INTRODUCTION
The treatment of patients with pancreatic cancer mainly depends on surgery, radiation therapy, chemotherapy or combined therapeutic methods. Due to aggressive tumor biology and lack of specific symptoms during the early stages, pancreatic cancer frequently presents as an incurable disease with approximately two-thirds of patients showing radiographically detectable metastasis at the time of diagnosis. Pancreatic ductal adenocarcinomas carry a dismal prognosis, with fewer than 5% of patients surviving 5 years or more[1].
For patients without metastasis, the only potentially curative treatment is surgery. However, even among the 10% of patients with resectable disease, only about 20% survive for 5 years or more, indicating a need for good adjuvant therapies. Approximately 40% of patients diagnosed have locally advanced, unresectable, non-metastatic disease known as locally advanced pancreatic cancer. These patients are treated with chemoradiation therapy in the United States[2]. However, advanced pancreatic cancer is often resistant to conventional chemotherapy or radiotherapy[3].
The dismal prognosis of pancreatic cancer is further accentuated by its poor response to chemotherapy and to radiation therapy[4]. Thus, therapies are needed to increase the efficiency of radiation and chemotherapy. The discovery of new therapeutic agents and approaches in patients with pancreatic cancer is of paramount importance.
Genetic changes in apoptotic regulatory mechanisms may result in an increase in cell numbers, as well as the preservation of genetically altered cells, which initiate the process of tumorigenesis[5]. Disordered apoptosis and abnormal proliferation have been linked to the development of malignancy and treatment resistance[6]. Apoptosis, also termed programmed cell death, occurs via extrinsic or intrinsic signal transduction pathways[7]. Therefore, further understanding of the molecular mechanisms of apoptosis and the relationship between pancreatic cancer chemoresistance and disordered apoptosis and abnormal proliferation is needed. Furthermore, apoptosis contributes to cell death in tumors treated with various anticancer agents. Chemotherapy, radiation therapy and immunotherapy all rely heavily on the induction of apoptosis to kill pancreatic cancer cells.
Many recent studies have revealed that certain garlic-derived organosulfur compounds can suppress the proliferation of cultured cancer cells by causing apoptosis and/or cell cycle arrest[8-10].
Garlic (Allium sativum) is a common plant used mainly as food and has recently been reported to have medicinal properties[11]. Garlic-derived organosulfur compounds including diallyl sulfide, diallyl disulfide (DADS) and/or diallyl trisulfide (DATS), which are major components of garlic, may be associated with a reduced risk of certain cancers[12].
These compounds are known to inhibit cell proliferation, cause cell cycle arrest and increase apoptosis in various cancer cell lines[13]. However, the cellular and molecular mechanisms underlying these activities have not yet been completely elucidated.
In the present study, we investigated the effects of the garlic constituent, DATS, in human pancreatic cancer cells (Capan-2) and non-tumorigenic pancreatic ductal epithelial cells (H6C7).
MATERIALS AND METHODS
Cell culture and drug treatment
The human pancreatic cancer cell line Capan-2, with wild-type p53 gene, and the normal pancreatic epithelial cell line H6C7 were obtained from ATCC (United States) and cultured in RPMI1640 (Gibco, CA, United States) containing 10% heat-inactivated fetal bovine serum (FBS), benzylpenicillin (100 KU/L), streptomycin (100 mg/L), transferrin (0.001 mg/mL), EGF (20 ng/μL) and hydrocortisone (500 ng/mL) at 37 °C in a humidified incubator containing 5% CO2 in air (Gibco). The cells were cultured in plastic culture dishes with a non-adhesive surface and digested with 0.25% trypsin for subculture.
DATS (99% purity) was purchased from Chia-Tai Tianqing Pharmaceutical Co., Ltd. (China). DATS was prepared at a concentration of 100 μmol/L and dissolved in suitable medium.
Methyl thiazolyl tetrazolium assay
Cell viability was detected using the methyl thiazolyl tetrazolium (MTT) (Sigma, St. Louis, MO, United States) assay. Briefly, cells in the logarithmic growth phase at a density of 1 × 104 cells/well in 100 μL of medium were seeded in a 96-well plate and cultured for 24 h prior to drug treatment. The medium was then changed to medium containing (100 μmol/L) DATS. After 24 h, 10 μL of 5 mg/mL MTT reagent was added to each well and incubated for 4 h. After incubation, 100 μL of detergent reagent was added to each well to dissolve the formazan crystals. The absorbance of the solution was determined at 570 nm (A570 nm) and the cell inhibition ratio was calculated as follows: cell inhibition ratio = [(A570 nm value of the control group - A570 nm value of the experimental group)/A570 nm value of the control group] × 100%. Cells treated with irradiation only were used as controls. Each assay was performed in triplicate and the standard deviation was determined.
TUNEL and DAPI staining
Apoptotic cells were determined by TUNEL assay using the In Situ Cell Death Detection kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Cells were cultured on 4-chamber slides (VWR, United States) at a density of 2 × 104 cells/chamber. After treatment with 100 μmol/L of DATS, the cells were washed with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich Corp., United States) for 20 min at 4 °C. The fixed cells were incubated with digoxigenin-conjugated dUTP in a terminal deoxynucleotide transferase recombinant (rTdT)-catalyzed reaction and nucleotide mixture for 1 h at 37 °C in a humidified atmosphere, and then immersed in stop/wash buffer for 15 min at room temperature. Cells were then washed with PBS to remove unincorporated fluorescein-12-dUTP. After washing with PBS, cells were incubated with 1 mg/mL DAPI (Sigma) solution for 30 min at 37 °C in the dark. Apoptotic cells were photographed under a fluorescence microscope (Nikon Inc., Tokyo, Japan).
Flow cytometric analysis
Cells were seeded at a density of 3-5 × 105/plate and incubated for 24 h before irradiation. The medium was changed to medium containing DATS (100 μmol/L) 30 min before irradiation. Cells were harvested by trypsinization after 24 h. The cells were washed with PBS and then fixed with ice-cold 70% ethanol while vortexing. Finally, the cells were washed and resuspended in PBS containing 5 μg/mL RNase A (Sigma) and 50 μg/mL propidium iodide (Sigma) for analysis. Cell cycle analysis was performed using a FACScan Flow Cytometer (Becton Dickson, United States) according to the manufacturer’s protocol.
Western blot analysis
The samples were normalized to monitor protein concentrations and the loading dye (50 mmol/L Tris-Cl pH 6.8, 100 mmol/L β-mercaptoethanol, 2% sodium dodecyl sulfate (SDS), 0.1% bromophenol blue, 10% glycerol) was added. The proteins were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, United States). After transferring and blocking, the membrane was incubated with an appropriate primary antibody (Santa Cruz Biotechnology) at a 1:1000 dilution in PBS-T containing 5% milk overnight at 4 °C. Before the transfer, the membrane was activated by incubation in methanol for 30 s, water for 2 min and then transfer buffer (48 mmol/L Tris base, 20% methanol, 0.04% SDS, 30 mmol/L glycine) until ready for transfer. A semi-dry transfer apparatus (Owl Separation Systems, Portsmouth, NH, United States) was used to transfer the proteins onto the membrane. The membrane was quickly washed three times, then two 5 min washes and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (goat anti-mouse antibody, Santa Cruz Biotechnology).
For Western blot analysis, mouse anti-human cyclin D1, p21, cyclin B1, caspase-3, caspase-8, Bcl-2, Bax, and Fas monoclonal antibodies (1:1000 for all) were used. Anti-glyceraldehyde phosphate dehydrogenase (GAPDH) and anti-β-actin antibodies (1:1000; Santa Cruz Biotechnology) were used to detect internal controls. All experiments were performed in triplicate.
Immunofluorescence
Capan-2 cells in 5% CS and 1% penicillin streptomycin-containing Dulbecco’s modification of Eagle’s medium Dulbecco (DMEM) were cultured in two-well chambers and then mounted on glass slides with plastic covers. At 70% confluence, the cells were switched to fresh 5% CS and 1% penicillin streptomycin-containing DMEM medium supplemented with or without 100 μmol/L DATS. After fixation and washing, the cells were incubated with the primary antibody Bp53-12 (Santa Cruz Biotechnology). Following overnight incubation, the cells were incubated with the secondary antibody (donkey anti-mouse IgG) (Santa Cruz Biotechnology). Finally, the cells were visualized and photographed at × 400 magnification using an immunofluorescence microscope (Nikon Inc., Tokyo, Japan). The images were merged using the advanced SPOT software (Diagnostic Instruments Inc., Sterling Heights, MI, United States). For the detection of Bax, the primary antibody which was diluted at 1:400 in 2% chicken serum/PBS was added. Cells were then washed three times with PBS and incubated with Texas Red-conjugated secondary antibody (Santa Cruz Biotechnology).
Quantitative real-time polymerase chain reaction
Capan-2 cells were cultured in 5% CS and 1% penicillin streptomycin-containing DMEM to 70% confluence and then switched to new culture medium supplemented with 100 μmol/L DATS. After 48 h, RNA was extracted using the RNAeasy kit according to the manufacturer’s protocol. The extracted total RNA was subjected to cDNA synthesis using the Biorad iScriptTM Select cDNA Synthesis kit (Biorad, Hercules, CA, United States) containing random and oligo (dT) primer mix. The cDNA produced was used to specifically quantify the transcript of interest using RT-PCR. Quantitative RT-PCR was performed using SYBR Green chemistry (SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, United States). The increase in fluorescence of the SYBR Green dye was monitored using a 7500 Real-Time PCR System (Applied Biosystems). The primers were: forward, 5’-ACC ACA GTC CAT GCC ATC AC-3’ and reverse, 5’-TCC ACC ACC CTG TTG CTG TA-3’ for the house-keeping gene GAPDH; forward, 5’-AAG CTG TGC ATC TAC ACC GA-3’ and reverse, 5’-CTT GAG CTT GTT CAC CAG GA-3’ for cyclin D1; forward, 5’-AAC ACC ATG GAC AGG GAG AG -3’ and reverse, 5’-CCC AGC AGC TTC AGG TAC TC -3’ for Akt; forward, 5’-GGC TCA GCA TGG TCG CTT-3’ and reverse, 5’-CTC CCG CCA GCT GTC ATT-3’ for Fas; forward, 5’-TTG AGT TCG GTG GGG TCA TG-3’ and reverse, 5’-GAT CCA GGT GTG CAG ATG CC-3’ for BcL-2; forward, 5’-CTG AGC TGA CCT TGG AGC-3’ and reverse, 5’-GAC TCC AGC CAC AAA GAT G-3’ for Bax. The SyBr green dye intercalates with the double-stranded cDNA formed during PCR, allowing easy quantification of cDNA to indirectly quantify the original RNA transcripts. Thermal cycling conditions were designed as follows: reverse transcription at 50 °C for 10 min followed by an initial denaturation at 95 °C for 5 min and 35 cycles of 95 °C for 15 s and 60 °C for 30 s. The comparative CT method (ΔΔCt) was used to study the relative quantification of gene expression. Each evaluation was performed in triplicate in three independent experiments.
Statistical analyses
All the assays described above were repeated more than once. The quantification assays were performed at least twice and data were calculated and presented as mean ± SD. Data were either analyzed by unpaired Student’s t test or one-way ANOVA. Differences were considered significant at P < 0.05.
RESULTS
DATS affects cell viability and induces cell apoptosis
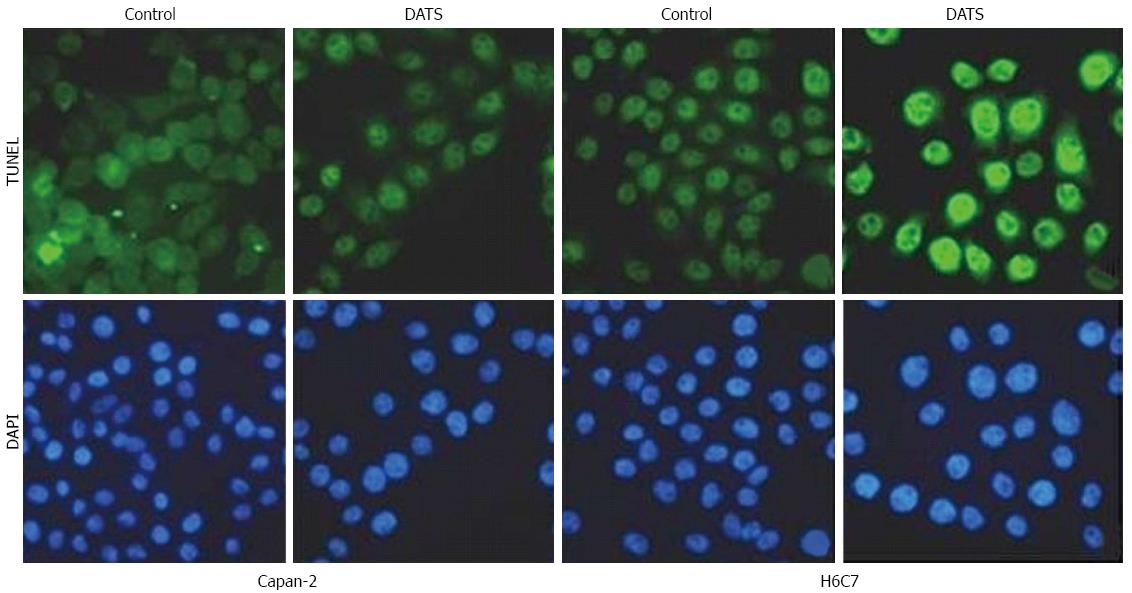
In Capan-2 cells and H6C7 cells, TUNEL assay were performed to ascertain the induction of apoptosis by 100 μmol/L DATS. Fewer TUNEL-positive cells were found in H6C7 cells that in Capan-2 cells after treatment with 100 μmol/L of DATS (Figure 1).
Figure 1 TUNEL assay to determine diallyl trisulfide-induced apoptosis of Capan-2 and H6C7 cells.
TUNEL assay was used to confirm induction of apoptosis in treated and untreated cells. Both Capan-2 and H6C7 cells were treated with 100 μmol/L diallyl trisulfide for 24 h and induction of apoptosis was confirmed by the appearance of TUNEL-positive cells; DATS: Diallyl trisulfide; DAPI: 4',6-diamidino-2-phenylindole.
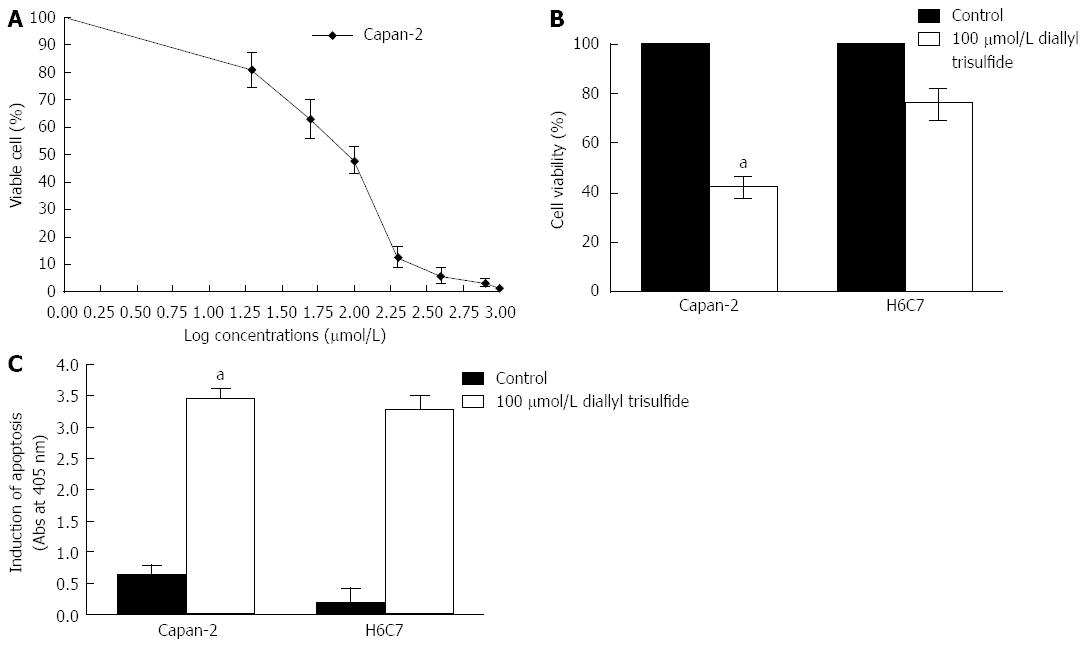
The effect of DATS on cell viability and cell apoptosis induction in Capan-2 cells was examined by MTT assay. A dose-response curve was constructed from which we chose 100 μmol/L for subsequent experiments (Figure 2A). The analysis revealed that 100 μmol/L of DATS decreased the viability of both Capan-2 cells (55%) and H6C7 cells (30%) compared with untreated control cells (P < 0.05) (Figure 2B). ELISA indicated that 100 μmol/L of DATS induced apoptosis of Capan-2 cells (about an 8-fold increase) compared with controls. In addition, the viability of H6C7 cells was significantly decreased by about 5 folds (P < 0.05) (Figure 2C).
Figure 2 Diallyl trisulfide induces apoptosis of Capan-2 cells and H6C7 cells.
A: Capan-2 cells were exposed to different concentrations of diallyl trisulfide (DATS) and the percentage of viable cells was determined by methyl thiazolyl tetrazolium (MTT) assay. Capan-2 and H6C7 cells were exposed to 100 μmol/L DATS for 24 h. Cells without DATS treatment were used as controls. Living cells was detected by MTT assay. Data points = mean ± SD of quadruplicate values for each independent experiment; B: The percentage survival of Capan-2 and H6C7 cells was significantly different (aP < 0.05); C: ELISA was used to determine apoptotic cells. Each condition was performed in quadruplicate. Data are presented as mean ± SD.
Impact of DATS on cell cycle progression
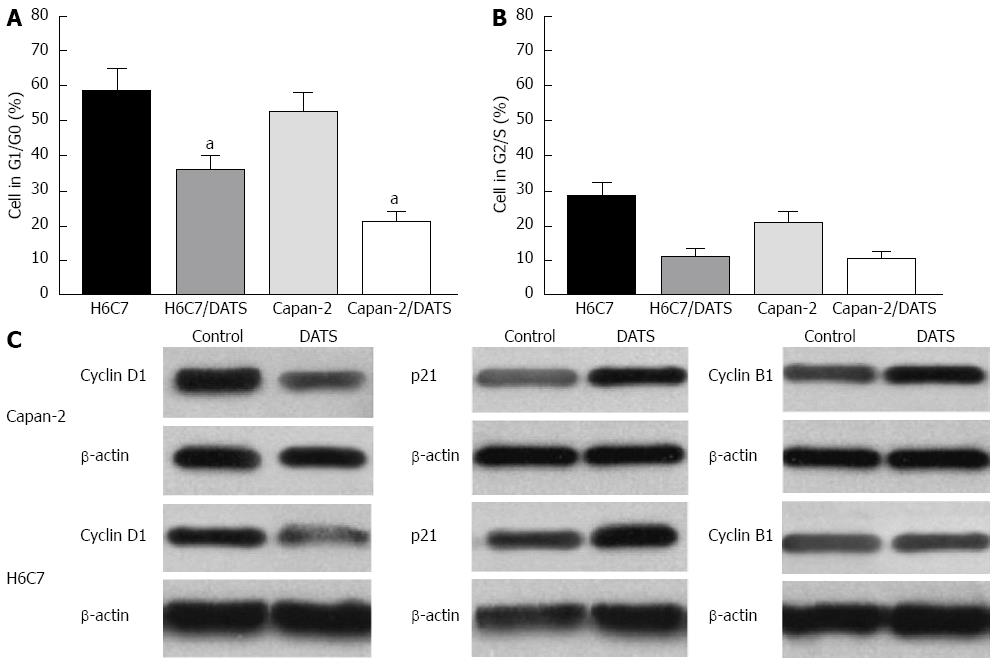
Flow cytometry was performed to study the effects of DATS on cell cycle progression. Treatment of both cell lines was carried out in three independent experiments and is represented in a histogram. The percentages of cells in G1/G0 and G2/S were determined after treatment with 100 μmol/L DATS for 24 h. Both Capan-2 and H6C7 cells treated with DATS and harvested after 24 h showed an decrease in the percentage of G1/G0 cells compared with control cells (P < 0.05), and the reduced value in Capan-2 cells was about 35% (Figure 3A). No significant difference was found in the percentage of cells in the G2/S phase in both Capan-2 and H6C7 cells compared with control cells (Figure 3B). The expression of cyclin D1, p21 and cyclin B1 was determined by Western blot in Capan-2 and H6C7 cells. The results showed that DATS decreased the level of cyclin D1 and increased the levels of cyclin B1 and p21 in both Capan-2 and H6C7 cells (Figure 3C).
Figure 3 Diallyl trisulfide modulates cell cycle progression in Capan-2 and H6C7 cells.
Diallyl trisulfide (DATS) (100 μmol/L) arrested Capan-2 and H6C7 cells in G1/G0 phase and G2/S phase. The percentage of cells in different phases was determined in Capan-2 and H6C7 cells after treatment with 100 μmol/L DATS for 24 h. A: DATS (100 μmol/L) arrested more Capan-2 cells in G1/G0 phase (aP < 0.05 vs H6C7 cells); B: No significant difference was found in the percentage of cells in the G2/S phase in both Capan-2 and H6C7 cells compared with control cells; C: DATS upregulated p21 and cyclin B1 expression and downregulated cyclin D1 expression in Capan-2 and H6C7 cells. Each data point is the mean of three independent experiments and expressed as mean ± SD.
DATS induced the expression of Bax and Bcl-2 in Capan-2 cells
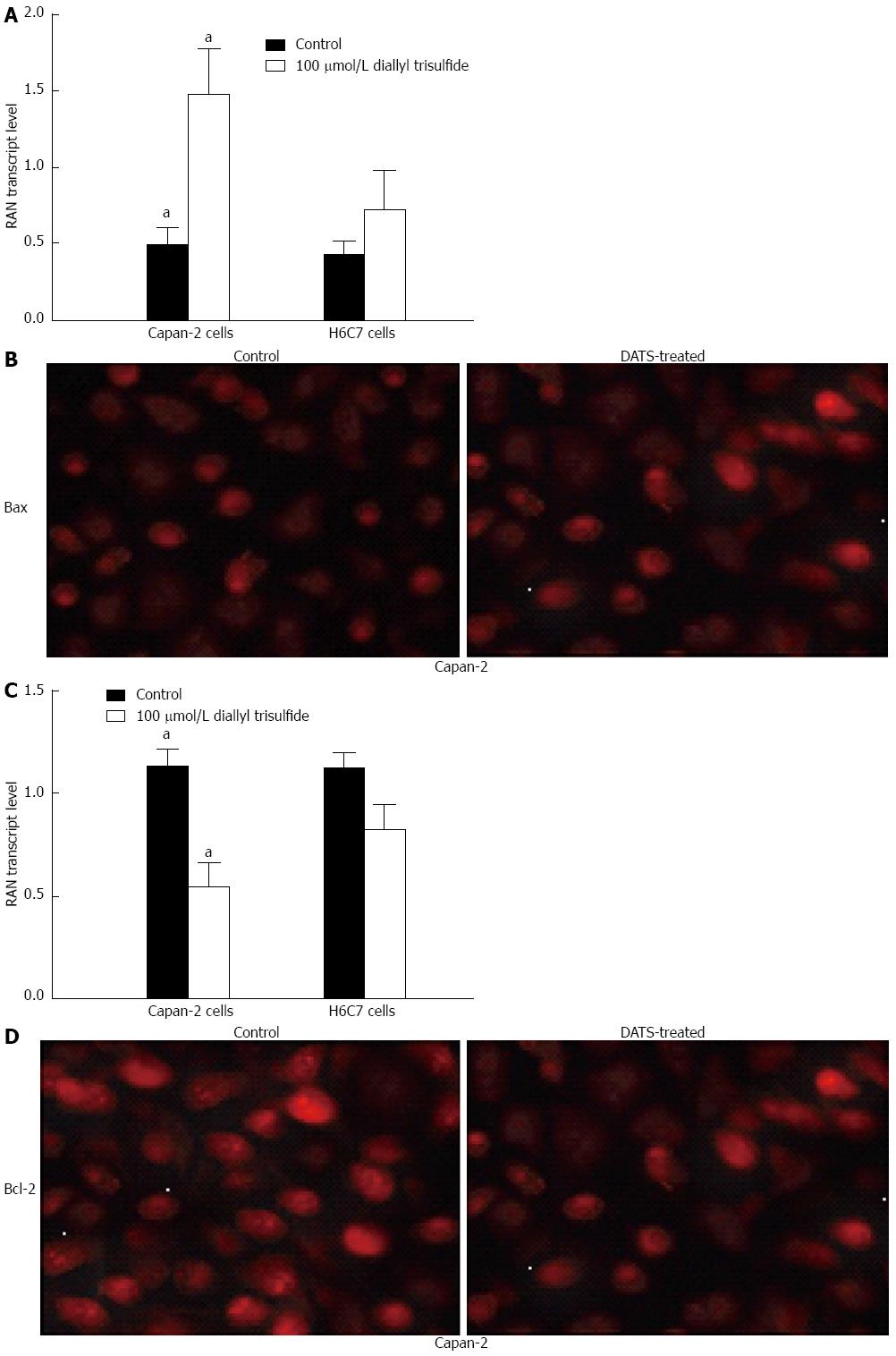
Capan-2 cells were treated with 100 μmol/L DATS for 24 h. Using RT-PCR and Western blot analyses, it was found that DATS significantly increased Bax expression at the mRNA level by 3 folds and decreased Bcl-2 expression at the mRNA level by 0.5 folds as indicated in Figure 4A and C (P < 0.05). These results were further confirmed by immunofluorescence (Figure 4B and D).
Figure 4 Diallyl trisulfide treatment induces Bax and bcl-2 expression in Capan-2 cells.
Capan-2 cells were treated with 100 μmol/L diallyl trisulfide (DATS) for 24 h. Untreated cells were used as controls. A, C: Total RNA was extracted and reversely transcribed to cDNA for RT-PCR analysis of Bax and Bcl-2 mRNA levels (aP < 0.05 vs H6C7 cells); B, D: Bax and Bcl-2 in Capan-2 cells were immunostained using anti-Bax and anti-Bcl-2 antibodies as the primary antibody and Texas Red-conjugated IgG as the secondary antibody. The individual images for Bax and Bcl-2 were taken at a magnification of × 200, under an immunofluorescence microscope. Representative data from three independent experiments are shown.
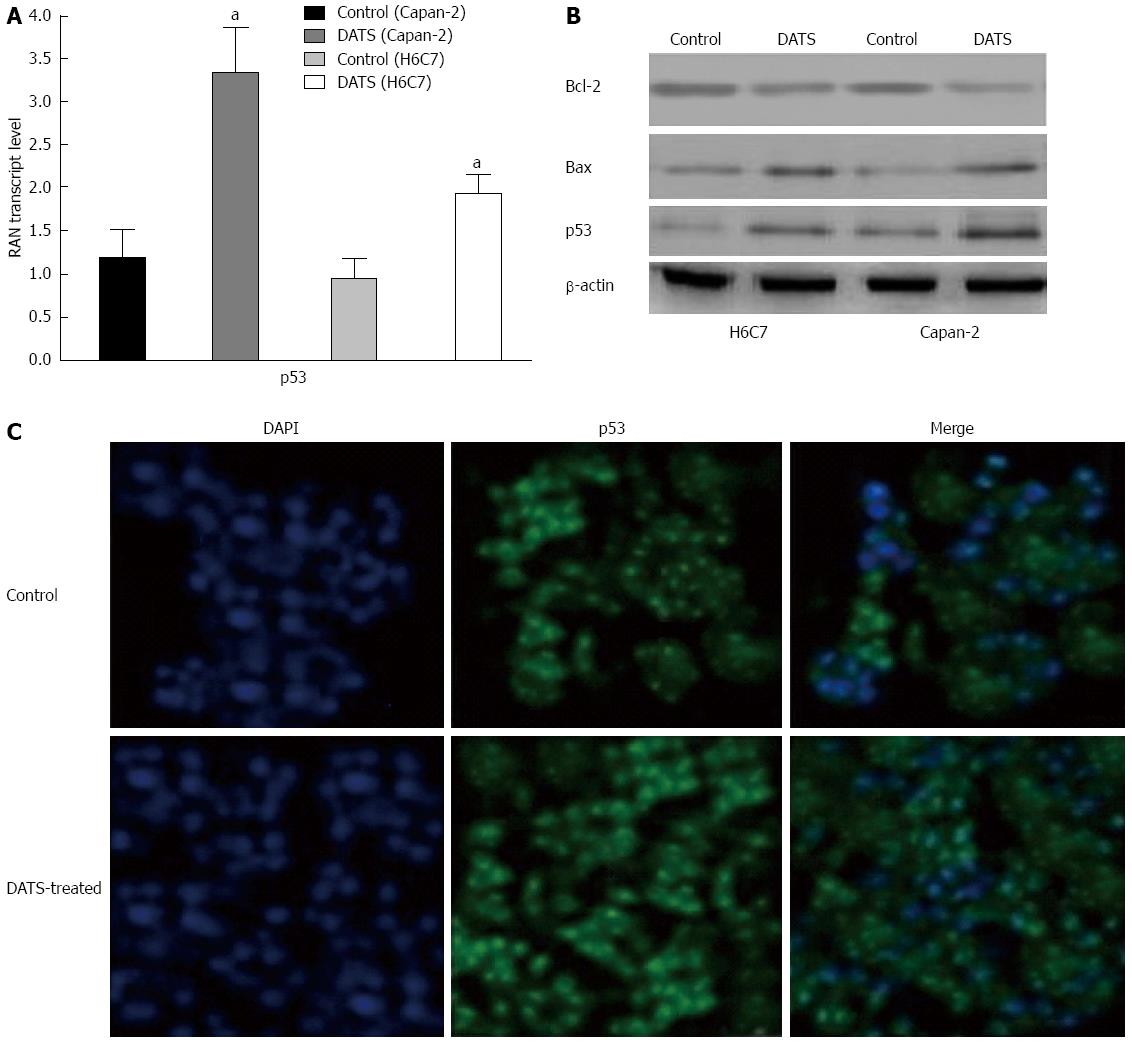
DATS induced p53 expression in Capan-2 cells
The p53 expression was up-regulated in Capan-2 cells compared with that in H6C7 cells after treatment with 100 μmol/L DATS. Bax expression was also elevated in Capan-2 cells by approximately 3 folds compared with the untreated control group (P < 0.05); however, Bcl-2 expression was reduced in Capan-2 cells (Figure 5A and B). Immunofluorescence showed that treatment with 100 μmol/L DATS induced p53 translocation from the cytoplasm (indicated in green) to the nucleus (indicated in blue) compared with the untreated control group (Figure 5C).
Figure 5 Diallyl trisulfide upregulates Bax expression and deregulates Bcl-2 expression and p53 translocation to the nucleus in Capan-2 cells.
A and B: Cells were treated with 100 μmol/L of diallyl trisulfide (DATS) for 24 h (aP < 0.05 vs control). Cells were lysed and total protein was collected and subjected to Western blot analysis to determine p53, Bax and Bcl-2 protein expression levels in treated and untreated control cells. β-actin was used as a loading control; C: The subcellular localization of p53 was indicated by the green FITC, while the position of the nucleus was shown by the blue DAPI staining. Individual images for p53 localization in the nucleus were taken at a magnification of × 400. Images were merged to display the subcellular localization of p53, using ECLIPSE. Representative data from three independent experiments are shown. DAPI: 4',6-diamidino-2-phenylindole.
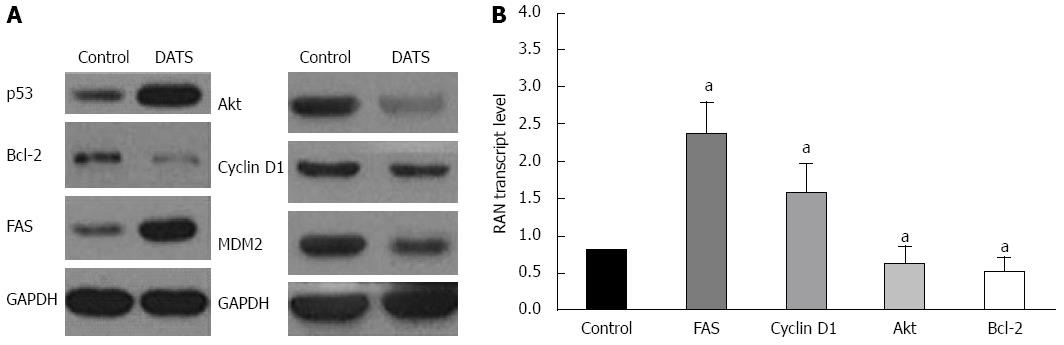
DATS affects apoptotic and survival signals
Akt inhibits molecular events that precede cytochrome C release, while Bcl-2 prevents its initial release. Treatment with 100 μmol/L DATS for 24 h significantly decreased the expression level of Akt by 40% compared with the control group (Figure 6B, P < 0.055). FAS expression level was increased after DATS treatment for 24 h by 4 folds compared with the control group (Figure 6, P < 0.05). Figure 6B also shows a 2-fold increase in the expression level of cyclin D1 (P < 0.05) and a 50% decrease in Bcl-2 expression level in response to 100 μmol/L DATS treatment for 24 h (P < 0.05) in Capan-2 cells. The results of RT-PCR also confirmed the above findings with a decrease in MDM2 level and an increase in p53 level as previously described (Figure 6A).
Figure 6 Diallyl trisulfide modulates survival and apoptotic signals in Capan-2 cells.
Capan-2 cells were treated with 100 μmol/L of diallyl trisulfide (DATS) for 24 h. A: Western blot was performed to determine the protein expression level of p53, Bcl-2, Fas, Akt, cyclin D1 and MDM2 in Capan-2 cells. Untreated cells were used as controls; B: mRNA was extracted and reversed transcribed to cDNA for real-time polymerase chain reaction analysis of mRNA levels. Each data point is an average of three independent experiments and expressed as mean ± SD (aP < 0.05 vs control).
DISCUSSION
Allium vegetables have been used in traditional medicine for centuries[14]. Recent scientific investigations have shown that allium vegetables and their constituents, garlic-derived organosulfur compounds (OSCs), including diallyl sulfide, diallyl disulfide and DATS, reduce the risk of cardiovascular disease and diabetes, stimulate the immune system, protect against infections, and have anti-aging as well as anti-cancer effects[13]. The anticancer effects of allium vegetables, especially garlic, are supported by epidemiological data[15-17]. It has been proved that garlic-derived OSCs, including diallyl sulfide, diallyl disulfide and DATS, show significant protection against different types of malignancies in animal models[18-24].
Numerous publications indicate that the suppression of cancer cell growth by OSCs correlates with apoptosis induction. Elucidation of the mechanism(s) of apoptosis induction by OSCs has been the topic of intense research in the last few years. Most studies indicate the involvement of Bcl-2 family proteins and p53 in the regulation of OSC-mediated apoptosis[9,24-27]. Our data showed that DATS treatment could induce apoptosis of Capan-2 cells via activation of p53. p53 is a tumor suppressor, is mutated in 50% of human cancers and regulates cell growth and sensitivity to irradiation and multiple anticancer agents[28-30]. Functional p53 can downregulate Bcl-2, which allows cells to survive a variety of fatal cellular events and protects cells from apoptosis[31]. p53 can also induce p21, and an increased level of p21 can in turn decrease the activity of cyclin-dependent kinases (CDKs), resulting in growth arrest[32-34]. Our results indicated that DATS increased the expression and translocation of p53 from the cytoplasm to the nucleus in Capan-2 cells following exposure to DATS, which was correlated with increased induction of apoptosis.
The tumor suppressor p53 is a key regulator of apoptosis and has pro-apoptotic activity. Under stress conditions, p53 is stabilized and acts as a transcription factor which may increase the expression of pro-apoptotic target genes, such as Puma, Noxa, Bax and Bid[35]. On the other hand, cytoplasmic p53 interacts with Bcl-2 family members, Bcl-2 or Bcl-xL, which results in activation and translocation of Bax and Bid to the mitochondrial outer membrane. Moreover, p53 also translocates to the mitochondria to activate the mitochondrial apoptosis pathway[35-37]. It was reported that DATS-induced apoptosis was attributed to the induction of Bax, downregulation of Bcl-xL and activation of caspase 3[8,38]. In addition, activated Akt can phosphorylate several apoptosis-regulating proteins including pro-apoptotic Bcl-2 family member, BAD[39,40]. BAD promotes cell death by interacting with anti-apoptotic Bcl-2 members such as Bcl-xL, which allows the multidomain pro-apoptotic Bcl-2 family members, Bax and Bak, to aggregate and cause release of apoptogenic molecules (e.g., cytochrome C) from mitochondria to the cytosol, culminating in caspase activation and cell death[41]. Our results support the conclusion that a significant decrease in the levels of survival signals is generated by both Bcl-2 and Akt (Figure 6).
It is well documented that inappropriate expression of cell-cycle regulatory proteins can contribute to human tumorigenesis[42]. Numerous studies have reported the relationship between carcinogenesis and cell cycle-related genes[43-47]. In experimental animals, DATS was capable of suppressing the growth of cancer cells in culture[43]. The DATS-mediated inhibition of cancer cell proliferation correlated with cell cycle arrest and apoptosis induction[42]. The mechanism of G2 phase arrest remains elusive. It was demonstrated that the DATS-mediated G2/M phase cell cycle arrest in the human prostate cancer cell line DU145 was transient and caused by differential kinetics of nuclear localization of CDK1 and cyclin B1[44]. The DATS-treated prostate cancer cells were arrested in G2 as well as the prometaphase[45]. This DATS-mediated prometaphase arrest was caused by checkpoint kinase 1-mediated inactivation of anaphase promoting complex/cyclosome characterized by accumulation of its substrates (e.g., securin)[42,46]. A fraction of cells arrested in prometaphase following treatment with DATS are ultimately driven to apoptotic cell death[46]. However, the mechanism underlying DATS-mediated G2 arrest is unclear.
The present study aimed to introduce DATS as an inducer of apoptosis in human pancreatic cancer cells (Capan-2) via a decrease in the percentage of cells in G2/M phase. DATS also increased the sensitivity of Capan-2 cells to radiation as indicated by the clonogenic survival assay and confirmed by flow cytometric analysis. Our data indicated that DATS increased the level of cyclin B1 in both Capan-2 and H6C7 cells, which was correlated with G2/M arrest, shown by a decrease in the percentage of cells in G2/M phase compared with the control group. Consistent with our data, it was recently reported that DATS suppresses the viability of cultured human lung cancer cells and normal human bronchial epithelial cells (BEAS-2B) by causing G2-M phase arrest and apoptotic cell death[47]. It is possible that the G2-M arrest is a priming mechanism for initiation of the cell death process in Capan-2 and H6C7 cells. A checkpoint kinase 1-dependent mechanism for DATS-induced mitotic arrest in human prostate cancer cells was previously reported[45]. Among the possible mechanisms for increased sensitivity to apoptosis in Capan-2 cells, overexpression of cyclin B1 may decrease the percentage of cells in G2/M phase. Our results also showed that elevated levels of cyclin B1 protein in Capan-2 and H6C7 cells due to DATS treatment increased apoptosis (Figure 4).
Cell cycle progression and cell division are driven by the sequential activation of a group of serine-threonine kinases called cyclin-dependent kinases (Cdks). Multiple Cdks control the cell cycle in mammals and have long been considered essential for normal proliferation, development and homeostasis[48]. Cyclin B1 is the regulatory subunit of the M-phase promoting factor, and correct regulation of cyclin B1 is essential for the initiation of mitosis. The deregulation of cyclin B1 is involved in neoplastic transformation, and the suppression of cyclin B1 could be an attractive strategy in antiproliferative therapy[49]. Our results also showed that elevated levels of cyclin B1 protein in Capan-2 and H6C7 cells due to DATS treatment increased apoptosis (Figure 4). Conversely, downregulation of cyclin B1 consequently reduced the activity of CDK1/cyclin B1 and blocked the aggressive proliferation of tumor cells[50].
There may be distinct differences in cyclin B1 signaling between cancer cell types, as a decrease in cyclin B1 protein signal pathways leads to apoptosis of cervical carcinoma cells, but not prostate cancer cells. Therefore, gene therapy strategies that reduce cyclin B1 protein are not likely to be effective in all types of cancer.
Cyclin D1 accumulates and activates its cognate CDK (CDK4/6) in response to mitogenic growth factors in early to mid G1 phase[51]. Perturbations in this step, which result in reduced growth factor requirements for cyclin D1/CDK activation, will provide cells with a distinct growth advantage over their normal counterparts, and thus likely represents an early event in neoplasia. Our data showed an increase in the expression level of cyclin D1, which was correlated with the percentage decrease in cells in the G2/M phase and an increase in the induction of apoptosis.
The enhanced induction of apoptosis after ionizing radiation was related to the induction of p53 and p21 by cyclin D1[52]. In the present study, cyclin D1-associated overexpression of p53 may facilitate DATS-induced death of Capan-2 cells. The mechanism for DATS-induced apoptosis has been studied in various cellular systems, and it is unclear if the DATS-induced apoptosis signaling in Capan-2 cancer cells resembles that in other cellular systems. The results of the present study point to noticeable differences in apoptosis signaling between Capan-2 cells and other cancer cells.
In conclusion, the present study indicated that DATS treatment inhibited the growth of human pancreatic cancer cells (Capan-2) by inducing apoptosis. In addition, non-tumorigenic pancreatic ductal epithelial cells (H6C7) were significantly more resistant to DATS-mediated growth suppression and apoptosis induction compared with pancreatic cancer cells (Capan-2). Also, our data showed that DATS increased the expression of p53 and induced its translocation to the nucleus in Capan-2 cells. DATS also increased the expression of Bax as a downstream target of p53. We also provided evidence to indicate the involvement of Fas, cyclin B1, cyclin D, Akt and Bcl-2 as possible critical mediators of DATS-induced apoptosis signaling in Capan-2 cells.
COMMENTS
Background
Many recent studies have revealed that certain garlic-derived organosulfur compounds can suppress proliferation of cultured cancer cells by causing apoptosis and/or cell cycle arrest. Garlic (Allium sativum) is a common plant used mainly as food and has recently been reported to have medicinal properties. Garlic-derived organosulfur compounds including diallyl sulfide, diallyl disulfide (DADS) and/or diallyl trisulfide (DATS), which are major components of garlic, may be associated with a reduced risk of certain cancers. These compounds are known to inhibit cell proliferation, cause cell cycle arrest and increase apoptosis in various cancer cell lines. However, the cellular and molecular mechanisms underlying these activities have not yet been completely elucidated.
Research frontiers
The dismal prognosis of pancreatic cancer is further accentuated by its poor response to chemotherapy and to radiation therapy. Thus, therapies are needed to increase the efficiency of radiation and chemotherapy. The discovery of new therapeutic agents and approaches in patients with pancreatic cancer is of paramount importance. DATS may be a novel strategy for the treatment of human pancreatic cancer through radio- or chemo-sensitization.
Innovations and breakthroughs
The present study indicates that DATS treatment inhibits the growth of human pancreatic cancer cells (Capan-2) by inducing apoptosis. In addition, non-tumorigenic pancreatic ductal epithelial cells (H6C7) are significantly more resistant to DATS-mediated growth suppression and apoptosis induction than Capan-2 cells. The data also showed that DATS increased the expression of p53 and induced its translocation to the nucleus in human Capan-2 cells. DATS also increased the expression of Bax as a downstream target of p53. This study provided evidence to indicate the involvement of Fas, cyclin B1, cyclin D, Akt and Bcl-2 as possible critical mediators of DATS-induced apoptosis signaling in Capan-2 cells.
Applications
This study demonstrates that DATS, a promising cancer chemopreventive constituent of processed garlic, induces apoptosis in human pancreatic cancer cells (Capan-2) and non-tumorigenic pancreatic ductal epithelial cells (H6C7). The results show, for the first time, that DATS administration might be a novel strategy for the treatment of human pancreatic cancer.
Terminology
Garlic-derived organosulfur compounds including diallyl sulfide, DADS and/or DATS, which are major components of garlic, may be associated with a reduced risk of certain cancers.
Peer review
In this report, the authors present data demonstrating a pattern of cell survival and gene expression changes suggesting that diallyl disulfide may provide therapeutic benefit against pancreatic ductal adenocarcinoma. The data are presented nicely and the findings merit publication.