Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8671
Revised: April 16, 2013
Accepted: May 7, 2013
Published online: December 14, 2013
AIM: To study, in intact male transgenic mice, the effects of three diets based on olive oil and olive oil diet supplemented with lovastatin and orlistat on hepatic lipogenic enzymes expression, considered markers of cell proliferation.
METHODS: Forty ApcMin/+ mice were randomly divided into 4 groups and fed for 10 wk: olive oil (OO) group, n = 10 animals received a diet with olive oil 12%; olive oil plus lovastatin (LOVA) group, n = 10 animals received the same diet with olive oil supplemented with lovastatin 5 mg/kg; olive oil plus orlistat (OR) group, n = 10 animals fed the diet with olive oil supplemented with orlistat 50 mg/kg and SD group, n = 10 animals fed a standard diet. The activity of lipogenic enzymes and their gene expression were evaluated by radiometric and real-time reverse transcription-polymerase chain reaction assay, respectively.
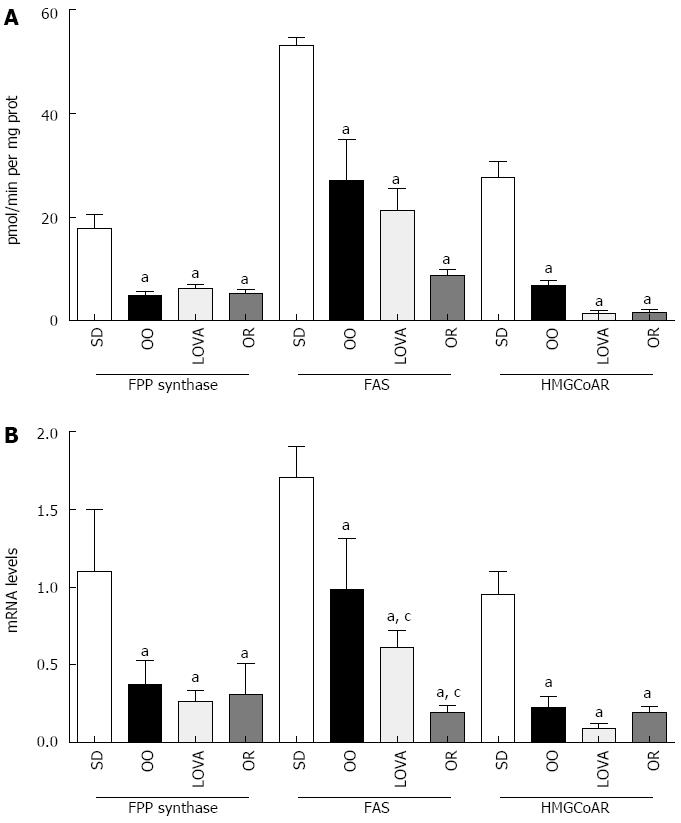
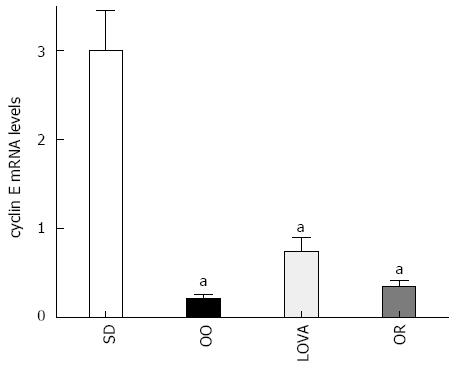
RESULTS: After 10 wk of dietary treatment, the body weight was no different among animal groups (21.3 ± 3.1 g for standard group, 22.1 ± 3.6 g for OO group, 22.0 ± 3.2 g for LOVA group and 20.7 ± 3.4 g for OR group, data expressed as mean ± SD), observing a generalized well-being in all animals. All the dietary managed treated groups presented significantly reduced hepatic levels of fatty acid synthase, farnesyl pyrophosphate synthase and 3-hydroxyl-3-methyl-glutaryl CoA reductase activity and gene expression when compared with the mice fed the standard diet. To evaluate cell proliferation in the liver of treated mice, the levels of cyclin E mRNA have been measured, demonstrating a significant reduction of cyclin E gene expression in all treated groups. Evidence of reduced hepatic cell proliferation was present overall in OO group mice.
CONCLUSION: We confirm the role of lipogenic enzymes as markers of cell proliferation, suggesting that appropriate dietary management alone or with drugs can be a feasible approach to counteract hepatic cell proliferation in mice.
Core tip: The olive oil diet significantly reduces the enzymatic activities, as well as the expression of hepatic cell cycle related genes. The addition of drugs as lovastatin and orlistat to olive oil diet more down-regulated the studied lipogenic enzymes, demonstrating that the inhibition of these enzymes with natural components of diet could have a potential benefit in association with canonical chemical substances to counteract hepatic cell proliferation in mice.
- Citation: Notarnicola M, Caruso MG, Tafaro A, Tutino V, Bianco G, Minoia M, Francavilla A. Dietary-suppression of hepatic lipogenic enzyme expression in intact male transgenic mice. World J Gastroenterol 2013; 19(46): 8671-8677
- URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8671.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8671
Several alterations of lipid metabolism are often found in tumors, where neoplastic lipogenesis is essential for cancer cell survival[1]. Cancer cells esterify fatty acids predominantly to phospholipids, essential component of cell membranes. The main pathway through which proliferating cells gain lipids for membrane synthesis is the endogenous mevalonate pathway[2,3]. Increased synthesis of mevalonate and mevalonate derived isoprenoids supports increased cell proliferation through the activation of growth-regulatory proteins and oncoproteins and by promoting DNA synthesis[4,5].
Endogenous fatty acid synthesis is dependent on the activity of fatty acid synthase (FAS). This enzyme is over-expressed in many types of malignancies, including prostate, breast, lung and colon cancer[6-8]. The tumor environment contains regions of poor oxygenation and high acidity, and FAS over-expression could confer a selective growth advantage upon these unfavorable conditions[9].
It is known that hydroxyl-3-methyl-glutaryl CoA reductase (HMGCoAR) activity is up-regulated severalfold in colon tumors and not regulated by feedback inhibition from cholesterol compared with normal mucosa[2,10,11]. Alterations in biosynthetic processes of the mevalonate pathway and in the levels of enzyme products participating in this biochemical system may contribute to the cell growth advantage acquired during carcinogenic process and to the development of malignancy.
In cancer, high levels of mevalonate-derived metabolites, such as isoprenoid compounds have been demonstrated[5,12,13]. Several HMGCoA metabolites, such as farnesyl pyrophosphate (FPP) and geranyl pyrophosphate are implicated in oncogene activation and tumorigenesis[14]. FPP, produced by activity of FPP synthase, is the substrate for the farnesylation of a wide number of proteins implicated as potential growth regulators. FPP synthase gene is over-expressed in different human tumors as well as an upregulation of FPP synthase has been detected in about 85% of hepatocellular carcinoma[15]. An elevated FPP synthase expression has also been observed in rat prostate tumor cell lines[16].
Previously, we demonstrated an high FPP synthase activity in human colorectal cancer[17].
The regulation of lipogenic enzymes abundance in cancer cells is complex and occurs at the transcriptional or posttranscriptional levels. Several studies show that blockade of these enzymes can attenuate the growth and survival of tumor cells[1,18,19], being potential target for cancer therapy.
Moreover, promotion and progression of carcinogenesis are susceptible to nutritional interventions aimed at counteracting cancer development[20]. In this respect, olive oil consumption has been demonstrated to reduce the incidence of aberrant crypt foci in azoxymethane-treated rats[21]. Furthermore, olive oil is able to down-regulate the expression of cyclooxygenase-2 and BCL-2 proteins that plays a crucial role in colorectal carcinogenesis[22]. Olive oil healthy effects can be attributed not only to the higher relationship between unsaturated and saturated fatty acids, but also to the antioxidant property of its phenolic compounds, as oleuropein and hydroxytyrosol (HT). As antioxidants, polyphenols may protect cell constituents against oxidative damage and act as highly effective chemopreventive agents[23,24].
Olive oil polyphenols are quickly absorbed by intestine, but the biotransformation of absorbed HT should take place mostly in the liver. Taking into account this point, the aim of the present study was to test in the ApcMin/+ mouse model three diets based on olive oil and olive oil diet supplemented with lovastatin and orlistat, known agents with antitumor activity and inhibitors of HMGCoAR and FAS, respectively. Since high serum concentration of the lipid and liver steatosis have been observed in ApcMin/+ mice[25,26], this experimental model was selected to evaluate a putative hepatic dietary-induced down-regulation of lipogenic enzymes.
Five-week-old C57BL/6J mice with an heterozygote mutation for the Apc gene (ApcMin/+) were obtained from Charles River (Calco, CO, Italy). The mice were maintained in the animal care facility at our Institute. They were kept in temperature, air- and light-controlled conditions and received food and water ad libitum. Animals did not receive any surgical or hormonal manipulation but were kept anatomically and physiologically intact. All animals received care in compliance to the “Guide for the Care and Use of Laboratory Animals”. The procedures related to animal use have been communicated to the Italian Ministry of Health and approved.
Forty ApcMin/+ mice were randomly divided into 4 groups and fed for 10 wk: olive oil (OO) group, n = 10 animals received a diet with olive oil 12% (12.5% protein, 12% oils and fats, 3% fibers); lovastatin (LOVA) group, n = 10 animals received the same diet with olive oil supplemented with lovastatin 5 mg/kg; orlistat (OR) group, n = 10 animals fed the diet with olive oil supplemented with orlistat 50 mg/kg and SD group, n = 10 animals fed a standard diet (18.5 % protein, 5% oils and fats, 4.2% fibers). Any diet was provided in pellets by Mucedola Srl, Settimo Milanese, Italy. Body weight and food intake were measured every 3 d.
After 10 wk of dietary treatment, the animals were killed by cervical dislocation. The liver from each animal was immediately excised and washed with phosphate buffered saline. Samples of fresh liver tissue were rapidly frozen and stored at -80 °C and the counterpart specimens were fixed in 10% buffered formalin to assess histological analysis.
FAS activity was determined on frozen liver samples. After tissue homogenization and centrifugation, an aliquot of supernatant (50 μL) was pre-incubated with 100 mmol/L potassium phosphate buffer, pH = 7 for 15 min at 37 °C. Subsequently, 20 μL of reaction mix (2.5 mmol/L NADPH, 1.25 mmol/L acetyl-CoA, 1.25 mmol/L malonyl-CoA and 0.02 mmol/L 2-14C-malonyl-CoA (52 mCi/mmol, Amersham Biosciences, United Kingdom) were added and samples were incubated for 10 min at 37 °C. Reactions were stopped by the addition of 500 μL 1 mol/L HCl/methanol (6:4, v:v); fatty acids were extracted with 1 mL of petroleum ether and incorporation of 2-14C-malonyl-CoA was analyzed by scintillation counting. FAS activity was expressed as picomoles of incorporated 2-14C-malonyl-CoA per minute per milligram of total proteins (pmol/min/mg prot).
Frozen hepatic tissue specimens were placed in cold homogenization buffer containing 0.3 mol/L sucrose, 10 mmol/L EDTA (pH = 7.4) and 1 mmol/L 2-β-mercaptoethanol. Each homogenate was centrifuged at 900 ×g for 5 min at 4 °C; the supernatant was further centrifuged at 8700 g for 10 min; the pellet was discarded and the supernatant was centrifuged at 10000 g for 10 min to obtain microsomal fraction. Each pellet was resuspended in 0.2 mL ice-cold buffer containing 20 mmol/L imidazol (pH = 7.4), 5 mmol/L dithiothreitol.
FPP synthase assay was carried out with some modifications of the procedure of Krisans et al[13] and described by Gupta et al[27]. Briefly, FPP synthase was assayed in 150 μL containing 25 mmol/L Hepes, pH = 7, 2 mmol/L MgCl2, 1 mmol/L dithiothreitol, 5 mmol/L KF, 1% noctyl-β-glucopyranoside, 3.3 μmol/L [4-14C] IPP (18 Ci/mmol), 3 μmol/L unlabeled IPP and 20 μmol/L geranyl diphosphate. Reactions were started by adding 40 μL of microsomal fraction containing 100 μg of total protein and incubated for 45 min at 37 °C. Reactions were stopped by the addition of 150 μL 2.5 mol/L HCl in 80% ethanol containing 100 μg/mL farnesol as a carrier. The samples were hydrolyzed for 30 min at 37 °C to convert the FPP to farnesol and neutralized by the addition of 150 μL of 10% NaOH. The reaction product (farnesol) was extracted into 1 mL of N-hexane and an aliquot (200 μL) of the organic phase was used for radioactivity counting. One unit of enzyme activity is defined as the amount of enzyme required to synthesize one pmol of FPP per min. Parallel samples were assayed to evaluate the total and the nonspecific radioactivity. In all experiments, enzyme assays were carried out in duplicate. The coefficient percentages of intra- and inter-assay variation were 3% and 4%, respectively.
HMGCoAR activity was measured by radiochemical assay using DL-3-hydroxy-3-methyl-[3-14C]-glutaryl-coenzyme A (14C-HMGCoA) as substrate. Briefly, 50 μL of microsomal fraction containing about 50 μg of total protein were pre-incubation for 10 min at 37 °C with 50 μL of cofactors solution containing 1 mol/L potassium-phosphate buffer (pH = 7.4), 100 mmol/L EDTA (pH = 7.4), 50 mmol/L dithiothreitol, 200 mmol/L glucose-6-phosphate, 25 mmol/L NADP and 0.5 U of glucose-6-phosphate dehydrogenase. Reactions were started by adding 10 μL of 14C-HMGCoA (specific activity 5.0 mCi/mmol) in each sample followed by an incubation for 20 min at 37 °C. Reactions were stopped by the addition of 20 μL of KOH 33% and incubated for 45 min to consent the hydrolysis of substrate. The subsequent addition of 50 μL of 5 mol/L HCl and the incubation for 60 min at 37 °C were performed to convert the formed [14C]-mevalonic acid in [14C]-mevalonolactone. The reaction product (14C-mevalonolactone) was extracted into 300 μL of N-hexane and an aliquot (200 μL) of the organic phase was used for radioactivity counting. HMGCoAR activity was expressed as picomoles of [14C]-mevalonate formed per minute per milligram of microsomal proteins (pmol/min/mg prot). In all experiments, enzyme assays were carried out in duplicate.
Total RNA from samples of liver tissue was isolated with TRI-Reagent (Mol. Res. Centre Inc. Cincinnati, O, United States), following the manufacturer’s instruction. Briefly, the tissue was homogenized in 0.25 mL of cold 0.9% NaCl; then, 0.75 mL of TRI-Reagent and 0.2 mL of chloroform were added to the homogenate. The samples were vigorously shaken and centrifuged and the RNA present in the aqueous phase was precipitated with 0.5 mL of isopropanol. The RNA pellet was washed once with 1 mL of 75% ethanol, dried, resuspended in sterile water and quantified by UV absorbance. 2 μg of total RNA were used for the reverse transcription reaction performed in 20 μL of final volume at 41 °C for 60 min, using 30 pmol of antisense primer (Table 1) for analyses of the FAS, FPP synthase, HMGCoAR, cyclin E and the β-actin gene. β-actin gene was utilized as reference gene. Real-time PCRs were performed in 25 μL of final volume containing 2 μL of cDNA, master mix with SYBR Green (iQ SYBR Green Supermix Bio-Rad, Milan, Italy) and sense and antisense primers for the FAS, FPP synthase, HMGCoAR, cyclin E and the β-actin gene (Table 1).
| Gene | Primer | |
| FPP synthase | Sense | 5’-AAAATTGGCACTGACATCCAGG-3’ |
| Antisense | 5’-GGGTGCTGCGTACTGTTCAATG-3’ | |
| FAS | Sense | 5’-GATCCTGGAACGAGAACACGA-3’ |
| Antisense | 5’-GAGACGTGTCACTCCTGGACTTG-3’ | |
| HMGCoAR | Sense | 5’-GCTTGAGCATCCTGACATAC-3’ |
| Antisense | 5’-GAACCATAGTTCCCACGTCT-3’ | |
| Cyclin E | Sense | 5’-GTCTTCGCAGATCGCAGA-3’ |
| Antisense | 5’-GAGACCTTCTGCGACTCCA-3’ | |
| β-actin | Sense | 5’-GCCTCTGGTCGTACCACTGGC-3’ |
| Antisense | 5’-AGGGAGGAAGAGGATGCGGCA-3’ |
Real-time polymerase chain reaction (PCR) was carried out in an CFX96 Real-time PCR Detection System (Bio-Rad Laboratories, Inc.) using the following protocol: 45 cycles at 95 °C for 3 min, 95 °C for 10 s, 55 °C for 30 s followed by a melting curve step at 65 °C-95 °C with a heating rate of 0.5 °C per cycle for 80 cycles. The PCR products were quantified by external calibration curves, one for each tested gene, obtained with serial dilutions of known copy number of molecules (102-107 molecules). All expression data were normalized by dividing the target amount by the amount of β-actin used as internal control for each sample. The specificity of the PCR product was confirmed by gel electrophoresis.
The significance of the differences among experimental groups was evaluated with one-way analysis of variance (ANOVA) and Tukey’s Multiple Comparison Test. Differences were considered significant at a 5% probability level.
After 10 wk of dietary treatment, the body weight (in grams) was no different among animal groups (21.3 ± 3.1 g for standard group, 22.1 ± 3.6 g for OO group, 22.0 ± 3.2 g for LOVA group and 20.7 ± 3.4 g for OR group, data expressed as mean ± SD), observing a generalized well-being in all animals.
All the dietary managed treated groups presented significantly reduced levels of hepatic lipogenic enzymes activity when compared with the mice fed the standard diet, being such reduction particularly marked in LOVA group for HMGCoAR and in OR group for FAS (one-way analysis of variance and Tukey’s Multiple Comparison Test, P < 0.05) (Figure 1A).
The levels of FPP synthase, FAS and HMGCoAR mRNA in liver tissue followed the same behavior of protein activities (Figure 1B). Differences statistically significant were observed between the three groups of treatment and the mice group treated with standard diet for all enzymes studied (one-way analysis of variance and Tukey’s Multiple Comparison Test, P < 0.05). For FAS mRNA levels, a significant reduction was also detected between LOVA group and OR group compared to OO group (Figure 1B).
To evaluate cell proliferation in the liver of treated mice, the levels of cyclin E mRNA have been measured, demonstrating a significant reduction of cyclin E gene expression in all treated groups. Evidence of reduced hepatic cell proliferation was present overall in OO group mice (Figure 2).
Our data support appropriate dietary management as a feasible approach to counteract hepatic cell proliferation in mice, targeted to the selective down regulation of FAS, FPP synthase and HMGCoAR considered biomarkers of cell proliferation.
Understanding the distribution of roles within a biochemical pathway is clearly important and it provides a rationale for selecting a particular reaction step suitable for therapeutic intervention.
Statins having biochemical effects on cholesterol synthesis, are considered as potential anti-tumor agents[28], inhibiting tumor cell growth by restricting either cholesterol availability or cholesterol synthesis[28,29]. Previously, we have demonstrated that the combined treatment with eicosapentaenoic acid (EPA) and lovastatin enhanced the regulatory effect on gene expression of HMGCoAR and low density lipoprotein receptor in HepG2 cell line[30]. Moreover, we detected a synergistic effect in the inhibition of cancer cell proliferation obtained by combination of EPA and Lovastatin, demonstrating an inhibition at the lower doses with respect to the substances used separately.
On the other hand, orlistat, as an anti-obesity drug, is a novel and selective FAS inhibitor in tumors[1]. Moreover, FAS inhibition by orlistat reduces proliferation and promotes apoptosis in prostate, breast and gastric cancer cell lines[31,32].
In our previous study[33], we showed a down-regulation of FAS observed after HT treatment in SW620 cell line, suggesting that FAS might be a molecular target for anti-proliferative activity of olive oil polyphenols in a metabolically defined subset of colon cancer.
In this study the reduction of cyclin E gene expression in mice liver by these compounds demonstrates their ability to inhibit cell proliferation. This finding supports the role of lipogenic enzymes as markers of cell growth.
The olive oil diet significantly reduces the enzymatic activities, as well as the expression of hepatic cell cycle related genes. The addition of drugs as lovastatin and orlistat to olive oil diet more down-regulated the studied lipogenic enzymes, demonstrating that the inhibition of these enzymes with natural components of diet could have a potential benefit in association with canonical chemical substances to counteract hepatic cell proliferation in mice.
The authors thank Mr. Vito Spilotro for his excellent technical assistance.
Several alterations of lipid metabolism are often found in tumors, where neoplastic lipogenesis is essential for cancer cell survival. Increased synthesis of mevalonate and mevalonate derived isoprenoids supports increased cell proliferation through the activation of growth-regulatory proteins and oncoproteins and by promoting DNA synthesis. Promotion and progression of carcinogenesis are susceptible to nutritional interventions aimed at counteracting cancer development. In this respect, olive oil consumption has been demonstrated to reduce the incidence of colorectal cancer. Olive oil healthy effects can be attributed not only to the higher relationship between unsaturated and saturated fatty acids, but also to the antioxidant property of its phenolic compounds, as oleuropein and hydroxytyrosol.
Alterations in biosynthetic processes of the mevalonate pathway and in the levels of enzyme products participating in this biochemical system may contribute to the cell growth advantage acquired during carcinogenic process and to the development of malignancy. The blockade of these enzymes can attenuate the growth and survival of tumor cells, being potential target for cancer therapy. Their paper demonstrates that the inhibition of lipogenic enzymes with natural components of diet could have a potential benefit in association with canonical chemical substances to counteract hepatic cell proliferation in mice.
The study supports the role of lipogenic enzymes as markers of cell growth. Reported data on possible benefits of olive oil to counteract hepatic cell proliferation in mice, highlight the importance and the innovation that an appropriate dietary treatment can be useful in cancer prevention.
Further studies will be designed to translate the findings in clinical practice. Olive oil through its ability to suppress the lipogenic enzymes may provide well-tolerated novel therapies, particularly in metabolic disorders-related tumors, as gastrointestinal cancers.
Lipogenic enzymes are involved in lipid metabolism. The lipogenesis is essential for cell membrane synthesis. The main pathway through which proliferating cells gain lipids for membrane synthesis is the endogenous mevalonate pathway, where the 3-hydroxyl-3-methyl-glutaryl CoA reductase (HMGCoAR) is the key enzyme. Among HMGCoAR metabolites, farnesyl pyrophosphate (FPP), produced by activity of FPP synthase, is the substrate for the farnesylation of a wide number of proteins considered potential growth regulators. Moreover, endogenous fatty acid synthesis is dependent on the activity of fatty acid synthase (FAS). FAS is a multi-enzyme protein containing domains for acyl-carrier peptide and the seven different catalytic activities required for the conversion of acetyl-CoA and malonyl-CoA to palmitate. Expression of FAS is linked to specific functions such as the conversion and storage of energy in the liver and in adipose tissue, and is possibly involved in the regulation of food intake.
The aim of this research is to study the effects of three diets based on olive oil and olive oil diet supplemented with lovastatin and orlistat on hepatic lipogenic enzymes expression in ApcMin/+ mice which are divided randomly into 4 groups of 10 animals per group that were fed for 10 wk: Concentration of olive oil used was 12%; lovastatin 5 mg/kg; orlistat 50 mg/kg and SD group that was fed a standard diet. The activity of lipogenic enzymes and their gene expression were evaluated by radiometric and real-time reverse transcription-polymerase chain reaction assay. Results show that all the dietary managed treated groups significantly reduced hepatic levels of fatty acid synthase, farnesyl pyrophosphate synthase and 3-hydroxyl-3-methyl-glutaryl CoA reductase activity and gene expression when compared with the mice fed the standard diet. The data are the potential interest and they confirm the role of lipogenic enzymes as markers of cell proliferation, suggesting that appropriate dietary management alone or with drugs can be a feasible approach to counteract hepatic cell proliferation in mice. The conclusions indicate that the observed effects could serve as markers for hepatic cell proliferation.
P- Reviewers: Chowdhury P, Grassi G, Zhong WX S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Chuang HY, Chang YF, Hwang JJ. Antitumor effect of orlistat, a fatty acid synthase inhibitor, is via activation of caspase-3 on human colorectal carcinoma-bearing animal. Biomed Pharmacother. 2011;65:286-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Notarnicola M, Messa C, Pricci M, Guerra V, Altomare DF, Montemurro S, Caruso MG. Up-regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in left-sided human colon cancer. Anticancer Res. 2004;24:3837-3842. [PubMed] [Cited in This Article: ] |
| 3. | Caruso MG, Notarnicola M. Biochemical changes of mevalonate pathway in human colorectal cancer. Anticancer Res. 2005;25:3393-3397. [PubMed] [Cited in This Article: ] |
| 4. | Siperstein MD. Role of cholesterogenesis and isoprenoid synthesis in DNA replication and cell growth. J Lipid Res. 1984;25:1462-1468. [PubMed] [Cited in This Article: ] |
| 5. | Holstein SA, Wohlford-Lenane CL, Hohl RJ. Isoprenoids influence expression of Ras and Ras-related proteins. Biochemistry. 2002;41:13698-13704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1925] [Cited by in F6Publishing: 2000] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 7. | Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 579] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 8. | Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977-5980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 474] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 9. | Hochachka PW, Rupert JL, Goldenberg L, Gleave M, Kozlowski P. Going malignant: the hypoxia-cancer connection in the prostate. Bioessays. 2002;24:749-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Caruso MG, Notarnicola M, Santillo M, Cavallini A, Di Leo A. Enhanced 3-hydroxy-3-methyl-glutaryl coenzyme A reductase activity in human colorectal cancer not expressing low density lipoprotein receptor. Anticancer Res. 1999;19:451-454. [PubMed] [Cited in This Article: ] |
| 11. | Caruso MG, Notarnicola M, Cavallini A, Di Leo A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity and low-density lipoprotein receptor expression in diffuse-type and intestinal-type human gastric cancer. J Gastroenterol. 2002;37:504-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Rao KN. The significance of the cholesterol biosynthetic pathway in cell growth and carcinogenesis (review). Anticancer Res. 1995;15:309-314. [PubMed] [Cited in This Article: ] |
| 13. | Krisans SK, Ericsson J, Edwards PA, Keller GA. Farnesyl-diphosphate synthase is localized in peroxisomes. J Biol Chem. 1994;269:14165-14169. [PubMed] [Cited in This Article: ] |
| 14. | Newmark HL. Squalene, olive oil, and cancer risk. Review and hypothesis. Ann N Y Acad Sci. 1999;889:193-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Sung YK, Hwang SY, Park MK, Farooq M, Han IS, Bae HI, Kim JC, Kim M. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94:259-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Jiang F, Yang L, Cai X, Cyriac J, Shechter I, Wang Z. Farnesyl diphosphate synthase is abundantly expressed and regulated by androgen in rat prostatic epithelial cells. J Steroid Biochem Mol Biol. 2001;78:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Notarnicola M, Messa C, Cavallini A, Bifulco M, Tecce MF, Eletto D, Di Leo A, Montemurro S, Laezza C, Caruso MG. Higher farnesyl diphosphate synthase activity in human colorectal cancer inhibition of cellular apoptosis. Oncology. 2004;67:351-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Diel IJ, Solomayer EF, Bastert G. Bisphosphonates and the prevention of metastasis: first evidences from preclinical and clinical studies. Cancer. 2000;88:3080-3088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Zeichner S, Mihos CG, Santana O. The pleiotropic effects and therapeutic potential of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in malignancies: a comprehensive review. J Cancer Res Ther. 2012;8:176-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Yang K, Edelmann W, Fan K, Lau K, Leung D, Newmark H, Kucherlapati R, Lipkin M. Dietary modulation of carcinoma development in a mouse model for human familial adenomatous polyposis. Cancer Res. 1998;58:5713-5717. [PubMed] [Cited in This Article: ] |
| 21. | Waterman E, Lockwood B. Active components and clinical applications of olive oil. Altern Med Rev. 2007;12:331-342. [PubMed] [Cited in This Article: ] |
| 22. | Corona G, Deiana M, Incani A, Vauzour D, Dessì MA, Spencer JP. Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem Biophys Res Commun. 2007;362:606-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Owen RW, Giacosa A, Hull WE, Haubner R, Würtele G, Spiegelhalder B, Bartsch H. Olive-oil consumption and health: the possible role of antioxidants. Lancet Oncol. 2000;1:107-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 439] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 24. | Gill CI, Boyd A, McDermott E, McCann M, Servili M, Selvaggini R, Taticchi A, Esposto S, Montedoro G, McGlynn H. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int J Cancer. 2005;117:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Niho N, Takahashi M, Kitamura T, Shoji Y, Itoh M, Noda T, Sugimura T, Wakabayashi K. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003;63:6090-6095. [PubMed] [Cited in This Article: ] |
| 26. | Mutoh M, Komiya M, Teraoka N, Ueno T, Takahashi M, Kitahashi T, Sugimura T, Wakabayashi K. Overexpression of low-density lipoprotein receptor and lipid accumulation in intestinal polyps in Min mice. Int J Cancer. 2009;125:2505-2510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Gupta SD, Mehan RS, Tansey TR, Chen HT, Goping G, Goldberg I, Shechter I. Differential binding of proteins to peroxisomes in rat hepatoma cells: unique association of enzymes involved in isoprenoid metabolism. J Lipid Res. 1999;40:1572-1584. [PubMed] [Cited in This Article: ] |
| 28. | Shibata MA, Kavanaugh C, Shibata E, Abe H, Nguyen P, Otsuki Y, Trepel JB, Green JE. Comparative effects of lovastatin on mammary and prostate oncogenesis in transgenic mouse models. Carcinogenesis. 2003;24:453-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 435] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 30. | Notarnicola M, Messa C, Refolo MG, Tutino V, Miccolis A, Caruso MG. Synergic effect of eicosapentaenoic acid and lovastatin on gene expression of HMGCoA reductase and LDL receptor in cultured HepG2 cells. Lipids Health Dis. 2010;9:135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070-2075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 440] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 32. | Dowling S, Cox J, Cenedella RJ. Inhibition of fatty acid synthase by Orlistat accelerates gastric tumor cell apoptosis in culture and increases survival rates in gastric tumor bearing mice in vivo. Lipids. 2009;44:489-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Notarnicola M, Pisanti S, Tutino V, Bocale D, Rotelli MT, Gentile A, Memeo V, Bifulco M, Perri E, Caruso MG. Effects of olive oil polyphenols on fatty acid synthase gene expression and activity in human colorectal cancer cells. Genes Nutr. 2011;6:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |