 1.2 pg/mg protein vs 7.4 ± 2.1 pg/mg protein, P < 0.05) and Stat-3 phosphorylation were also observed. Systemic alterations in iron homeostasis, hepcidin levels and anemia were not detected in colitic rats. Iron deposition in the colon was only observed during colitis. Hepcidin gene expression was increased in HT-29 cells after IL-6 and lipopolysaccharide [a toll-like receptor 4 (TLR-4) ligand] treatment. Deferoxamine, ferric citrate and peptidoglycan (a TLR-2 ligand) were unable to alter the in vitro expression of hepcidin in HT-29 cells.
CONCLUSION: Colitis increased local hepcidin-25 expression, which was associated with the IL-6/Stat-3 signaling pathway. An increase in local iron sequestration was also observed, but additional studies are needed to determine whether this sequestration is a defensive or pathological response to intestinal inflammation.
Core tip: Hepcidin is an endogenous peptide with weak antimicrobial properties that regulates changes in iron metabolism during inflammation. However, infection-associated cytokines, pathogen-derived molecules or whole pathogens can induce hepcidin synthesis as part of the host response to infection. This is the first study to describe that colitis induces hepcidin expression in colons associated with the Interleukin-6/Stat-3 signaling pathways and local iron sequestration. This finding suggests a host response to infection because reducing the iron available to pathogens is an important antimicrobial mechanism. However, we could not exclude the possibility that hepcidin expression contributes to increased local inflammation by stimulating pro-inflammatory macrophages.
- Citation: Gotardo &MF, Ribeiro GA, Clemente TRL, Moscato CH, Tomé RBG, Rocha T, Pedrazzoli Jr J, Ribeiro ML, Gambero A. Hepcidin expression in colon during trinitrobenzene sulfonic acid-induced colitis in rats. World J Gastroenterol 2014; 20(15): 4345-4352
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4345
In addition to intestinal inflammation, Crohn’s disease (CD) and ulcerative colitis (UC) patients present with several extraintestinal symptoms, including systemic iron deficiency. Iron deficiency is the main cause of anemia in inflammatory bowel disease (IBD) patients, which occurs as result of several factors, such as intestinal blood loss, absorptive deficiencies and/or undertreatment of anemia[1]. Anemia of chronic disease (ACD), also known as anemia of inflammation, is the second most common cause of anemia in IBD patients; it frequently occurs with iron deficiency[2,3]. ACD is normally associated with low serum iron levels and low transferrin saturation. However, in this case, hypoferremia is a consequence of macrophage iron sequestration; therefore, the total body iron content may be increased[4].
Inflammatory cytokines produced during inflammation, most notably interleukin-6 (IL-6), have been hypothesized to contribute to systemic iron deficiency through their effects on hepcidin synthesis[5]. Hepcidin is a 25-amino acid cysteine-rich peptide with weak antimicrobial properties that is regulated by a number of factors such as liver iron levels, inflammation, hypoxia and anemia. Hepcidin is mainly expressed and released by the liver in response to increased circulating iron levels, but hepcidin synthesis also occurs to a lesser degree in adipose tissue, the heart, placenta and kidneys. However, the functions of hepcidin in these tissues are currently unknown[6]. Furthermore, hepcidin decreases intestinal iron absorption and blocks macrophage iron efflux, thus protecting the body against excessive iron levels[7].
Infection-associated cytokines, pathogen-derived molecules and whole pathogens can also induce hepcidin synthesis as part of the host response to infection because reducing available iron is an important antimicrobial mechanism[8]. Hepcidin has been proposed to be directly involved in IBD pathogenesis because in Hfe knockout mice, which have low hepcidin expression, the severity of lipopolysaccharide (LPS)- and Salmonella-induced enterocolitis is attenuated[9]. On the other hand, antimicrobial peptides, such as hepcidin, are induced in response to gut microbe invasion during colonic inflammation and affect colitis progression[10,11]. Furthermore, hepcidin has been found to be expressed in human gastric parietal cells and colorectal cancer tissue[12,13], but no data are currently available regarding the expression of hepcidin in the colon during IBD.
Therefore, we investigated rat colonic hepcidin expression in trinitrobenzene sulfonic acid (TNBS)-induced colitis[14] and correlated hepcidin expression with local IL-6 production and iron levels. This experimental model included periods of relapse and remission that resembled the colonic inflammation present in human IBD[14]. The ability of HT-29 cells[15] to express hepcidin was also assessed.
Specific pathogen-free male Wistar rats (200-250 g, 6-8 wk old) were obtained from CEMIB (State University of Campinas, Campinas, SP, Brazil). All experiments were performed in accordance with the principles outlined by the Brazilian College for Animal Experimentation and received approval from the Ethics Committee of São Francisco University, Bragança Paulista, SP, Brazil (Protocol 002.09.09). Rats were maintained in a room with controlled humidity and temperature in collective cages and were exposed to 12 h light-dark cycles. Twelve hours prior to experimental procedures, the animals were deprived of food (standard chow) but not water. Each study used 5-7 rats per group.
Animals were anesthetized with ketamine/xylazine (1:1, v/v), and colitis was induced by the intracolonic administration of 3 mg TNBS dissolved in 0.3 mL of 50% ethanol (Sigma, St. Louis, MO, United States). The solution was injected into the colon 8 cm proximal to the anus using a catheter. TNBS administration lasted only a few seconds, and the rats were maintained in a vertical position until they recovered from anesthesia. The same procedure was repeated 14 and 28 d after the first TNBS administration, and the rats were sacrificed on 35th day. Control rats received saline through the same route. An additional colitis induction protocol was performed, and the rats were evaluated 24 h after the first TNBS administration.
Blood samples were collected from the rat vena cava under anesthesia (1:1, v/v of xylazine 2%-ketamine 10%). Blood/ethylenediamine tetraacetic acid (EDTA) was used to analyze the hematological parameters (ABX Pentra 120, Horiba Instruments Brazil, Jundiai, SP, Brazil), and serum samples were obtained from collected blood lacking anticoagulant solution for the enzymatic assay (EIA) quantification of transferrin (GenWay, San Diego, CA, United States) and hepcidin (USCN Life Science, Wuhan, China) levels. Iron levels and the iron binding capacity were also measured in serum samples using colorimetric commercial kits (Bioclin, Belo Horizonte, MG, Brazil).
Colons were immediately removed from the animals, opened lengthwise and evaluated for macroscopically visible damage by two observers who were blinded to the experimental groups. The criteria for macroscopic colonic damage were as follows: no damage (0 points), hyperemia without ulcers (1 point), linear ulcer with no significant inflammation (2 points), linear ulcer with inflammation at one site (3 points), two or more sites of ulceration/inflammation (4 points), and two or more major sites of ulceration and inflammation or one site of ulceration/inflammation extending 1 cm along the length of the colon (5 points). If the damage extended at least a 2 cm length of colon tissue, the score was increased by 1 point for each additional centimeter of involvement (6-10 points).
Lengthwise colon samples obtained from a site of macroscopically detectable inflammation (or a corresponding tissue site with no macroscopically detectable inflammation) were homogenized in 0.5% (w/v) hexadecyltrimethylammonium bromide in 50 mmol/L potassium phosphate buffer, pH 6.0. For the myeloperoxidase assay, 50 μL of each sample was added to 200 μL of o-dianisidine solution (0.167 mg/mL o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide in 50 mmol/L phosphate buffer, pH 6.0) immediately prior to measuring the absorbance at 460 nm over a 5-min period using a microplate reader (Multiscan MS, Labsystems).
To measure cytokine concentrations by EIA, colon tissue samples were collected as described above, excised and immediately homogenized in solubilization buffer at 4 °C (1% Triton X-100, 100 mmol/L Tris-HCl, 100 mmol/L sodium pyrophosphate, 100 mmol/L sodium fluoride, 10 mmol/L EDTA, 10 mmol/L sodium orthovanadate, 2.0 mmol/L phenylmethylsulfonyl fluoride, and 0.1 mg/mL aprotinin). The insoluble fraction was removed by centrifugation at 9000 g for 20 min at 4 °C. The protein supernatant concentrations were determined using the Biuret method. Hepcidin, tumor necrosis factor alpha (TNF-α), IL-10 and IL-6 levels were quantified using commercial kits (hepcidin, USCN Life Science; TNF-α and IL-10, GE Healthcare, United Kingdom; and IL-6, R and D Systems, Minneapolis, MN, United States). Liver biopsies were also used for hepcidin quantification as described above for colon samples.
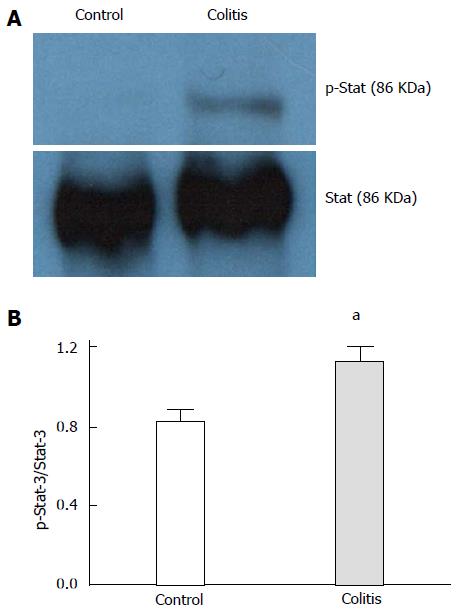
For protein analysis by Western blot (WB) assay, supernatant aliquots were treated with Laemmli sample buffer containing 100 mmol/L dithiothreitol, and the samples were then heated in a boiling water bath for 5 min. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a Bio-Rad miniature slab gel apparatus (Mini-Protean). For WB experiments, 0.15 mg of protein extracted from tissue samples was separated by SDS-PAGE, transferred to nitrocellulose membranes and blocked for 2 h with 5% blocking agent (GE Healthcare, United Kingdom) in Tris buffer solution. The membranes were then incubated overnight with anti-Stat-3 and anti-phospho-Stat-3 antibodies (1:1000; Santa Cruz Biotechnology, CA, United States) and developed using a commercial chemiluminescent kit (GE Healthcare, United Kingdom). The band intensities were quantified by optical densitometry (Scion Image software, ScionCorp, Frederick, MD) from the developed autoradiography.
Hydrated, 5.0 μm sections of paraformaldehyde-fixed, paraffin-embedded colon tissue were used for immunohistochemistry (IHC) and the Prussian blue reaction. Sections stained with hematoxylin and eosin (HE) were used to microscopically score determination. IHC sections were probed with an anti-rabbit hepcidin-25 antibody (1:100; Abcam, Cambridge, MA, United States) and biotinylated anti-rabbit secondary antibody, and developed with DAB (ImmunoCruz ABC Staining System, Santa Cruz Biotechnology, Santa Cruz, CA, United States). Additionally, hematoxylin staining was used to reveal the nuclear morphology. For the Prussian blue reaction, slides were incubated with a 5% potassium ferrocyanide aqueous solution and a 5% hydrochloric acid aqueous solution. After washing in distilled water, sections were counterstained with Nuclear Fast Red. Microscopic tissue damage was scored on a scale from 0 to 20 according to the criteria previously described by Rodríguez-Cabezas et al[16]. Six parameters were scored: neutrophil infiltration into the epithelium (0-2), lamina propria (0-2), submucosa (0-2), muscularis propria (0-2) and serosa (0-2); fibrin deposition into the mucosa (0-1) and submucosa (0-1); submucosa neutrophil margination (0-1); submucosal edema (0-2); epithelial necrosis (0-2); and epithelial ulceration (0-1).
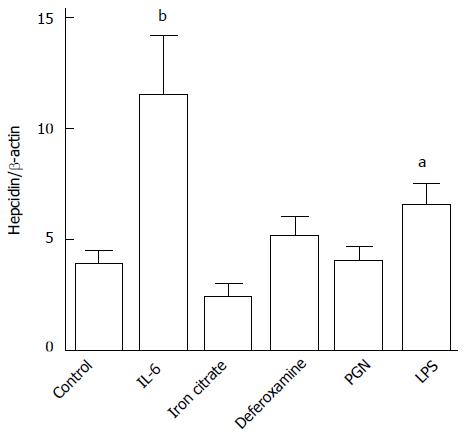
HT-29 cells (Rio de Janeiro Cell Bank, RJ, Brazil) were incubated with 20 μmol/L ferric citrate (Sigma) for 72 h, 30 mmol/L deferoxamine (Sigma) for 4 h, 40 ng/mL IL-6 (Peprotech, Rocky Hill, NJ, United States)[12], 1 μg/mL of the toll-like receptor 2 (TLR-2) ligand peptidoglycan [peptidoglycan (PGN); Sigma] or 10 μg/mL of the TLR-4 ligand lipopolysaccharide (LPS; Sigma) for 6 h[12]. Then, cells were harvested, and total RNA was isolated according to the manufacturer’s instructions (RNeasy Mini Kit; Qiagen Valencia, CA, United States). cDNA was synthesized with a High-capacity cDNA archive kit (Applied Biosystems, Foster City, CA, United States) according to the manufacturer’s protocol. Quantitative polymerase chain reaction and melting curve analyses were performed as previously described[17]. The primer sequences were as follows: hepcidin sense, 5’-CAGGGCAGGTAGGTTCTACG-3’; hepcidin antisense, 5’-CACTTCCCCATCTGCATTTT-3’; β-actin sense, 5’-ACACTGGCTCGTGTGACAAGG-3’; and β-actin antisense, 5’-CGGCTAATACACACTCCAAGGCG-3’.
All data are expressed as means ± SE. Non-parametric data (histological scores) are expressed as the median (range) and were analyzed using the Mann-Whitney test. Comparisons between groups were performed using the unpaired Student’s test. Statistical analyses were performed using GraphPad InStat (GraphPad Software, La Jolla, CA, United States). An associated probability (P value) of less than 0.05 was considered significant.
Repeated intracolonic TNBS administration to Wistar rats resulted in macroscopic lesions characterized by hyperemia and small ulcers in the colon. In association with these observed macroscopic lesions, the colonic myeloperoxidase levels, a marker of neutrophil infiltration, were increased (Table 1). Histological assessment of colon tissues revealed the presence of ulcers and extensive neutrophil infiltration in the mucosa and submucosa. Severe edema and, in some cases, transmural inflammation were observed. The median score of the colitis group was elevated compared with the control group (Table 1). Additionally, colitis development corresponded with a low body weight at the end of the experimental protocol (Table 1). The red blood cell count, hemoglobin and hematocrit levels in colitic rats were similar to the levels observed in control rats. Leukocytosis was observed in colitic rats (Table 2).
| Control | Colitis | |
| Damage score | 0 (0-1) | 5 (4-9)a |
| Myeloperoxidase activity (U/g tissue) | 2.2 ± 0.6 | 18.2 ± 4.8b |
| Initial body weight (g) | 299 ± 8 | 303 ± 3 |
| Final body weight (g) | 405 ± 9 | 368 ± 4a |
| Microscopic score | 2 (0-3) | 11 (6-14)a |
| TNF-α (pg/mg protein) | 1.8 ± 1.2 | 7.4 ± 2.1a |
| IL-6 (pg/mg protein) | 8.2 ± 1.7 | 14.7 ± 0.7a |
| IL-10 (pg/mg protein) | 23.5 ± 3.4 | 12.1 ± 1.7b |
In colon extracts, hepcidin levels were increased in colitic animals compared with control animals (Figure 1G), and hepcidin was predominantly expressed in the surface epithelium of colitic animals (Figure 1).
In the colon, hepcidin levels were correlated with increased levels of IL-6 and TNF-α and decreased levels of IL-10 (Table 1). In an additional group, rats were evaluated 24 h after the first TNBS administration, and the level of IL-6 was significantly higher in the colitic group compared with the control group (9.1 ± 0.8 pg/mg protein and 39.8 ± 7.1 pg/mg protein for the control and colitis groups, respectively; P < 0.01, n = 5), but the hepcidin levels were not significantly different (11.8 ± 4.2 pg/mg protein and 30.9 ± 11.8 pg/mg protein for the control and colitis groups, respectively; P = 0.22, n = 5).
The serum hepcidin levels were similar between the colitic and control rats, and the iron and transferrin levels and iron saturation were also unchanged (Table 3). Furthermore, the liver hepcidin levels were not modified by colitis (653 ± 27 pg/mg protein and 675 ± 27 pg/mg protein for the control and colitis groups, respectively, n = 4).
| Control | Colitis | |
| Hepcidin (pg/mL) | 241.9 ± 59.5 | 280.4 ± 65.8 |
| Transferrin (ng/mL) | 18.0 ± 0.4 | 20.4 ± 1.5 |
| Iron (μg/dL) | 69.0 ± 4.5 | 72.9 ± 10.0 |
| Transferrin saturation (%) | 26.1 ± 2.4 | 25.5 ± 1.2 |
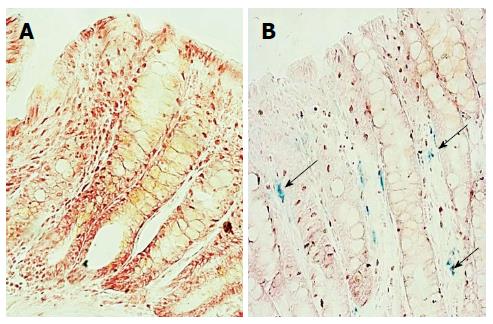
In colitic rats, Stat-3 phosphorylation was increased compared to the controls (Figure 2). Iron deposition in the colons of colitic animals was observed using the Prussian blue reaction, and no reaction was evident in control colon tissues (Figure 3). In colitic rats, the observed blue precipitate was not localized to the surface or crypt epithelium, which suggested that iron might have accumulated in immune cells.
The human colon adenocarcinoma cell line HT-29 was used to model colonic hepcidin expression. Upon stimulation with IL-6 and LPS (a TLR-4 ligand), hepcidin expression significantly increased (Figure 4). Deferoxamine, ferric citrate and PGN (a TLR-2 ligand) were unable to modify hepcidin expression in these human colon cells (Figure 4).
Iron is an important factor in the competition for nutritional resources between microbial pathogens and their hosts. In humans, host defense responses to infectious agents modulate local and systemic iron availability, interfering with infections such as malaria and tuberculosis[8]. Hepcidin is the major regulator of iron homeostasis in humans and other mammals. Increased iron levels and innate immunity (most likely through Toll-like receptor activation/IL-6 induction) can induce hepcidin expression[8,18], Conversely, macrophages also produce hepcidin, and this autocrine production is related to the downregulation of ferroportin expression and iron sequestration in these cells[19]. Macrophage iron accumulation correlates with a more pro-inflammatory phenotype and consequent increased cytokine release[20]. In this study, we used a well-established experimental colitis model, induced by repeated TNBS administration, to examine hepcidin expression in the infected/inflamed colon. In this experimental model, moderate colonic inflammation and alterations in mesenteric adipose tissue are observed, simulating some aspects of CD[14]. Although increased hepcidin expression has been described in adipose tissue in obese patients[21] and in subcutaneous adipose tissue during cardiac surgery[22], no alterations in hepcidin expression were observed in the mesenteric adipose tissue in our study (data not shown). We demonstrated that colonic hepcidin expression was increased in experimental inflammation. Previously, in two experimental models of the acute-phase response, increased hepcidin gene expression was observed in both the small intestine and colon[23]. Hepcidin immunoreactivity in colorectal tissue and increased urinary hepcidin levels have also been described in cancer patients[13]. Here, the increased colonic hepcidin expression observed during inflammation was associated with elevated levels of pro-inflammatory cytokines such as IL-6 and a significant increase in Stat-3 phosphorylation. Increased IL-6 levels were observed 24 h after the first TNBS administration, in the absence of increased hepcidin levels, suggesting that pro-inflammatory cytokines may induce hepcidin expression. Our in vitro experiments demonstrating that IL-6 induced hepcidin expression in HT-29 cells support this hypothesis. A direct activation of TLR-4 but not TLR-2 also induced hepcidin expression, suggesting that PAMPs and cytokines could stimulate colonic hepcidin expression. Several pro-inflammatory cytokines are candidates for this effect, but the most well-characterized is IL-6, which signals through the Jak/Stat-3 pathway[24]. Colonic hepcidin production did not alter serum or liver hepcidin, iron or transferrin levels. Additionally, our experimental rats were not anemic, suggesting a localized role for hepcidin in intestinal inflammation. In an initial report, low serum hepcidin-25 levels were found in IBD patients with or without iron deficiency anemia[25]. A subsequent report demonstrated that serum hepcidin-25 levels are increased in UC and CD patients compared with healthy control patients, and in UC patients, this increase was related to disease activity but not iron deficiency[26]. Furthermore, in a recent report, serum hepcidin-25 levels were implicated in the differential diagnosis of IBD-associated anemia, but hepcidin-20 levels were independently regulated by inflammation[27]. Moreover, increased IL-6 and hepcidin serum levels were measured in CD patients with ACD[28], but no studies have examined colonic hepcidin expression in human IBD.
The main site of dietary iron absorption in adults is the proximal small intestine, whereas the distal small intestine and colon are absorb only small amounts of iron[29]. Curiously, the Prussian blue reaction in the colons of the control group did not reveal the presence of iron, as observed in inflamed tissue. Hepcidin acts by triggering ferroportin internalization and degradation through a mechanism most likely involving ferroportin ubiquitination[30]. Ferroportin downregulation results in intracellular iron accumulation and unavailable local or plasma iron. As previously discussed, local iron sequestration could participate in an antimicrobial response associated with intestinal inflammation, but we could not exclude the possibility that it contributes to increased local inflammation by recruiting macrophages with a pro-inflammatory phenotype. In the treatment of iron deficiency anemia in patients with IBD, oral iron supplementation induced adverse gastrointestinal events that resulted in drug discontinuation in 20.8% of patients[31]. A recent study also demonstrated that dietary iron intake was negatively associated with quality of life in mildly active IBD patients[32].
In conclusion, we are the first to demonstrate that colonic inflammation can increase local hepcidin-25 expression in association with IL-6 production and Stat-3 activation. Although local iron deposition was also observed, additional studies are necessary to understand whether this observation constitutes a defensive or pathological response to intestinal inflammation.
Hepcidin is an endogenous peptide with weak antimicrobial properties that regulates iron metabolism during inflammation. Hepcidin expression in intestinal inflammation has not been previously studied and could aid in the understanding of the pathophysiology of inflammatory bowel diseases.
Hepcidin expression appears to be induced by pathogen-associated molecular patterns, likely through interleukin-6 (IL-6) induction, as part of the host immune response. In this study, authors described colitis-induced colonic hepcidin expression that was associated with the IL-6/Stat-3 signaling pathway and local iron sequestration. These observations suggest a host response to infection that reduces the iron available for pathogen use as an important antimicrobial mechanism. However, authors could not exclude the possibility that hepcidin expression contributed to increased local inflammation by recruiting pro-inflammatory macrophages.
Recent reports have highlighted the importance of hepcidin as the major regulator of iron homeostasis in humans and other mammals. Iron is an important factor in the competition for nutritional resources between microbial pathogens and their hosts. Here, authors described colitis-induced colonic hepcidin expression associated with the IL-6/Stat-3 signaling pathway and local iron sequestration. Local iron sequestration could participate in an antimicrobial response associated with intestinal inflammation. Furthermore, human colon adenocarcinoma HT-29 cells increased hepcidin expression after IL-6 and lipopolysaccharide (a toll-like receptor 4 ligand) treatment, suggesting that inflammation and microbial pathogens could participate in authors’in vivo observations.
By demonstrating that intestinal inflammation induced hepcidin expression and that hepcidin could function in a protective role, this study contributes to a better understanding of the pathophysiology of inflammatory bowel disease.
Hepcidin is a peptide that is mainly expressed and released by the liver in response to increased circulating iron levels. Inflammation and infections are also able to induce hepcidin synthesis resulting in hypoferremia. Hepcidin acts by triggering ferroportin internalization and degradation by a mechanism that most likely involves ferroportin ubiquitination. Downregulation of ferroportin results in the intracellular accumulation of iron and its unavailability locally and in plasma.
This manuscript focuses on hepcidin in an animal model of gut inflammation. The management of iron, and the mechanisms leading to iron deficiency in inflammatory bowel disease are important issues. This work adds further to this area.
P- Reviewers: Day AS, Horiguchi H, Pantopoulos K, Yamakawa M S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
| 1. | Mullin GE. Micronutrients and inflammatory bowel disease. Nutr Clin Pract. 2012;27:136-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Kent AJ, Blackwell VJ, Travis SP. What is the optimal treatment for anemia in inflammatory bowel disease? Curr Drug Deliv. 2012;9:356-366. [PubMed] [Cited in This Article: ] |
| 3. | Sun CC, Vaja V, Babitt JL, Lin HY. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol. 2012;87:392-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Finberg KE. Regulation of systemic iron homeostasis. Curr Opin Hematol. 2013;20:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Tussing-Humphreys L, Pusatcioglu C, Nemeth E, Braunschweig C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet. 2012;112:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425-4433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 603] [Cited by in F6Publishing: 628] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 8. | Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 475] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 9. | Wang L, Johnson EE, Shi HN, Walker WA, Wessling-Resnick M, Cherayil BJ. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol. 2008;181:2723-2731. [PubMed] [Cited in This Article: ] |
| 10. | Koon HW, Shih DQ, Chen J, Bakirtzi K, Hing TC, Law I, Ho S, Ichikawa R, Zhao D, Xu H. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology. 2011;141:1852-1863.e1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Ho S, Pothoulakis C, Koon HW. Antimicrobial peptides and colitis. Curr Pharm Des. 2013;19:40-47. [PubMed] [Cited in This Article: ] |
| 12. | Schwarz P, Kübler JA, Strnad P, Müller K, Barth TF, Gerloff A, Feick P, Peyssonnaux C, Vaulont S, Adler G. Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. Gut. 2012;61:193-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Ward DG, Roberts K, Brookes MJ, Joy H, Martin A, Ismail T, Spychal R, Iqbal T, Tselepis C. Increased hepcidin expression in colorectal carcinogenesis. World J Gastroenterol. 2008;14:1339-1345. [PubMed] [Cited in This Article: ] |
| 14. | Gambero A, Maróstica M, Abdalla Saad MJ, Pedrazzoli J. Mesenteric adipose tissue alterations resulting from experimental reactivated colitis. Inflamm Bowel Dis. 2007;13:1357-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Funakoshi T, Yamashita K, Ichikawa N, Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T, Ichihara S. A novel NF-κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J Crohns Colitis. 2012;6:215-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Rodríguez-Cabezas ME, Gálvez J, Lorente MD, Concha A, Camuesco D, Azzouz S, Osuna A, Redondo L, Zarzuelo A. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. J Nutr. 2002;132:3263-3271. [PubMed] [Cited in This Article: ] |
| 17. | Clemente TR, Dos Santos AN, Sturaro JN, Gotardo EM, de Oliveira CC, Acedo SC, Caria CR, Pedrazzoli J, Ribeiro ML, Gambero A. Infliximab modifies mesenteric adipose tissue alterations and intestinal inflammation in rats with TNBS-induced colitis. Scand J Gastroenterol. 2012;47:943-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Layoun A, Santos MM. Bacterial cell wall constituents induce hepcidin expression in macrophages through MyD88 signaling. Inflammation. 2012;35:1500-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, Zoller H, Bellmann-Weiler R, Niederegger H, Talasz H. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111:2392-2399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 20. | Recalcati S, Locati M, Gammella E, Invernizzi P, Cairo G. Iron levels in polarized macrophages: regulation of immunity and autoimmunity. Autoimmun Rev. 2012;11:883-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 22. | Vokurka M, Lacinová Z, Kremen J, Kopecký P, Bláha J, Pelinková K, Haluzík M, Necas E. Hepcidin expression in adipose tissue increases during cardiac surgery. Physiol Res. 2010;59:393-400. [PubMed] [Cited in This Article: ] |
| 23. | Sheikh N, Dudas J, Ramadori G. Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat. Lab Invest. 2007;87:713-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Gaffney-Stomberg E, McClung JP. Inflammation and diminished iron status: mechanisms and functional outcomes. Curr Opin Clin Nutr Metab Care. 2012;15:605-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Arnold J, Sangwaiya A, Bhatkal B, Geoghegan F, Busbridge M. Hepcidin and inflammatory bowel disease: dual role in host defence and iron homoeostasis. Eur J Gastroenterol Hepatol. 2009;21:425-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Oustamanolakis P, Koutroubakis IE, Messaritakis I, Malliaraki N, Sfiridaki A, Kouroumalis EA. Serum hepcidin and prohepcidin concentrations in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:262-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Bergamaschi G, Di Sabatino A, Albertini R, Costanzo F, Guerci M, Masotti M, Pasini A, Massari A, Campostrini N, Corbella M. Serum hepcidin in inflammatory bowel diseases: biological and clinical significance. Inflamm Bowel Dis. 2013;19:2166-2172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Basseri RJ, Nemeth E, Vassilaki ME, Basseri B, Enayati P, Shaye O, Bourikas LA, Ganz T, Papadakis KA. Hepcidin is a key mediator of anemia of inflammation in Crohn’s disease. J Crohns Colitis. 2013;7:e286-e291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Frazer DM, Wilkins SJ, Anderson GJ. Elevated iron absorption in the neonatal rat reflects high expression of iron transport genes in the distal alimentary tract. Am J Physiol Gastrointest Liver Physiol. 2007;293:G525-G531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Qiao B, Sugianto P, Fung E, Del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T, Nemeth E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15:918-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 31. | Schröder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge U, Schrott M. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503-2509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Powell JJ, Cook WB, Hutchinson C, Tolkien Z, Chatfield M, Pereira DIa, Lomer MC. Dietary fortificant iron intake is negatively associated with quality of life in patients with mildly active inflammatory bowel disease. Nutr Metab (Lond). 2013;10:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |